A Novel System for Real-Time, In Situ Monitoring of CO2 Sequestration in Photoautotrophic Biofilms
Abstract
1. Introduction
2. Materials and Methods
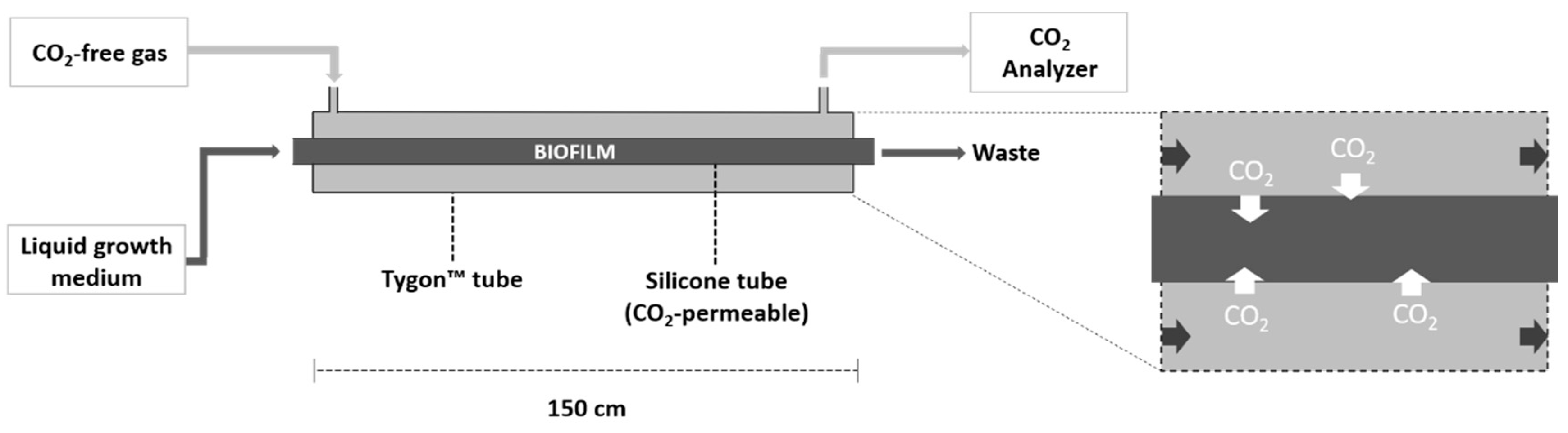
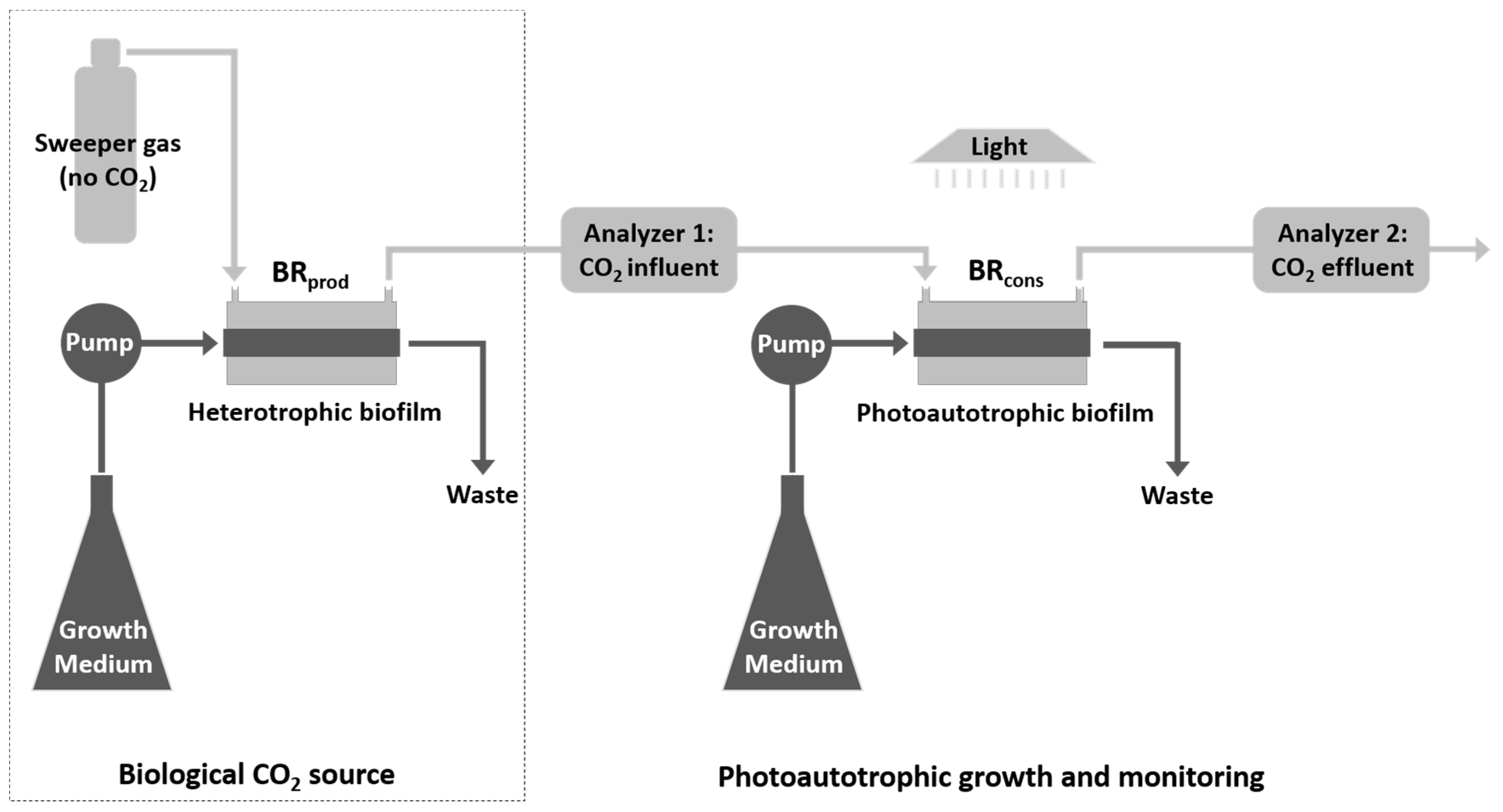
2.1. System Configuration
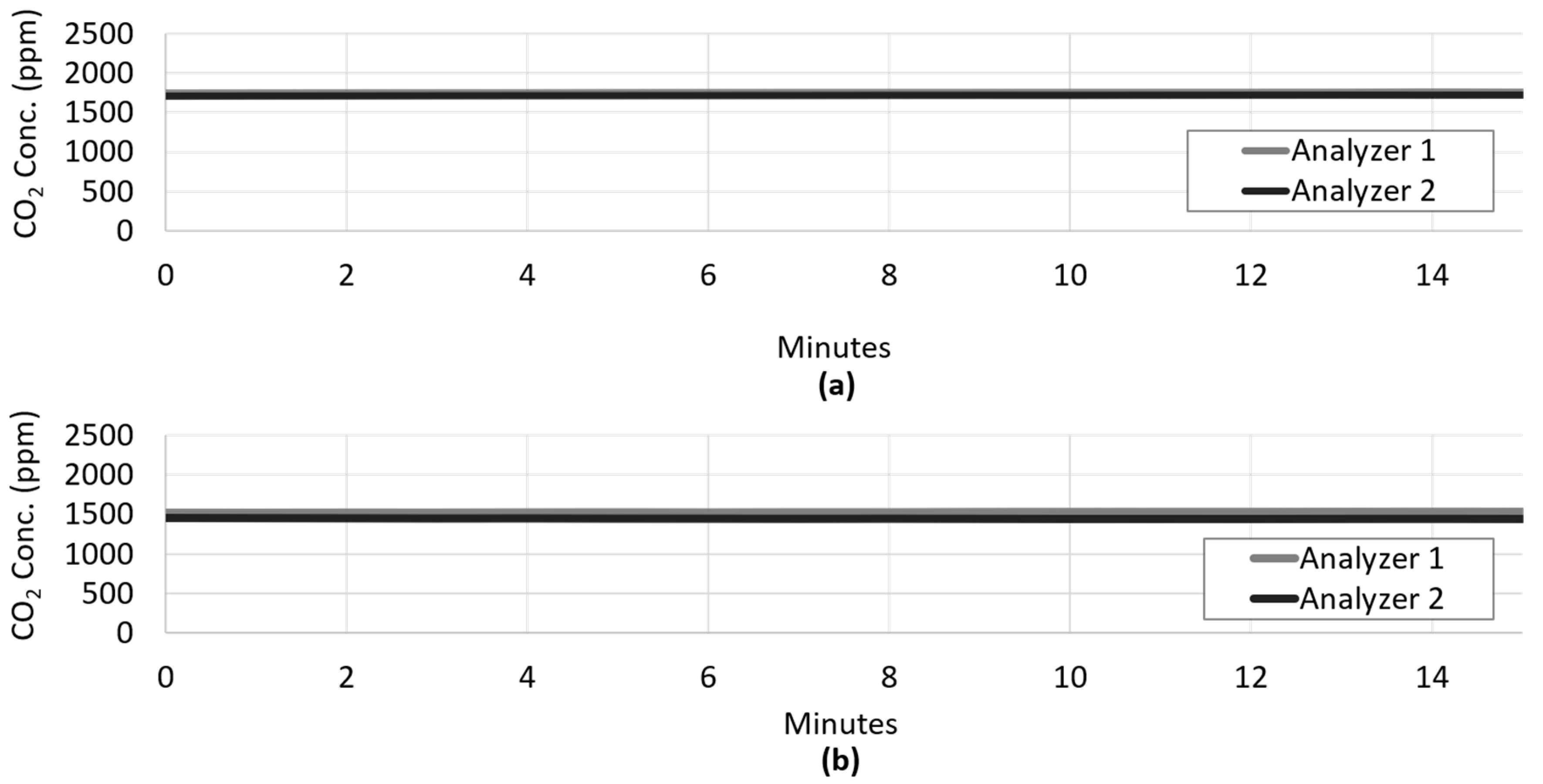
2.2. Gas Channeling and Monitoring
2.3. Light Exposure and Intensity
2.4. Growth Media
2.5. Test Cultures and System Inoculation
2.6. Assessing CO2 Uptake during Photoautotrophic Biofilm Development
2.7. Light Manipulations
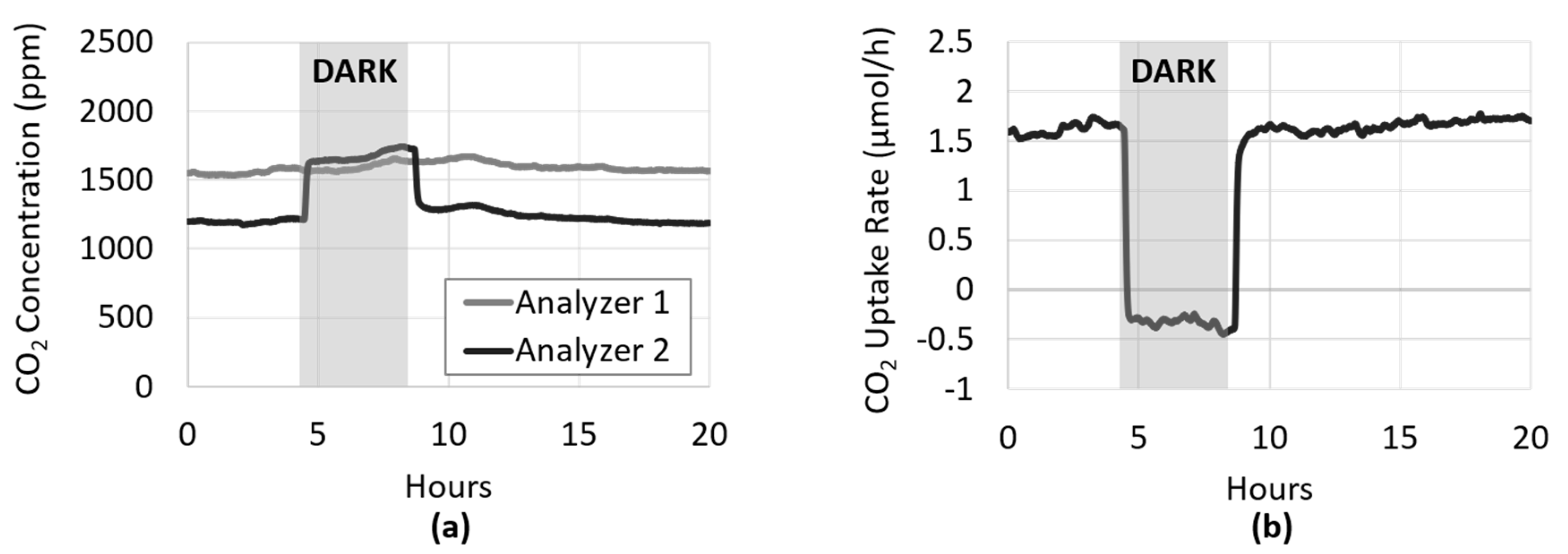
2.7.1. CO2 Uptake during Light–Dark Cycling
2.7.2. CO2 Uptake during Changes in Light Intensity
2.8. Variation in Biofilm Biomass
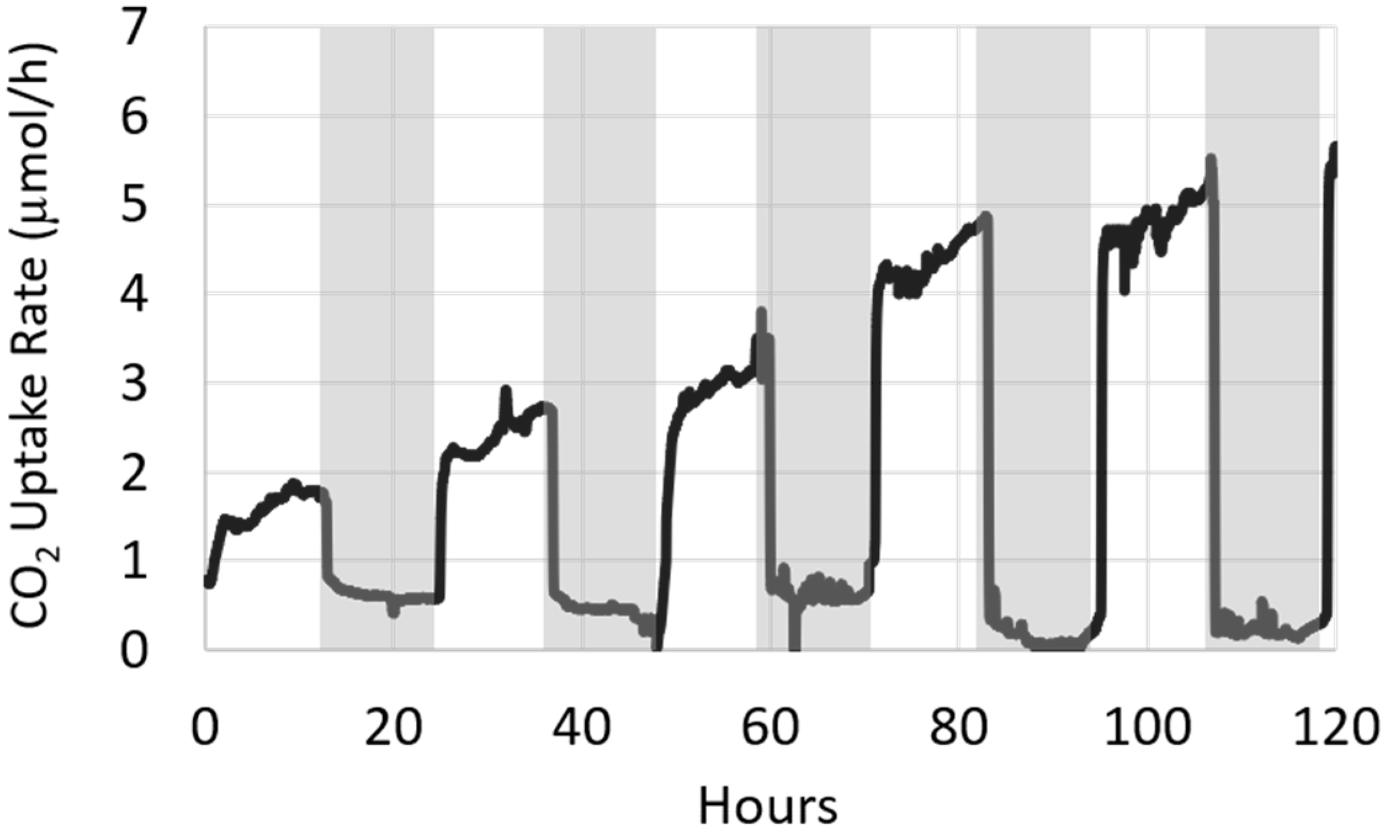
2.8.1. Assessing CO2 Uptake with Supplementary Photoautotrophic Biofilm
2.8.2. Assessing CO2 Uptake with Increased Photoautotrophic Biofilm Length
3. Results and Discussion
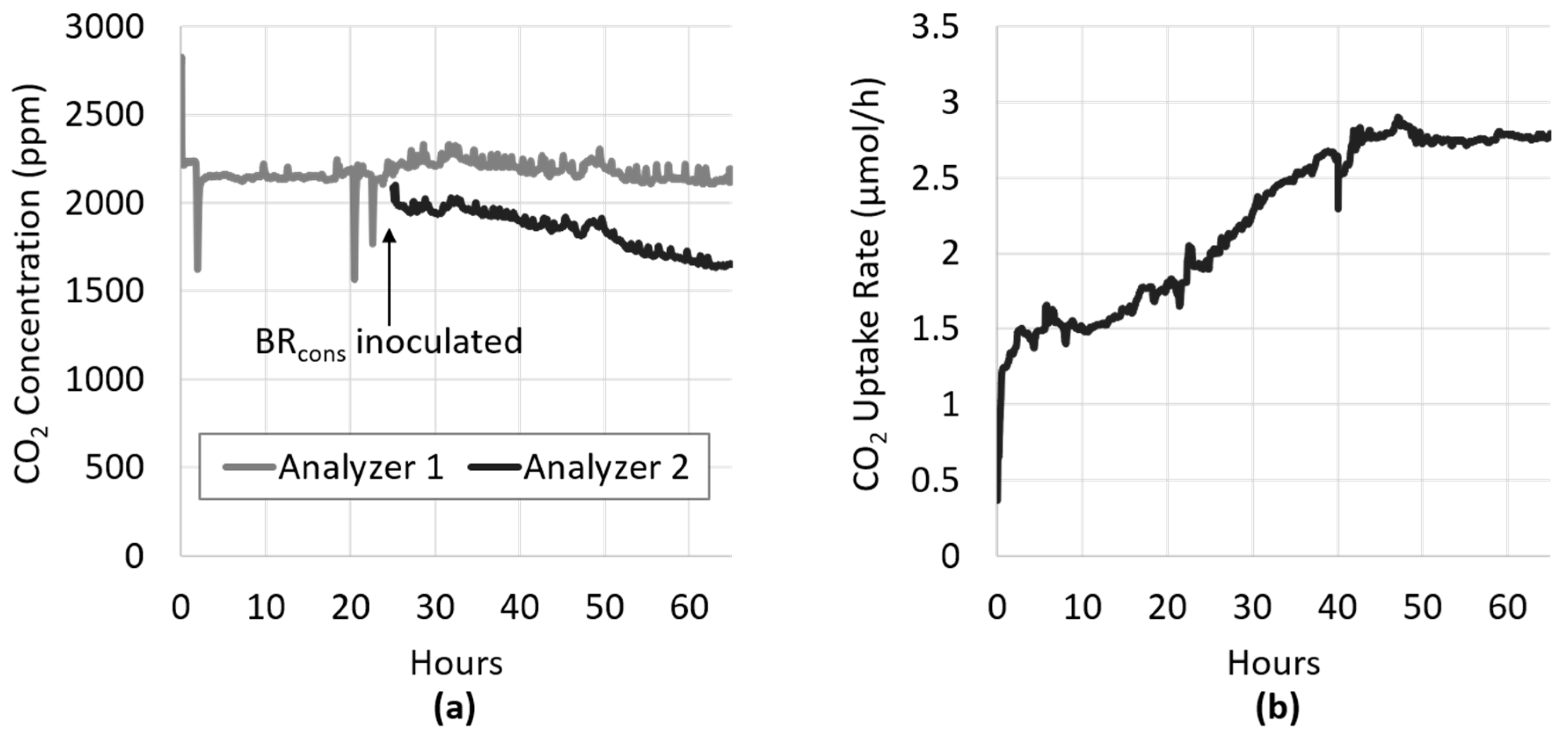
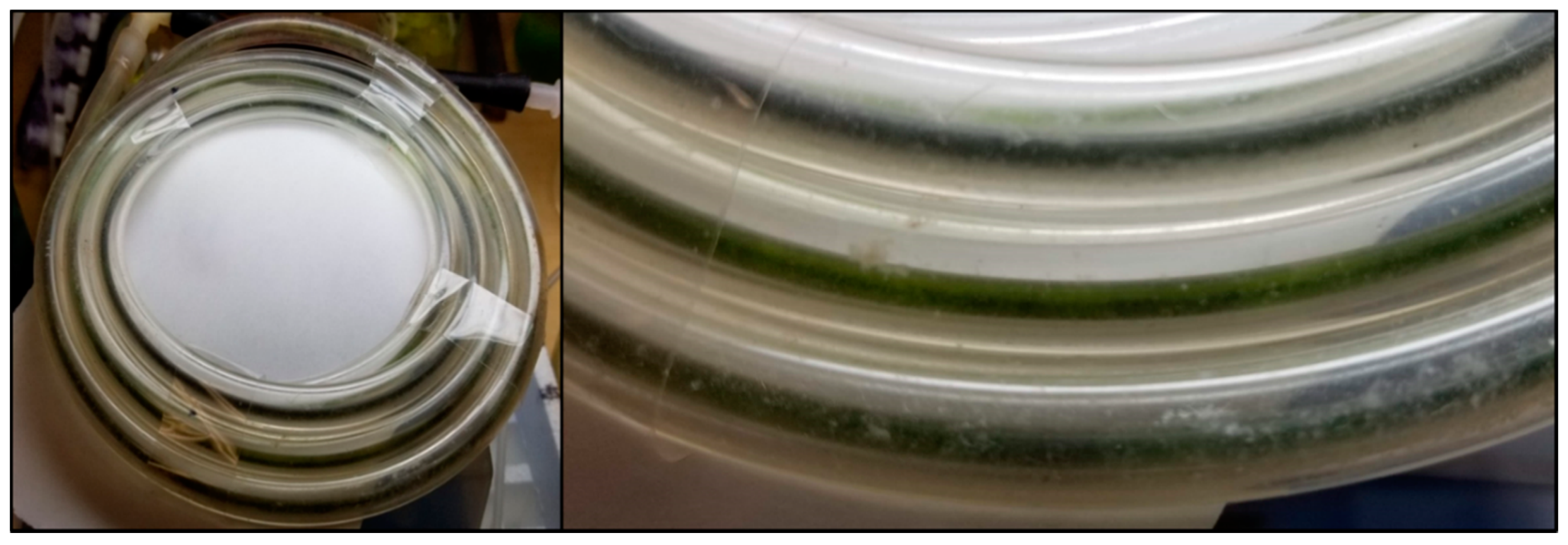
3.1. Development of Algal Biofilms
3.2. Light Manipulation
3.3. Variation in Biofilm Biomass
3.4. System Utility and Future Considerations
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Steffen, W.; Broadgate, W.; Deutsch, L.; Gaffney, O.; Ludwig, C. The trajectory of the Anthropocene: The Great Acceleration. Anthr. Rev. 2015, 2, 81–98. [Google Scholar] [CrossRef]
- Solomon, K.; Plattner, G.-K.; Knutti, R.; Friedlingstein, P. Irreversible climate change due to carbon dioxide emissions. Proc. Natl. Acad. Sci. USA 2009, 106, 1704–1709. [Google Scholar] [CrossRef]
- Hosseini, N.S.; Shang, H.; Scott, J.A. Biosequestration of industrial off-gas CO2 for enhanced lipid productivity in open microalgae cultivation systems. Renew. Sustain. Energy Rev. 2018, 92, 458–469. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, J.; Chen, P.; Ji, C.; Kang, Q.; Lu, B.; Li, K.; Liu, J.; Ruan, R. Bio-mitigation of carbon dioxide using microalgal systems: Advances and perspectives. Renew. Sustain. Energy Rev. 2017, 76, 1163–1175. [Google Scholar] [CrossRef]
- Bhola, V.; Swalaha, F.M.; Kumar, R.R.; Singh, M.; Bux, F. Overview of the potential of microalgae for CO2 sequestration. Int. J. Environ. Sci. Technol. 2014, 11, 2103–2118. [Google Scholar] [CrossRef]
- Gouveia, L.; Batista, A.P.; Sousa, I.; Raymundo, A.; Bandarra, N.M. Microalgae in novel food products. In Food Chemistry Research Developments; Papadopoulos, K.N., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2008; Chapter 2; pp. 75–111. [Google Scholar]
- Judd, S.; Broeke, L.J.V.D.; Shurair, M.; Kuti, Y.; Znad, H. Algal remediation of CO2 and nutrient discharges: A review. Water Res. 2015, 87, 356–366. [Google Scholar] [CrossRef]
- Jais, N.M.B.; Mohamed, R.; Al-Gheethi, A.; Hashim, M.K.A. The dual roles of phycoremediation of wet market wastewater for nutrients and heavy metals removal and microalgae biomass production. Clean Technol. Environ. Policy 2016, 19, 37–52. [Google Scholar] [CrossRef]
- Deshmukh, S.; Kumar, R.; Bala, K. Microalgae biodiesel: A review on oil extraction, fatty acid composition, properties and effect on engine performance and emissions. Fuel Process. Technol. 2019, 191, 232–247. [Google Scholar] [CrossRef]
- Goh, B.H.H.; Ong, H.C.; Cheah, M.Y.; Chen, W.-H.; Yu, K.L.; Mahlia, T.M.I. Sustainability of direct biodiesel synthesis from microalgae biomass: A critical review. Renew. Sustain. Energy Rev. 2019, 107, 59–74. [Google Scholar] [CrossRef]
- Nagappan, S.; Devendran, S.; Tsai, P.-C.; Dahms, H.-U.; Ponnusamy, V.K. Potential of two-stage cultivation in microalgae biofuel production. Fuel 2019, 252, 339–349. [Google Scholar] [CrossRef]
- Kesaano, M.; Sims, R.C. Algal biofilm based technology for wastewater treatment. Algal Res. 2014, 5, 231–240. [Google Scholar] [CrossRef]
- Hoffmann, J.P. Wastewater Treatment with Suspended and Nonsuspended Algae. J. Phycol. 1998, 34, 757–763. [Google Scholar] [CrossRef]
- Schnurr, P.J.; Allen, D.G. Factors affecting algae biofilm growth and lipid production: A review. Renew. Sustain. Energy Rev. 2015, 52, 418–429. [Google Scholar] [CrossRef]
- Donlan, R.M. Biofilms: Microbial Life on Surfaces. Emerg. Infect. Dis. 2002, 8, 881–890. [Google Scholar] [CrossRef]
- Choudhary, P.; Prajapati, S.K.; Kumar, P.; Malik, A.; Pant, K.K. Development and performance evaluation of an algal biofilm reactor for treatment of multiple wastewaters and characterization of biomass for diverse applications. Bioresour. Technol. 2017, 224, 276–284. [Google Scholar] [CrossRef]
- Katarzyna, L.; Sai, G.; Singh, O.A. Non-enclosure methods for non-suspended microalgae cultivation: Literature review and research needs. Renew. Sustain. Energy Rev. 2015, 42, 1418–1427. [Google Scholar] [CrossRef]
- Salih, F.M. Microalgae Tolerance to High Concentrations of Carbon Dioxide: A Review. J. Environ. Prot. 2011, 2, 648–654. [Google Scholar] [CrossRef]
- Chisti, Y. Constraints to commercialization of algal fuels. J. Biotechnol. 2013, 167, 201–214. [Google Scholar] [CrossRef]
- El-Sheekh, M.; Bedaiwy, M.Y.; E Osman, M.; Ismail, M.M. Mixotrophic and heterotrophic growth of some microalgae using extract of fungal-treated wheat bran. Int. J. Recycl. Org. Waste Agric. 2012, 1, 12. [Google Scholar] [CrossRef]
- Padmanabhan, P. Technical insight on the requirements for CO2-saturated growth of microalgae in photobioreactors. 3 Biotech 2017, 7, 119. [Google Scholar] [CrossRef][Green Version]
- Kroukamp, O.; Wolfaardt, G.M. CO2 Production as an Indicator of Biofilm Metabolism. Appl. Environ. Microbiol. 2009, 75, 4391–4397. [Google Scholar] [CrossRef]
- Krzemińska, I.; Pawlik-Skowrońska, B.; Trzcińska, M.; Tys, J. Influence of photoperiods on the growth rate and biomass productivity of green microalgae. Bioprocess Biosyst. Eng. 2013, 37, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Liberton, M.; Cliften, P.F.; Head, R.D.; Jacobs, J.M.; Smith, R.D.; Koppenaal, D.W.; Brand, J.J.; Pakrasi, H.B. Synechococcus elongatus UTEX 2973, a fast growing cyanobacterial chassis for biosynthesis using light and CO2. Sci. Rep. 2015, 5, 8132. [Google Scholar] [CrossRef]
- Chang, H.-X.; Huang, Y.; Fu, Q.; Liao, Q.; Zhu, X. Kinetic characteristics and modeling of microalgae Chlorella vulgaris growth and CO2 biofixation considering the coupled effects of light intensity and dissolved inorganic carbon. Bioresour. Technol. 2016, 206, 231–238. [Google Scholar] [CrossRef]
- Ghadakpour, M.; Bester, E.; Liss, S.N.; Gardam, M.; Droppo, I.; Hota, S.; Wolfaardt, G.M. Integration and Proliferation of Pseudomonas aeruginosa PA01 in Multispecies Biofilms. Microb. Ecol. 2014, 68, 121–131. [Google Scholar] [CrossRef]
- Lambertsen, L.; Sternberg, C.; Molin, S. Mini-Tn7 transposons for site-specific tagging of bacteria with fluorescent proteins. Environ. Microbiol. 2004, 6, 726–732. [Google Scholar] [CrossRef]
- Revsbech, N.P.; Jørgensen, B.B.; Brix, O. Primary production of microalgae in sediments measured by oxygen microprofile, H14CO3- fixation, and oxygen exchange methods. Limnol. Oceanogr. 1981, 26, 717–730. [Google Scholar] [CrossRef]
- Revsbech, N.P.; Jørgensen, B.B. Photosynthesis of benthic microflora measured with high spatial resolution by the oxygen microprofile method: Capabilities and limitations of the method. Limnol. Oceanogr. 1983, 28, 749–756. [Google Scholar] [CrossRef]
- Spaeth, E.E.; Friedlander, S.K. The Diffusion of Oxygen, Carbon Dioxide, and Inert Gas in Flowing Blood. Biophys. J. 1967, 7, 827–851. [Google Scholar] [CrossRef]
- Robb, W.L. Thin Silicone Membranes-Their Permeation Properties and some Applications. Ann. N. Y. Acad. Sci. 1968, 146, 119–137. [Google Scholar] [CrossRef]
- Hu, Q.; Sommerfield, M.; Jarvis, E.; Ghirardi, M.; Posewitz, M.; Seibert, M.; Darzins, A. Microalgal triaceylglycerols as feedstocks for biofuel production: Perspectives and advances. Plant J. 2008, 54, 621–639. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.-S.; Li, K.; Wang, Q.-M.; Song, X.-Y.; Su, H.-N.; Xie, B.-B.; Zhang, X.-Y.; Huang, F.; Chen, X.-L.; Zhou, B.-C.; et al. Nitrogen Starvation Impacts the Photosynthetic Performance of Porphyridium cruentum as Revealed by Chlorophyll a Fluorescence. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Liefer, J.; Garg, A.; Campbell, D.A.; Irwin, A.J.; Finkel, Z.V. Nitrogen starvation induces distinct photosynthetic responses and recovery dynamics in diatoms and prasinophytes. PLoS ONE 2018, 13, e0195705. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Chen, J.; Qin, S.; Zeng, M.; Jiang, Y.; Hu, L.; Xiao, P.; Hao, W.; Hu, Z.; Lei, A.; et al. Growth and lipid accumulation by different nutrients in the microalga Chlamydomonas reinhardtii. Biotechnol. Biofuels 2018, 11, 40. [Google Scholar] [CrossRef]
- Carvalho, A.; Malcata, F. Transfer of Carbon Dioxide within Cultures of Microalgae: Plain Bubbling versus Hollow-Fiber Modules. Biotechnol. Prog. 2001, 17, 265–272. [Google Scholar] [CrossRef]
- Blanken, W.; Schaap, S.; Theobald, S.; Rinzema, A.; Wijffels, R.H.; Janssen, M. Optimizing carbon dioxide utilization for microalgae biofilm cultivation. Biotechnol. Bioeng. 2016, 114, 769–776. [Google Scholar] [CrossRef]
- Lee, Y.-K.; Hing, H.-K. Supplying CO2 to photosynthetic algal cultures by diffusion through gas-permeable membranes. Appl. Microbiol. Biotechnol. 1989, 31, 298–301. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, Y.; Chen, Y.; Spalding, M.H.; Dong, L. Microfluidic chip for automated screening of carbon dioxide conditions for microbial cell growth. Biomicrofluidics 2017, 11, 1–9. [Google Scholar] [CrossRef]


| Inner Silicone Tube | Outer Tygon™ Tube | |
|---|---|---|
| Colonizable surface area | 74 cm2 | n/a |
| Volume | 2.90 mL | 19.85 mL |
| Flow rate | 0.25 mL/min | 2.5 mL/min |
| Retention time | 11.6 min | 7.9 min |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ronan, P.; Kroukamp, O.; Liss, S.N.; Wolfaardt, G. A Novel System for Real-Time, In Situ Monitoring of CO2 Sequestration in Photoautotrophic Biofilms. Microorganisms 2020, 8, 1163. https://doi.org/10.3390/microorganisms8081163
Ronan P, Kroukamp O, Liss SN, Wolfaardt G. A Novel System for Real-Time, In Situ Monitoring of CO2 Sequestration in Photoautotrophic Biofilms. Microorganisms. 2020; 8(8):1163. https://doi.org/10.3390/microorganisms8081163
Chicago/Turabian StyleRonan, Patrick, Otini Kroukamp, Steven N. Liss, and Gideon Wolfaardt. 2020. "A Novel System for Real-Time, In Situ Monitoring of CO2 Sequestration in Photoautotrophic Biofilms" Microorganisms 8, no. 8: 1163. https://doi.org/10.3390/microorganisms8081163
APA StyleRonan, P., Kroukamp, O., Liss, S. N., & Wolfaardt, G. (2020). A Novel System for Real-Time, In Situ Monitoring of CO2 Sequestration in Photoautotrophic Biofilms. Microorganisms, 8(8), 1163. https://doi.org/10.3390/microorganisms8081163





