Biofilm Mode of Cultivation Leads to an Improvement of the Entomotoxic Patterns of Two Aspergillus Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Mosquito Breeding
2.2. Aspergillus Cultivation
2.3. Acute Toxicity Test
2.4. Proteomic Analysis
2.5. Metabolomic Analysis
2.6. Statistical Analysis
3. Results
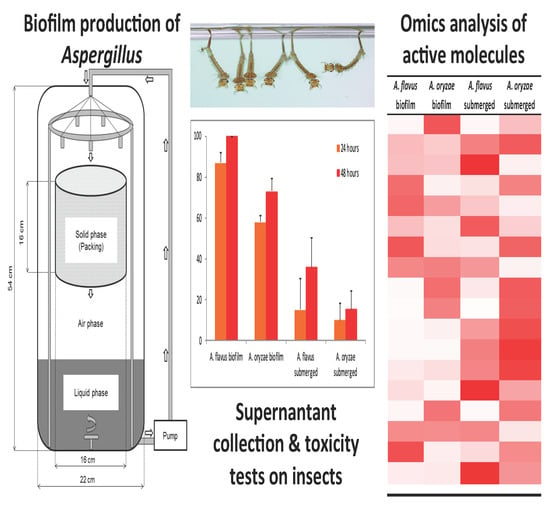
3.1. Biomass Production of the Two Species of Aspergillus
3.2. Acute Toxicity Test of Aspergillus Supernatants
3.3. Proteomic Analysis of Aspergillus Supernatants
3.4. Secondary Metabolomic Analysis of Aspergillus Supernatants
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Scholte, E.-J.; Knols, B.G.J.; Samson, R.A.; Takken, W. Entomopathogenic fungi for mosquito control: A review. J. Insect Sci. 2004, 4, 1–24. [Google Scholar] [CrossRef]
- Bawin, T.; Seye, F.; Boukraa, S.; Zimmer, J.-Y.; Delvigne, F.; Francis, F. La lutte contre les moustiques (Diptera: Culicidae): Diversité des approches et application du contrôle biologique. Can. Entomol. 2014, 147, 476–500. [Google Scholar] [CrossRef]
- Bawin, T.; Seye, F.; Boukraa, S.; Zimmer, J.-Y.; Raharimalala, F.N.; Zune, Q.; Ndiaye, M.; Delvigne, F.; Francis, F. Production of two entomopathogenic Aspergillus species and insecticidal activity against the mosquito Culex quinquefasciatus compared to Metarhizium anisopliae. Biocontrol Sci. Technol. 2016, 26, 617–629. [Google Scholar] [CrossRef]
- Seye, F.; Bawin, T.; Boukraa, S.; Zimmer, J.-Y.; Ndiaye, M.; Delvigne, F.; Francis, F. Pathogenicity of Aspergillus clavatus produced in a fungal biofilm bioreactor toward Culex quinquefasciatus (Diptera: Culicidae). J. Pestic. Sci. 2014, 39, 127–132. [Google Scholar] [CrossRef]
- WHO-IARC. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; Report No.: 82; WHO-IARC: Leone, France, 2002. [Google Scholar]
- Woloshuk, C.P.; Shim, W.B. Aflatoxins, fumonisins, and trichothecenes: A convergence of knowledge. FEMS Microbiol. Rev. 2013, 37, 94–109. [Google Scholar] [CrossRef] [PubMed]
- Abbas, H.K.; Zablotowicz, R.M.; Horn, B.W.; Phillips, N.A.; Johnson, B.J.; Jin, X. Comparison of major biocontrol strains of non-aflatoxigenic Aspergillus flavus for the reduction of aflatoxins and cyclopiazonic acid in maize. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2011, 28, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, R.; Ortega-Beltran, A.; Akande, A.; Mutegi, C.; Atehnkeng, J.; Kaptoge, L.; Senghor, A.; Adhikari, B.; Cotty, P. Biological control of aflatoxins in Africa: Current status and potential challenges in the face of climate change. World Mycotoxin J. 2016, 9, 771–789. [Google Scholar] [CrossRef]
- Kagot, V.; Okoth, S.; De Boevre, M.; De Saeger, S. Biocontrol of Aspergillus and Fusarium Mycotoxins in Africa: Benefits and Limitations. Toxins 2019, 11, 109. [Google Scholar] [CrossRef] [PubMed]
- Medina, M.L.; Haynes, P.A.; Breci, L.; Francisco, W.A. Analysis of secreted proteins from Aspergillus flavus. Proteomics 2005, 5, 3153–3161. [Google Scholar] [CrossRef]
- Kang, D.; Son, G.H.; Park, H.M.; Kim, J.; Choi, J.N.; Kim, H.Y.; Lee, S.; Hong, S.-B.; Lee, C.H. Culture condition-dependent metabolite profiling of Aspergillus fumigatus with antifungal activity. Fungal Boil. 2013, 117, 211–219. [Google Scholar] [CrossRef]
- Calvo, A.M.; Cary, J.W. Association of fungal secondary metabolism and sclerotial biology. Front. Microbiol. 2015, 6, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Barrios-González, J. Solid-state fermentation: Physiology of solid medium, its molecular basis and applications. Process Biochem. 2012, 47, 175–185. [Google Scholar] [CrossRef]
- Zacchetti, B.; Wösten, H.A.; Claessen, D. Multiscale heterogeneity in filamentous microbes. Biotechnol. Adv. 2018, 36, 2138–2149. [Google Scholar] [CrossRef] [PubMed]
- Khalesi, M.; Zune, Q.; Telek, S.; Riveros-Galan, D.; Verachtert, H.; Toye, D.; Gebruers, K.; Derdelinckx, G.; Delvigne, F. Fungal biofilm reactor improves the productivity of hydrophobin HFBII. Biochem. Eng. J. 2014, 88, 171–178. [Google Scholar] [CrossRef]
- Zune, Q.; Delepierre, A.; Gofflot, S.; Bauwens, J.; Twizere, J.-C.; Punt, P.J.; Francis, F.; Toye, D.; Bawin, T.; Delvigne, F. A fungal biofilm reactor based on metal structured packing improves the quality of a Gla: GFP fusion protein produced by Aspergillus oryzae. Appl. Microbiol. Biotechnol. 2015, 99, 6241–6254. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tremblay, Y.D.N.; Hathroubi, S.; Jacques, M. Bacterial biofilms: Their importance in animal health and public health]. Can. J. Vet. Res. 2014, 78, 110–116. [Google Scholar]
- Biesebeke, R.T.; Ruijter, G.; Rahardjo, Y.S.; Hoogschagen, M.; Heerikhuisen, M.; Levin, A.; A Van Driel, K.G.; Schutyser, M.; Dijksterhuis, J.; Zhu, Y.; et al. Aspergillus oryzae in solid-state and submerged fermentations. Progress report on a multi-disciplinary project. FEMS Yeast Res. 2002, 2, 245–248. [Google Scholar] [CrossRef][Green Version]
- Van Hartingsveldt, W.; Mattern, I.E.; Van Zeijl, C.M.J.; Pouwels, P.H.; Hondel, C.A.M.J.J.V.D. Development of a homologous transformation system for Aspergillus niger based on the pyrG gene. Mol. Genet. Genom. 1987, 206, 71–75. [Google Scholar] [CrossRef]
- Kakahi, F.B.; Ly, S.; Tarayre, C.; Deschaume, O.; Bartic, C.; Wagner, P.; Compère, P.; Derdelinckx, G.; Blecker, C.; Delvigne, F. Modulation of fungal biofilm physiology and secondary product formation based on physico-chemical surface properties. Bioprocess Biosyst. Eng. 2019, 42, 1935–1946. [Google Scholar] [CrossRef]
- Ly, S.; Kakahi, F.B.; Mith, H.; Phat, C.; Fifani, B.; Kenne, T.; Fauconnier, M.-L.; Delvigne, F. Engineering Synthetic Microbial Communities through a Selective Biofilm Cultivation Device for the Production of Fermented Beverages. Microorganisms 2019, 7, 206. [Google Scholar] [CrossRef]
- OMS. Guidelines for Laboratory and Field Testing of Mosquito Larvicides; World Health Organization: Geneva, Switzerland, 2015; pp. 1–41. [Google Scholar]
- Dagnelie, P. Théorie et Méthodes Statistiques: Applications Agronomiques (Vol. 2); Presses Agronomiques: Gembloux, Belgium, 1994; 451p. [Google Scholar]
- De Moraes, A.M.L.; Da Costa, G.L.; Barcellos, M.Z.D.C.; De Oliveira, R.L.; De Oliveira, P.C. The entomopathogenic potential ofAspergillus spp. in mosquitoes vectors of tropical diseases. J. Basic Microbiol. 2001, 41, 45–49. [Google Scholar] [CrossRef]
- Zhang, P.; You, Y.; Song, Y.; Wang, Y.; Zhang, L. First record of Aspergillus oryzae (Eurotiales: Trichocomaceae) as an entomopathogenic fungus of the locust, Locusta migratoria (Orthoptera: Acrididae). Biocontrol Sci. Technol. 2015, 25, 1–20. [Google Scholar] [CrossRef]
- Vijayan, V.; Balaraman, K. Metabolites of fungi & actinomycetes active against mosquito larvae. Indian J. Med. Res. 1991, 93, 115–117. [Google Scholar] [PubMed]
- Oda, K.; Kakizono, D.; Yamada, O.; Iefuji, H.; Akita, O.; Iwashita, K. Proteomic Analysis of Extracellular Proteins from Aspergillus oryzae Grown under Submerged and Solid-State Culture Conditions Proteomic Analysis of Extracellular Proteins from Aspergillus oryzae Grown under Submerged and Solid-State Culture Conditi. Appl. Environ. Microbiol. 2006, 72, e3448. [Google Scholar] [CrossRef]
- Hata, Y.; Ishida, H.; Ichikawa, E.; Kawato, A.; Suginami, K.; Imayasu, S. Nucleotide sequence of an alternative glucoamylase-encoding gene (glaB) expressed in solid-state culture of Aspergillus oryzae. Gene 1998, 207, 127–134. [Google Scholar] [CrossRef]
- Meurer, F.; Do, H.T.; Sadowski, G.; Held, C. Standard Gibbs energy of metabolic reactions: II. Glucose-6-phosphatase reaction and ATP hydrolysis. Biophys. Chem. 2017, 223, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Urquiza, A.; Riveiro-Miranda, L.; Santiago-Alvarez, C.; Quesada-Moraga, E. Insect-toxic secreted proteins and virulence of the entomopathogenic fungus Beauveria bassiana. J. Invertebr. Pathol. 2010, 105, 270–278. [Google Scholar] [CrossRef]
- Stalinski, R.; Laporte, F.; Després, L.; Tetreau, G. Alkaline phosphatases are involved in the response ofAedes aegyptilarvae to intoxication withBacillus thuringiensissubsp.israelensis Cry toxins. Environ. Microbiol. 2016, 18, 1022–1036. [Google Scholar] [CrossRef]
- Berthold, C.L.; Toyota, C.G.; Richards, N.G.J.; Lindqvist, Y. Reinvestigation of the Catalytic Mechanism of Formyl-CoA Transferase, a Class III CoA-transferase. J. Boil. Chem. 2007, 283, 6519–6529. [Google Scholar] [CrossRef]
- Mullins, E.; Starks, C.M.; Francois, J.A.; Sael, L.; Kihara, D.; Kappock, T.J. Formyl-coenzyme A (CoA): Oxalate CoA-transferase from the acidophile Acetobacter aceti has a distinctive electrostatic surface and inherent acid stability. Protein Sci. 2012, 21, 686–696. [Google Scholar] [CrossRef]
- Andrade, P.; Caldas, E. Aflatoxins in cereals: Worldwide occurrence and dietary risk assessment. World Mycotoxin J. 2015, 8, 415–431. [Google Scholar] [CrossRef]
- Blaney, B.J.; Green, P. Insecticidal Fungal Metabolites: Cyclopiazonic Acid and Kojic Acid Contribute to the Toxicity of “Aspergillus flavus” to Sheep Blowfly “Lucilia Cuprina.”; General and Applied Entomology: Sydney, Australia, 1989; Available online: http://search.informit.com.au/documentSummary;dn=236829195107453;res=IELHSS (accessed on 3 February 2020).
- Geiser, D.M.; Dorner, J.W.; Horn, B.W.; Taylor, J.W. The Phylogenetics of Mycotoxin and Sclerotium Production in Aspergillus flavus and Aspergillus oryzae. Fungal Genet. Boil. 2000, 31, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Frisvad, J.C.; Skouboe, P.; Samson, R.A. Taxonomic comparison of three different groups of aflatoxin producers and a new efficient producer of aflatoxin B1, sterigmatocystin and 3-O-methylsterigmatocystin, Aspergillus rambellii sp. nov. Syst. Appl. Microbiol. 2005, 28, 442–453. [Google Scholar] [CrossRef] [PubMed]
- Lebar, M.; Cary, J.W.; Majumdar, R.; Carter-Wientjes, C.H.; Mack, B.M.; Wei, Q.; Uka, V.; De Saeger, S.; Di Mavungu, J.D. Identification and functional analysis of the aspergillic acid gene cluster in Aspergillus flavus. Fungal Genet. Boil. 2018, 116, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Nishie, K.; Cole, R.; Dorner, J. Toxicity and neuropharmacology of cyclopiazonic acid. Food Chem. Toxicol. 1985, 23, 831–839. [Google Scholar] [CrossRef]
- Batt, C.A.; Tortorello, M.L. Encyclopedia of Food Microbiology, 2nd ed.; Academic Press: Cambridge, MA, USA; 3248p.
- Fremy, J.-M. Évaluation des risques liés à la présence de mycotoxines dans les chaînes alimentaires humaine et animale Rapport final. Afssa 2009, 200–224. [Google Scholar]
- Tokuoka, M.; Kikuchi, T.; Shinohara, Y.; Koyama, A.; Iio, S.-I.; Kubota, T.; Kobayashi, J.; Koyama, Y.; Totsuka, A.; Shindo, H.; et al. Cyclopiazonic acid biosynthesis gene cluster gene cpaM is required for speradine A biosynthesis. Biosci. Biotechnol. Biochem. 2015, 79, 2081–2085. [Google Scholar] [CrossRef]
- Nishimura, A.; Yoshizako, F.; Chubachi, M. Purification and Characterization of an Enzyme That Catalyzes Ring Cleavage of Aspergillic Acid, from Tvichoderma koningii ATCC 76666. Biosci. Biotechnol. Biochem. 1997, 61, 1527–1530. [Google Scholar] [CrossRef]
- Macdonald, J.C. Biosynthesis of hydroxyaspergillic acid. J. Boil. Chem. 1962, 237, 1977–1981. [Google Scholar]
- Micetich, R.G.; Macdonald, J.C. Biosynthesis of Neoaspergillic and Neohydroxyaspergillic acids. J. Boil. Chem. 1965, 240, 1–5. [Google Scholar]
- Zhu, F.; Wu, J.; Chen, G.; Lu, W.; Pan, J. Biosynthesis, characterization and biological evalutation of Fe(III) and Cu(II) complexes of neoaspergillic acid, a hydroxamate siderophore produced by co-cultures of two marine-derived mangrove epiphytic fungi. Nat. Prod. Commun. 2011, 6, 1137–1140. [Google Scholar] [CrossRef] [PubMed]
- Arroyo-Manzanares, N.; Di Mavungu, J.D.; Uka, V.; Malysheva, S.V.; Cary, J.W.; Ehrlich, K.C.; Vanhaecke, L.; Bhatnagar, D.; De Saeger, S. Use of UHPLC high-resolution Orbitrap mass spectrometry to investigate the genes involved in the production of secondary metabolites in Aspergillus flavus. Food Addit. Contam. Part A 2015, 32, 1–18. [Google Scholar] [CrossRef] [PubMed]

| A. flavus Biofilm | A. oryzae Biofilm | A. flavus Submerged | A. oryzae Submerged | Protein Identification | Accession Number | Mascot Score | Coverage | Molecular Weight | pI | Organisms |
|---|---|---|---|---|---|---|---|---|---|---|
| glucoamylase | BDHI01000015.1 | 52 | 15 | 68,837 | 4.1 | Aspergillus awamori | ||||
| phosphohydrolase | WP_066372067.1 | 84 | 23 | 24,463 | 5.2 | Bacillus | ||||
| hypothetical protein | WP_027406331.1 | 87 | 31 | 13,278 | 3.9 | Anaerovibrio sp. | ||||
| cell division protein | WP_062096136.1 | 72 | 31 | 24,637 | 5 | Arthrobacter sp. | ||||
| sulfite reductase (NADPH) | SDC68807.1 | 95 | 22 | 63,455 | 6.1 | Aspergillus awamori | ||||
| hypothetical protein | BAE63852.1 | 91 | 47 | 26,086 | 4.7 | Aspergillus oryzae | ||||
| hypothetical protein | BAE63852.1 | 87 | 37 | 26,086 | 4.7 | Aspergillus oryzae | ||||
| formyl-CoA transferase | WP_066772048.1 | 88 | 22 | 44,240 | 5 | Croceicoccus mobilis | ||||
| hypothetical protein | WP_064318324.1 | 93 | 44 | 21,046 | 4.7 | Bibersteinia trehalosi | ||||
| exosome exonuclease | WP_048094625.1 | 93 | 29 | 28,821 | 5.1 | Geoglobus ahangari |

| Chemical Class | Name | Formule | Exact Mass (Da) | [M+H+] Measured (Da) | RRT Measured | A. flavus Biofilm | A. oryzae Biofilm | A. flavus Submerged | A. oryzae Submerged |
|---|---|---|---|---|---|---|---|---|---|
| Pyrazines | Aspergillic acid | C12H20N2O2 | 22,415,248 | 22,515,975 | 129,351 | ||||
| Indole-tetramates | Beta-cyclopiazonic acid | C20H22N2O3 | 33,816,306 | 35,517,030 | 109,803 | ||||
| Indole-tetramates | Cyclopiazonic acid | C20H20N2O3 | 33,614,740 | 33,715,467 | 145,486 | ||||
| Pyrazines | Ferrineaspergillin | C36H57FeN6O6 | 72,536,890 | 72,637,617 | 210,616 | ||||
| Pyrazines | Flavacol | C12H20N2O | 20,815,756 | 20,916,516 | 125,951 | ||||
| Indole-tetramates | Speradine A | C21H22N2O4 | 36,615,796 | 36,716,523 | 142,600 |

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Francis, F.; Druart, F.; Mavungu, J.D.D.; De Boevre, M.; De Saeger, S.; Delvigne, F. Biofilm Mode of Cultivation Leads to an Improvement of the Entomotoxic Patterns of Two Aspergillus Species. Microorganisms 2020, 8, 705. https://doi.org/10.3390/microorganisms8050705
Francis F, Druart F, Mavungu JDD, De Boevre M, De Saeger S, Delvigne F. Biofilm Mode of Cultivation Leads to an Improvement of the Entomotoxic Patterns of Two Aspergillus Species. Microorganisms. 2020; 8(5):705. https://doi.org/10.3390/microorganisms8050705
Chicago/Turabian StyleFrancis, Frédéric, Florent Druart, José Diana Di Mavungu, Marthe De Boevre, Sarah De Saeger, and Frank Delvigne. 2020. "Biofilm Mode of Cultivation Leads to an Improvement of the Entomotoxic Patterns of Two Aspergillus Species" Microorganisms 8, no. 5: 705. https://doi.org/10.3390/microorganisms8050705
APA StyleFrancis, F., Druart, F., Mavungu, J. D. D., De Boevre, M., De Saeger, S., & Delvigne, F. (2020). Biofilm Mode of Cultivation Leads to an Improvement of the Entomotoxic Patterns of Two Aspergillus Species. Microorganisms, 8(5), 705. https://doi.org/10.3390/microorganisms8050705