Skin Bacteria Mediate Glycerol Fermentation to Produce Electricity and Resist UV-B
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Bacteria Identification
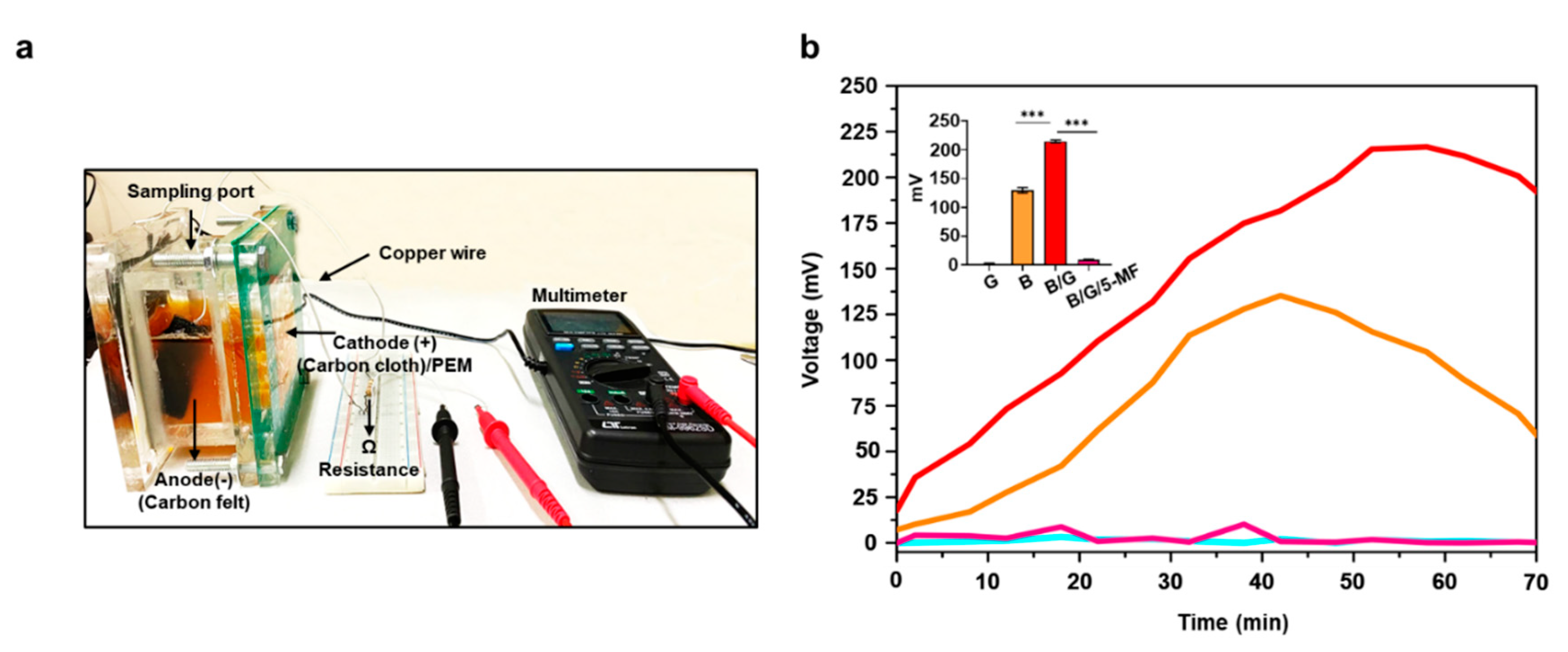
2.3. Microbial Fuel Cells (MFC)
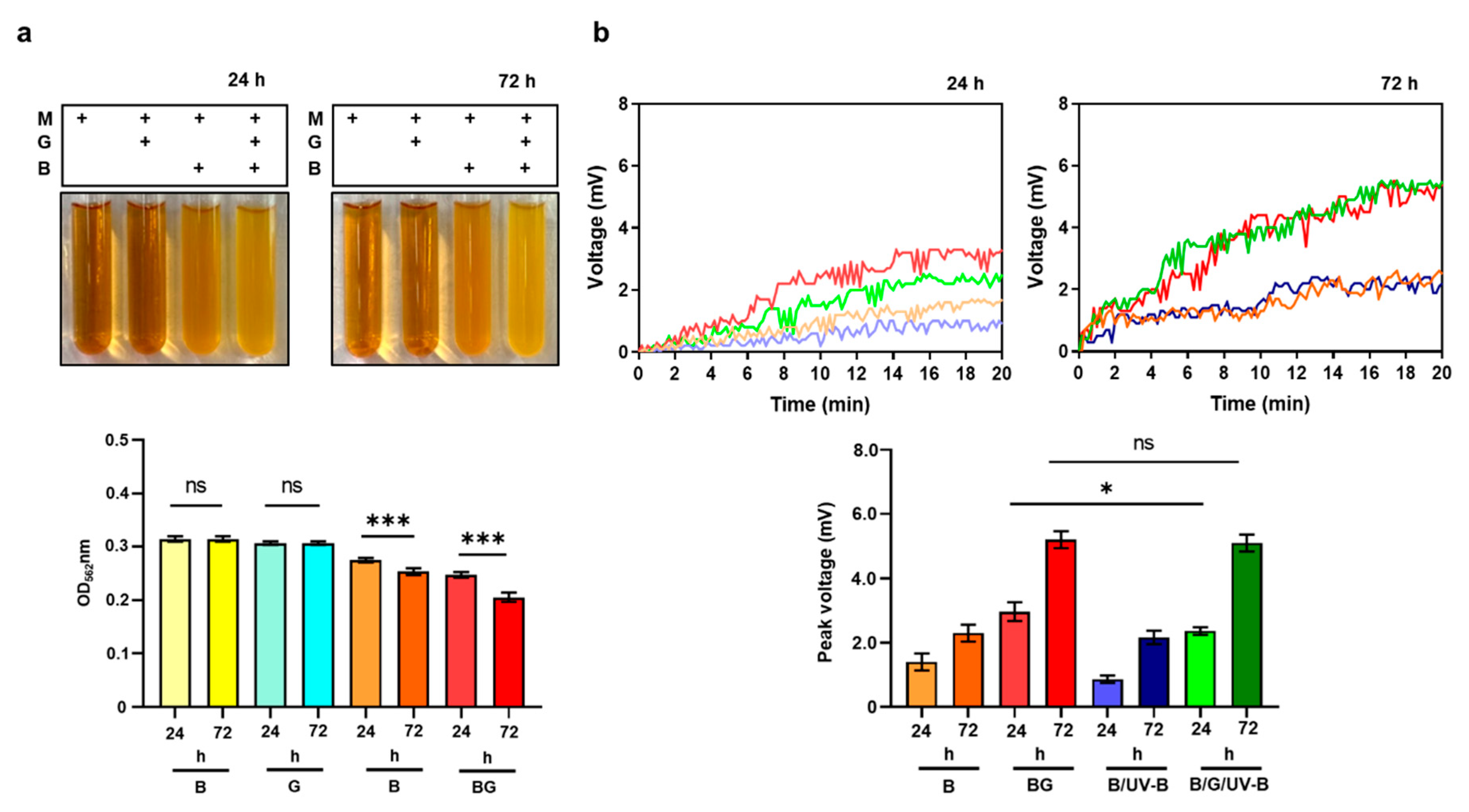
2.4. Bacterial Fermentation
2.5. Electricity Detection
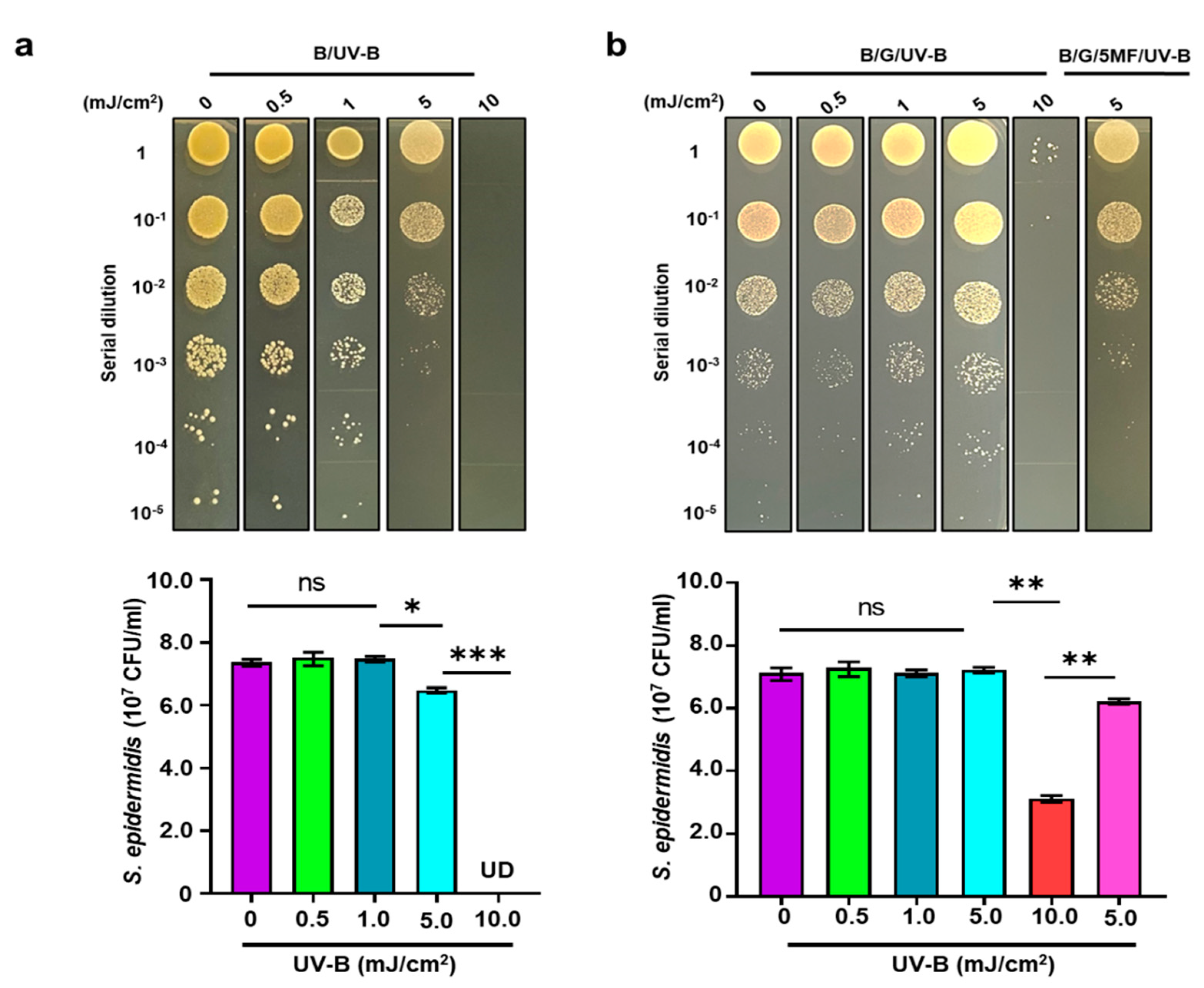
2.6. UV-B Irradiation
2.7. Statistical Analysis
3. Results
3.1. A TSB Agar-Based Ferrozine Overlay Assay for Screening Electrogenic Bacteria
3.2. Electricity Production of S. epidermidis in MFCs
3.3. The Effect of UV-B on Electricity Production of S. epidermidis
3.4. Enhancement of Bacterial Resistance to UV-B by Glycerol Fermentation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| S. epidermidis | Staphylococcus epidermidis |
| S. hominis | Staphylococcus hominis |
| E. faecalis | Enterococcus faecalis |
| E. avium | Enterococcus avium |
| P. vagans | Pantoea vagans |
| S. capitis | Staphylococcus capitis |
| P. aeruginosa | Pseudomonas aeruginosa |
| EET | Extracellular electron transfer |
| UV-B | Ultraviolet-B |
| MFC | Microbial fuel cell |
| FAC | Ferric ammonium citrate |
| TSB | Tryptic soy broth |
| CFU | C M Colony-forming unit |
| EF | Electro-fermentation |
| SCFA | Short-chain fatty acid |
| DMK | Demethylmenaquinone |
| ADH | Alcohol dehydrogenase |
| ALDH | Aldehyde dehydrogenase |
| PDH | Pyruvate dehydrogenase |
| ROS | Reactive oxygen species |
| FFAR2 | Free fatty acid receptor 2 |
| IL-6 | Interleukin-6 |
| NAG | N-acetyl-d glucosamine |
References
- Grice, E.A.; Segre, J.A. The skin microbiome. Nat. Rev. Microbiol. 2011, 9, 244–253. [Google Scholar] [CrossRef]
- Mohammadifar, M.; Tahernia, M.; Yang, J.H.; Koh, A.; Choi, S. Biopower-on-Skin: Electricity generation from sweat-eating bacteria for self-powered E-Skins. Nano Energy 2020, 75, 104994. [Google Scholar] [CrossRef]
- Elsner, P. Antimicrobials and the skin physiological and pathological flora. Curr. Probl. Dermatol. 2006, 33, 35–41. [Google Scholar] [CrossRef]
- Shi, L.; Dong, H.; Reguera, G.; Beyenal, H.; Lu, A.; Liu, J.; Yu, H.-Q.; Fredrickson, J.K. Extracellular electron transfer mechanisms between microorganisms and minerals. Nat. Rev. Microbiol. 2016, 14, 651–662. [Google Scholar] [CrossRef]
- Byrne, J.M.; Klueglein, N.; Pearce, C.; Rosso, K.M.; Appel, E.; Kappler, A. Redox cycling of Fe (II) and Fe (III) in magnetite by Fe-metabolizing bacteria. Science 2015, 347, 1473–1476. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.Y.; Kim, C.; Ainala, S.K.; Bae, H.; Jeon, B.-H.; Park, S.; Kim, J.R. Metabolic shift of Klebsiella pneumoniae L17 by electrode-based electron transfer using glycerol in a microbial fuel cell. Bioelectrochemistry 2019, 125, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Light, S.H.; Su, L.; Rivera-Lugo, R.; Cornejo, J.A.; Louie, A.; Iavarone, A.T.; Ajo-Franklin, C.M.; Portnoy, D.A. A flavin-based extracellular electron transfer mechanism in diverse Gram-positive bacteria. Nature 2018, 562, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Du, Y.; Yang, S.; Du, X.; Li, M.; Lin, B.; Zhou, J.; Lin, L.; Song, Y.; Li, J. Bacterial Extracellular Electron Transfer Occurs in Mammalian Gut. Anal. Chem. 2019, 91, 12138–12141. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Singh, L.; Zularisam, A.; Hai, F.I. Microbial fuel cell is emerging as a versatile technology: A review on its possible applications, challenges and strategies to improve the performances. Int. J. Energy Res. 2018, 42, 369–394. [Google Scholar] [CrossRef]
- Kumar, S.S.; Kumar, V.; Malyan, S.K.; Sharma, J.; Mathimani, T.; Maskarenj, M.S.; Ghosh, P.C.; Pugazhendhi, A. Microbial fuel cells (MFCs) for bioelectrochemical treatment of different wastewater streams. Fuel 2019, 254, 115526. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, L.; Zularisam, A. Exoelectrogens: recent advances in molecular drivers involved in extracellular electron transfer and strategies used to improve it for microbial fuel cell applications. Renew. Sustain. Energy Rev. 2016, 56, 1322–1336. [Google Scholar] [CrossRef]
- Keshari, S.; Balasubramaniam, A.; Myagmardoloonjin, B.; Herr, D.R.; Negari, I.P.; Huang, C.M. Butyric Acid from Probiotic Staphylococcus epidermidis in the Skin Microbiome Down-Regulates the Ultraviolet-Induced Pro-Inflammatory IL-6 Cytokine via Short-Chain Fatty Acid Receptor. Int. J. Mol. Sci. 2019, 20, 4477. [Google Scholar] [CrossRef] [PubMed]
- Nakatsuji, T.; Chen, T.H.; Butcher, A.M.; Trzoss, L.L.; Nam, S.-J.; Shirakawa, K.T.; Zhou, W.; Oh, J.; Otto, M.; Fenical, W.; et al. A commensal strain of Staphylococcus epidermidis protects against skin neoplasia. Sci. Adv. 2018, 4, eaao4502. [Google Scholar] [CrossRef] [PubMed]
- Oviedo, C.; Contreras, D.; Freer, J.; Rodríguez, J. A screening method for detecting iron reducing wood-rot fungi. Biotechnol. Lett. 2003, 25, 891–893. [Google Scholar] [CrossRef]
- Choi, O.; Kim, T.; Woo, H.M.; Um, Y. Electricity-driven metabolic shift through direct electron uptake by electroactive heterotroph Clostridiumpasteurianum. Sci. Rep. 2014, 4, 6961. [Google Scholar] [CrossRef]
- Wang, Y.; Kuo, S.; Shu, M.; Yu, J.; Huang, S.; Dai, A.; Two, A.; Gallo, R.L.; Huang, C.M. Staphylococcus epidermidis in the human skin microbiome mediates fermentation to inhibit the growth of Propionibacterium acnes: implications of probiotics in acne vulgaris. Appl. Microbiol. Biotechnol. 2014, 98, 411–424. [Google Scholar] [CrossRef]
- Velasquez, S.; Yu, E.; Katuri, K.; Head, I.; Curtis, T.; Scott, K. Evaluation of hydrolysis and fermentation rates in microbial fuel cells. Appl. Microbiol. Biotechnol. 2011, 90, 789–798. [Google Scholar] [CrossRef]
- Palmqvist, E.; Hahn-Hägerdal, B. Fermentation of lignocellulosic hydrolysates. II: inhibitors and mechanisms of inhibition. Bioresour. Technol. 2000, 74, 25–33. [Google Scholar] [CrossRef]
- Keogh, D.; Lam, L.N.; Doyle, L.E.; Matysik, A.; Pavagadhi, S.; Umashankar, S.; Low, P.M.; Dale, J.L.; Song, Y.; Ng, S.P.; et al. Extracellular Electron Transfer Powers Enterococcus faecalis Biofilm Metabolism. Mbio 2018, 9, e00626-17. [Google Scholar] [CrossRef]
- Pankratova, G.; Leech, D.; Gorton, L.; Hederstedt, L. Extracellular Electron Transfer by the Gram-Positive Bacterium Enterococcus faecalis. Biochemistry 2018, 57, 4597–4603. [Google Scholar] [CrossRef]
- Nueno-Palop, C.; Narbad, A. Probiotic assessment of Enterococcus faecalis CP58 isolated from human gut. Int. J. Food Microbiol. 2011, 145, 390–394. [Google Scholar] [CrossRef]
- Al Atya, A.K.; Drider-Hadiouche, K.; Ravallec, R.; Silvain, A.; Vachee, A.; Drider, D. Probiotic potential of Enterococcus faecalis strains isolated from meconium. Front. Microbiol. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Kao, M.S.; Huang, S.; Chang, W.L.; Hsieh, M.F.; Huang, C.J.; Gallo, R.L.; Huang, C.M. Microbiome precision editing: Using PEG as a selective fermentation initiator against methicillin-resistant Staphylococcus aureus. Biotechnol. J. 2017, 12. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.; Lee, Y.; Huang, Y.H.; Lim, H.W.; Jang, K.; Kim, D.D.; Lim, C.J. Probiotic fermentation augments the skin anti-photoaging properties of Agastache rugosa through up-regulating antioxidant components in UV-B-irradiated HaCaT keratinocytes. BMC Complement. Altern. Med. 2018, 18, 196. [Google Scholar] [CrossRef] [PubMed]
- Fiscus, E.L.; Booker, F.L. Is increased UV-B a threat to crop photosynthesis and productivity? Photosynth. Res. 1995, 43, 81–92. [Google Scholar] [CrossRef]
- Tao, J.-X.; Zhou, W.-C.; Zhu, X.-G. Mitochondria as Potential Targets and Initiators of the Blue Light Hazard to the Retina. Oxidative Med. Cell. Longev. 2019, 2019, 6435364. [Google Scholar] [CrossRef]
- Fluhr, J.W.; Darlenski, R.; Surber, C. Glycerol and the skin: holistic approach to its origin and functions. Br. J. Dermatol. 2008, 159, 23–34. [Google Scholar] [CrossRef]
- Yang, J.J.; Chang, T.W.; Jiang, Y.; Kao, H.J.; Chiou, B.H.; Kao, M.S.; Huang, C.M. Commensal Staphylococcus aureus Provokes Immunity to Protect against Skin Infection of Methicillin-Resistant Staphylococcus aureus. Int. J. Mol. Sci. 2018, 19, 1290. [Google Scholar] [CrossRef]
- Moscoviz, R.; Toledo-Alarcón, J.; Trably, E.; Bernet, N. Electro-Fermentation: How to Drive Fermentation Using Electrochemical Systems. Trends Biotechnol. 2016, 34, 856–865. [Google Scholar] [CrossRef]
- Alexandriaand, B.B.; Lauren, M.S. Enterococci as Indicators of Environmental Fecal Contamination. In Enterococci: From Commensals to Leading Causes of Drug Resistant Infection; Gilmore, M.S., Clewell, D.B., Ike, Y., Shankar, N., Eds.; Massachusetts Eye and Ear Infirmary: Boston, MA, USA, 2014. [Google Scholar]
- Frankenberg, L.; Brugna, M.; Hederstedt, L. Enterococcus faecalis heme-dependent catalase. J. Bacteriol. 2002, 184, 6351–6356. [Google Scholar] [CrossRef]
- Winstedt, L.; Frankenberg, L.; Hederstedt, L.; von Wachenfeldt, C. Enterococcus faecalis V583 contains a cytochrome bd-type respiratory oxidase. J. Bacteriol. 2000, 182, 3863–3866. [Google Scholar] [CrossRef] [PubMed]
- Baureder, M.; Hederstedt, L. Heme proteins in lactic acid bacteria. In Advances in Microbial Physiology; Elsevier: Amsterdam, The Netherlands, 2013; Volume 62, pp. 1–43. [Google Scholar]
- Borisov, V.B.; Gennis, R.B.; Hemp, J.; Verkhovsky, M.I. The cytochrome bd respiratory oxygen reductases. Biochim. Biophys. Acta Bioenergy 2011, 1807, 1398–1413. [Google Scholar] [CrossRef] [PubMed]
- Safarian, S.; Rajendran, C.; Müller, H.; Preu, J.; Langer, J.D.; Ovchinnikov, S.; Hirose, T.; Kusumoto, T.; Sakamoto, J.; Michel, H. Structure of a bd oxidase indicates similar mechanisms for membrane-integrated oxygen reductases. Science 2016, 352, 583–586. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Tsai, T.-H.; Wu, P.-S.; Tsao, S.-E.; Huang, Y.-S.; Chung, Y.-C. Selection of electrogenic bacteria for microbial fuel cell in removing Victoria blue R from wastewater. J. Environ. Sci. Health Part A 2018, 53, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Myagmardoloonjin, B.; Keshari, S.; Negari, I.P.; Huang, C.-M.J.M. 5-Methyl Furfural Reduces the Production of Malodors by Inhibiting Sodium l-Lactate Fermentation of Staphylococcus epidermidis: Implication for Deodorants Targeting the Fermenting Skin Microbiome. Microorganisms 2019, 7, 239. [Google Scholar] [CrossRef] [PubMed]
- Pankratova, G.; Gorton, L. Electrochemical communication between living cells and conductive surfaces. Curr. Opin. Electrochem. 2017, 5, 193–202. [Google Scholar] [CrossRef]
- Santos, A.L.; Gomes, N.C.; Henriques, I.; Almeida, A.; Correia, A.; Cunha, Â. Contribution of reactive oxygen species to UV-B-induced damage in bacteria. J. Photochem. Photobiol. B Biol. 2012, 117, 40–46. [Google Scholar] [CrossRef]
- Jagger, J. Solar-UV Actions on Living Cells; Praeger Publishers: New York, NY, USA, 1985. [Google Scholar]
- Oschman, J.L. Can electrons act as antioxidants? A review and commentary. J. Altern. Complement. Med. 2007, 13, 955–967. [Google Scholar] [CrossRef]
- Liu, H.; Cheng, S.; Logan, B.E. Production of electricity from acetate or butyrate using a single-chamber microbial fuel cell. Environ. Sci. Technol. 2005, 39, 658–662. [Google Scholar] [CrossRef]
- Liu, H.; Fang, H.J.W.S. Hydrogen production from wastewater by acidogenic granular sludge. Water Sci. Technol. 2003, 47, 153–158. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balasubramaniam, A.; Adi, P.; Do Thi, T.M.; Yang, J.-H.; Labibah, A.S.; Huang, C.-M. Skin Bacteria Mediate Glycerol Fermentation to Produce Electricity and Resist UV-B. Microorganisms 2020, 8, 1092. https://doi.org/10.3390/microorganisms8071092
Balasubramaniam A, Adi P, Do Thi TM, Yang J-H, Labibah AS, Huang C-M. Skin Bacteria Mediate Glycerol Fermentation to Produce Electricity and Resist UV-B. Microorganisms. 2020; 8(7):1092. https://doi.org/10.3390/microorganisms8071092
Chicago/Turabian StyleBalasubramaniam, Arun, Prakoso Adi, Tra My Do Thi, Jen-Ho Yang, Asy Syifa Labibah, and Chun-Ming Huang. 2020. "Skin Bacteria Mediate Glycerol Fermentation to Produce Electricity and Resist UV-B" Microorganisms 8, no. 7: 1092. https://doi.org/10.3390/microorganisms8071092
APA StyleBalasubramaniam, A., Adi, P., Do Thi, T. M., Yang, J.-H., Labibah, A. S., & Huang, C.-M. (2020). Skin Bacteria Mediate Glycerol Fermentation to Produce Electricity and Resist UV-B. Microorganisms, 8(7), 1092. https://doi.org/10.3390/microorganisms8071092




