Each Mycobacterium Requires a Specific Culture Medium Composition for Triggering an Optimized Immunomodulatory and Antitumoral Effect
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Different Culture Media
2.2. Bacterial Strains and Culture Conditions
2.3. Preparation for Ultrastructural Assessment of Mycobacterial Pellicles and Bacilli
2.4. Cell Culture Conditions
2.5. Tumor Growth Inhibition Experiments
2.6. Mycobacterial Survival inside Macrophages
2.7. Cytokine Analysis
2.8. Statistics
3. Results
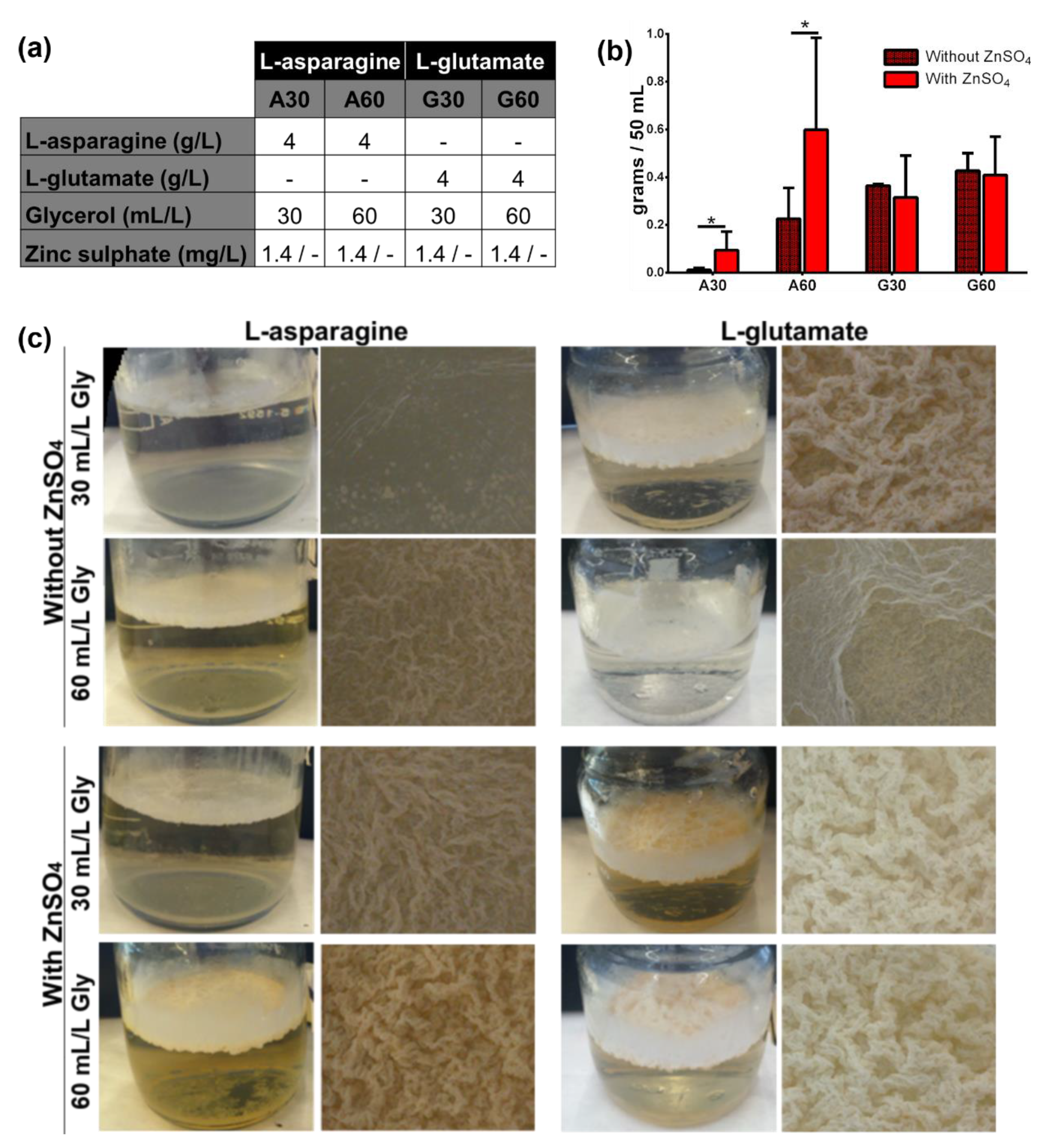
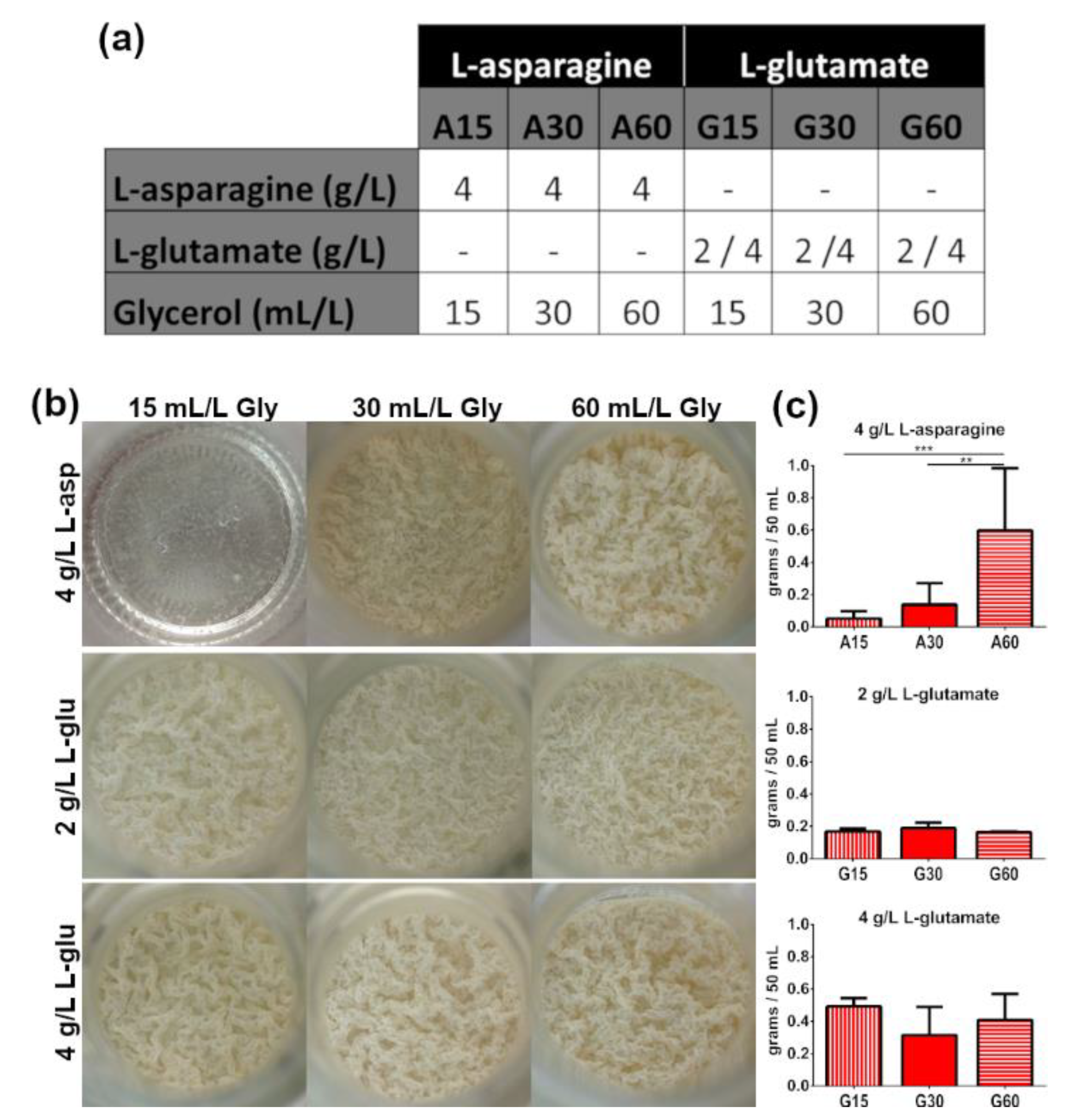
3.1. Presence of ZnSO4 and L-Asparagine and Increasing Glycerol Concentrations in Culture Media Determine the Growth of M. brumae
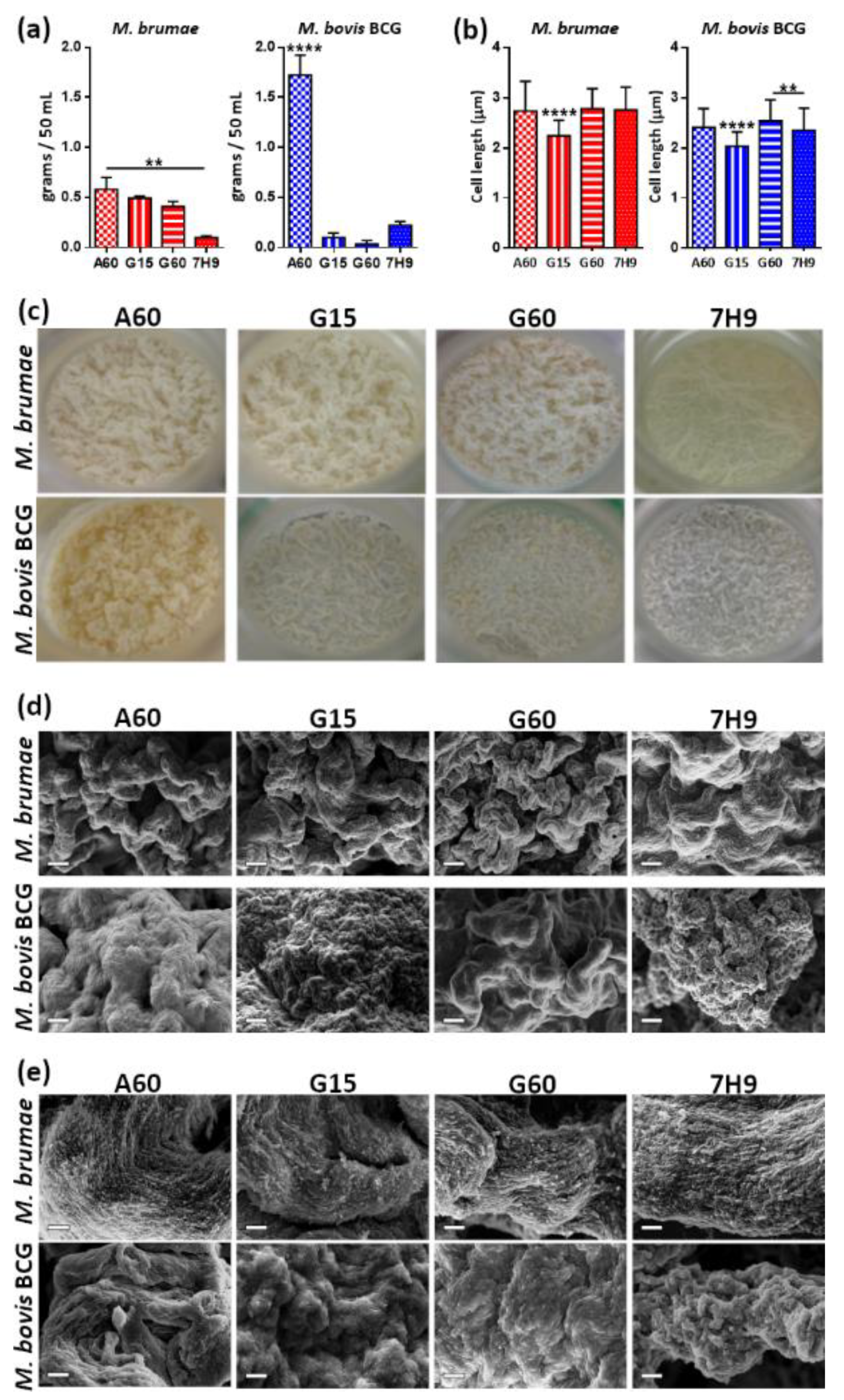
3.2. Culture Composition Differentially Influences M. brumae and M. bovis BCG Biomass Production but Not in Terms of Microscopic Appearance
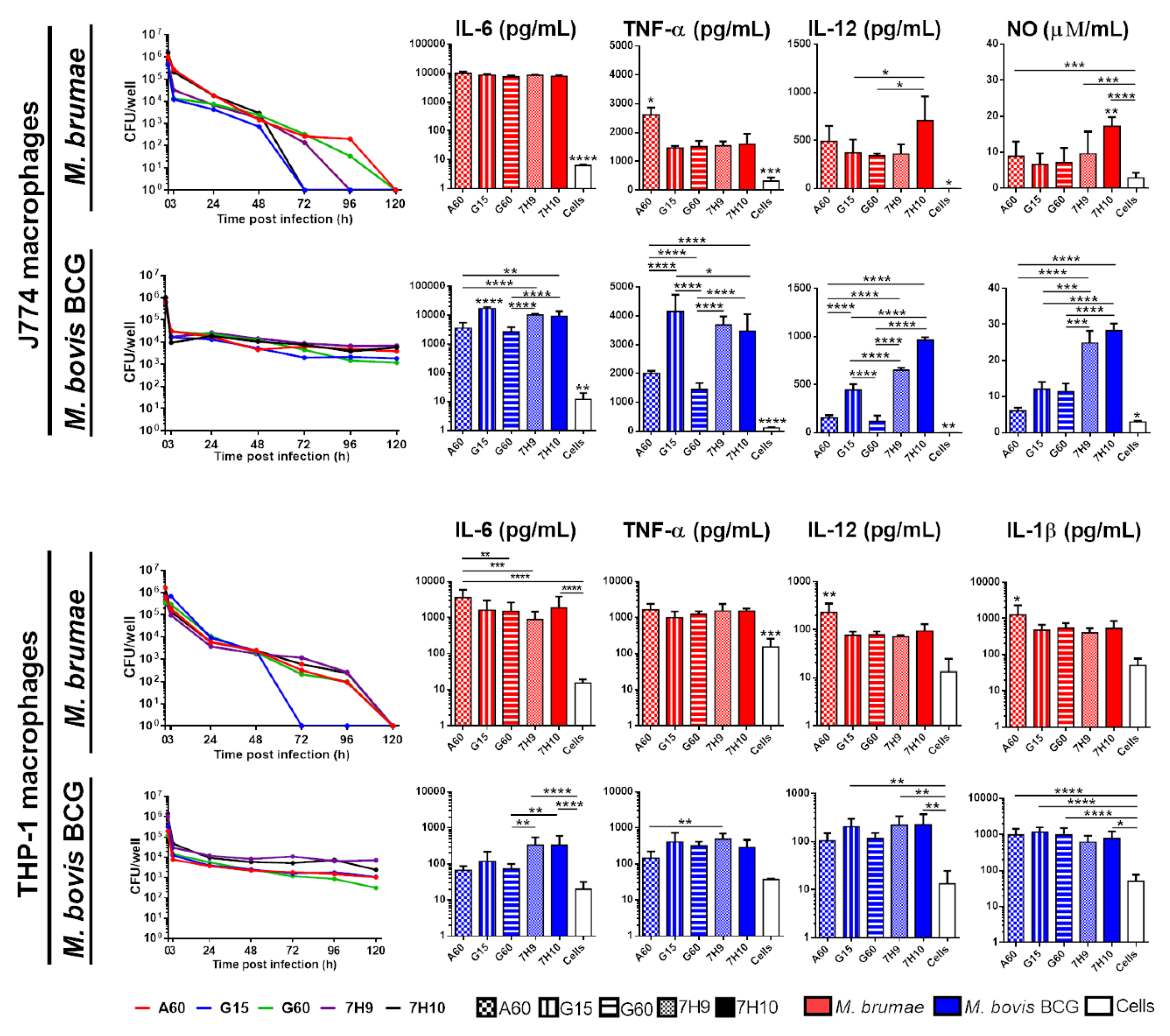
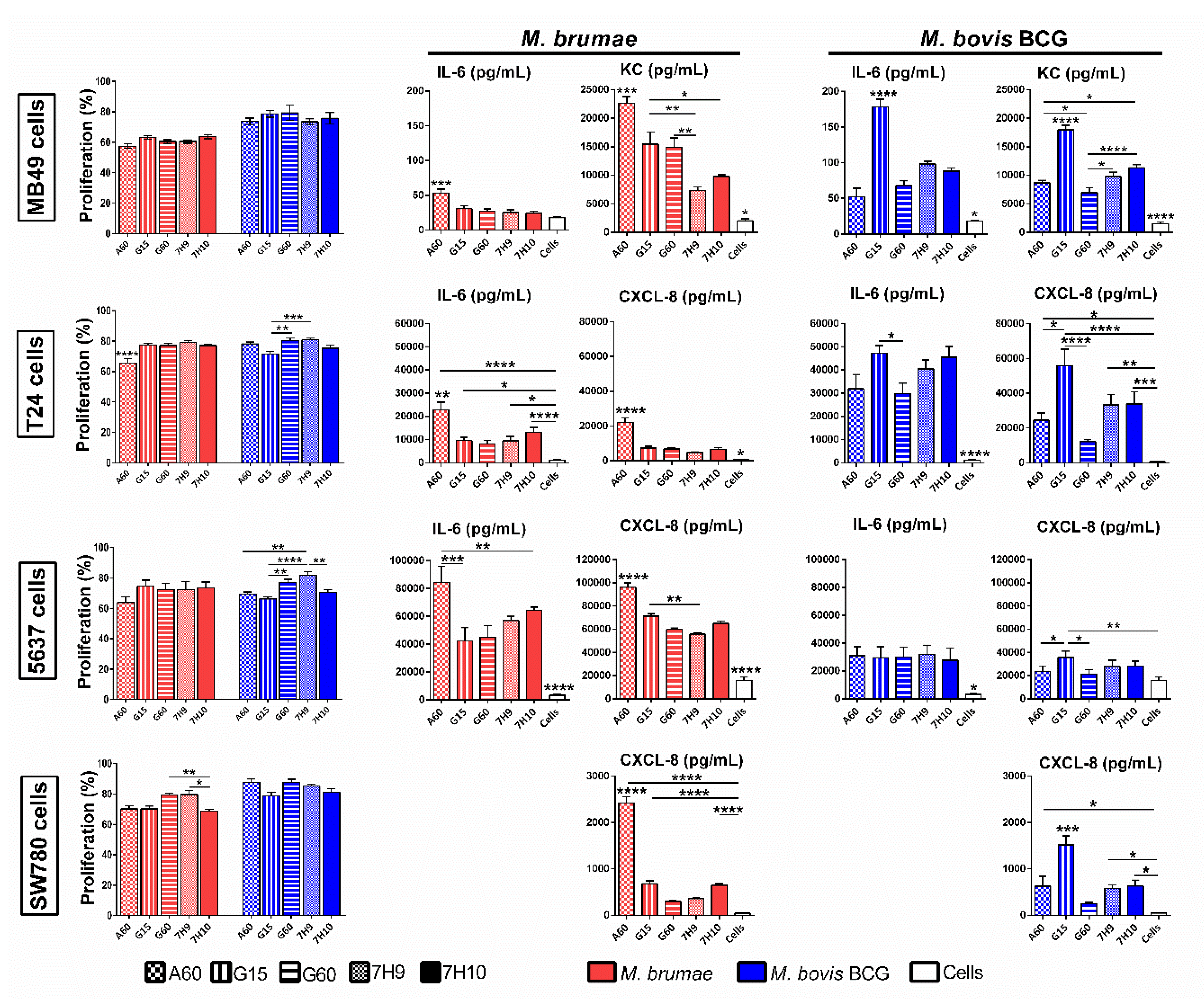
3.3. Culture Conditions Affect the Immune Response Triggered by Mycobacteria but Do Not Modify Their Survival in Macrophages
3.4. Antitumor Activity is Enhanced in L-Asparagine-Containing Medium for M. brumae and in L-Glutamate-Containing Media for M. bovis BCG
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Appendix A
References
- WHO World Health Organization 2019 Global Tuberculosis Report. Available online: https://apps.who.int/iris/bitstream/handle/10665/329368/9789241565714-eng.pdf (accessed on 30 April 2020).
- Dietrich, G.; Mollenkopf, H.-J.; Weber, H.; Knapp, B.; Diehl, K.-D.; Hess, J.; Blackkolb, F.; Bröker, M.; Kaufmann, S.H.E.; Hundt, E. Cultivation of Mycobacterium bovis BCG in bioreactors. J. Biotechnol. 2002, 96, 259–270. [Google Scholar] [CrossRef]
- Gontero, P.; Bohle, A.; Malmstrom, P.U.; O’Donnell, M.A.; Oderda, M.; Sylvester, R.; Witjes, F. The role of bacillus calmette-guérin in the treatment of non-muscle-invasive bladder cancer. Eur. Urol. 2010, 57, 410–429. [Google Scholar] [CrossRef] [PubMed]
- Babjuk, M.; Böhle, A.; Burger, M.; Capoun, O.; Cohen, D.; Compérat, E.M.; Hernández, V.; Kaasinen, E.; Palou, J.; Rouprêt, M.; et al. EAU guidelines on non–muscle-invasive urothelial carcinoma of the bladder: Update 2016. Eur. Urol. 2017, 71, 447–461. [Google Scholar] [CrossRef]
- Ziegler, J.; Ho, J.; Gibson, I.W.; Nayak, J.G.; Stein, M.; Walkty, A.; Orr, P. Disseminated Mycobacterium bovis infection post-kidney transplant following remote intravesical BCG therapy for bladder cancer. Transpl. Infect. Dis. 2018, 20, e12931. [Google Scholar] [CrossRef] [PubMed]
- Moussa, M.; Abou Chakra, M. Granulomatous hepatitis caused by Bacillus Calmette-Guerin (BCG) infection after BCG bladder instillation: A case report. Urol. Case Reports 2018, 20, 3–4. [Google Scholar] [CrossRef] [PubMed]
- Noguera-Ortega, E.; Blanco-Cabra, N.; Rabanal, R.M.; Sánchez-Chardi, A.; Roldán, M.; Guallar-Garrido, S.; Torrents, E.; Luquin, M.; Julián, E. Mycobacteria emulsified in olive oil-in-water trigger a robust immune response in bladder cancer treatment. Sci. Rep. 2016, 6, 27232. [Google Scholar] [CrossRef]
- Noguera-Ortega, E.; Secanella-Fandos, S.; Eraña, H.; Gasión, J.; Rabanal, R.M.; Luquin, M.; Torrents, E.; Julián, E. Nonpathogenic Mycobacterium brumae inhibits bladder cancer growth in vitro, ex vivo, and in vivo. Eur. Urol. Focus 2016, 2, 67–76. [Google Scholar] [CrossRef]
- Noguera-Ortega, E.; Rabanal, R.M.; Gómez-Mora, E.; Cabrera, C.; Luquin, M.; Julián, E. Intravesical Mycobacterium brumae triggers both local and systemic immunotherapeutic responses against bladder cancer in mice. Sci. Rep. 2018, 8, 15102. [Google Scholar] [CrossRef]
- Eickhoff, T.C. The current status of BCG immunization against tuberculosis. Annu. Rev. Med. 1977, 28, 411–423. [Google Scholar] [CrossRef]
- Venkataswamy, M.M.; Goldberg, M.F.; Baena, A.; Chan, J.; Jacobs, W.R.; Porcelli, S.A. In vitro culture medium influences the vaccine efficacy of Mycobacterium bovis BCG. Vaccine 2012, 30, 1038–1049. [Google Scholar] [CrossRef]
- Sauton, B. Sur la nutrition minirale du bacille tuberculeux. Comptes Rendus Lebdomadaires des Sci. L′Academie des Sci. 1912, 92, 85–93. [Google Scholar]
- Kusunose, E.; Ichihara, K.; Noda, Y.; Kusunose, M. Superoxide dismutase from Mycobacterium tuberculosis. J. Biochem. 1976, 1352, 1343–1352. [Google Scholar] [CrossRef] [PubMed]
- Harth, G.; Horwitz, M.A. Expression and efficient export of enzymatically active Mycobacterium tuberculosis glutamine synthetase in Mycobacterium smegmatis and evidence that the information for export is contained within the protein. J. Biol. Chem. 1997, 272, 22728–22735. [Google Scholar] [CrossRef][Green Version]
- Petricevich, V.; Ueda, C.; Alves, R.; Da Silva, M.; Moreno, C.; Melo, A.; Dias da Silva, W. A single strain of Mycobacterium bovis bacillus Calmette-Guérin (BCG) grown in two different media evokes distinct humoral immune responses in mice. Braz. J. Med. Biol. Res. 2001, 34, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Larsen, M.H.; Biermann, K.; Jacobs, W.R. Laboratory maintenance of Mycobacterium tuberculosis. Curr. Protoc. Microbiol. 2007, 6, 10A.1.1–10A.1.8. [Google Scholar] [CrossRef] [PubMed]
- Mehra, A.; Philips, J. Analysis of mycobacterial protein secretion. Bio-Protoc. 2014, 4, e1159. [Google Scholar] [CrossRef]
- Li, Y.; Mortuza, R.; Milligan, D.L.; Tran, S.L.; Strych, U.; Cook, G.M.; Krause, K.L. Investigation of the essentiality of glutamate racemase in Mycobacterium smegmatis. J. Bacteriol. 2014, 196, 4239–4244. [Google Scholar] [CrossRef]
- Teknova Sauton’s Defined Broth, 1 liter, Sterile; Sauton’s Medium Cat. No. S0351; Teknova Inc.: Hollister, CA, USA, 2016.
- Leal, M.B.B.; Baruque-Ramos, J.; Hiss, H.; Paz, M.F.D.; Sakai, M.C.; Vassoler, U.M.; Arauz, L.J.D.; Raw, I. Influence of initial L-asparagine and glycerol concentrations on the batch growth kinetics of Mycobacterium bovis BCG. Braz. J. Microbiol. 2004, 35, 337–344. [Google Scholar] [CrossRef]
- Florio, W.; Batoni, G.; Esin, S.; Bottai, D.; Maisetta, G.; Favilli, F.; Brancatisano, F.L.; Campa, M. Influence of culture medium on the resistance and response of Mycobacterium bovis BCG to reactive nitrogen intermediates. Microbes Infect. 2006, 8, 434–441. [Google Scholar] [CrossRef]
- Biering-Sørensen, S.; Jensen, K.J.; Aamand, S.H.; Blok, B.; Andersen, A.; Monteiro, I.; Netea, M.G.; Aaby, P.; Benn, C.S.; Hasløv, K.R. Variation of growth in the production of the BCG vaccine and the association with the immune response. An observational study within a randomised trial. Vaccine 2015, 33, 2056–2065. [Google Scholar] [CrossRef]
- De Bruyn, J.; Weckx, M.; Beumer-Jochmans, M.P. Effect of zinc deficiency on Mycobacterium tuberculosis var. bovis (BCG). J. Gen. Microbiol. 1981, 124, 353–357. [Google Scholar] [PubMed]
- Tepper, B.S. Differences in the utilization of glycerol and glucose by Mycobacterium phlei. J. Bacteriol. 1968, 95, 1713–1717. [Google Scholar] [CrossRef] [PubMed]
- Julián, E.; Roldán, M.; Sánchez-Chardi, A.; Astola, O.; Agustí, G.; Luquin, M. Microscopic cords, a virulence-related characteristic of Mycobacterium tuberculosis, are also present in nonpathogenic mycobacteria. J. Bacteriol. 2010, 192, 1751–1760. [Google Scholar] [CrossRef] [PubMed]
- Salamaga, B.; Prajsnar, T.K.; Jareño-Martinez, A.; Willemse, J.; Bewley, M.A.; Chau, F.; Ben Belkacem, T.; Meijer, A.H.; Dockrell, D.H.; Renshaw, S.A.; et al. Bacterial size matters: Multiple mechanisms controlling septum cleavage and diplococcus formation are critical for the virulence of the opportunistic pathogen Enterococcus faecalis. PLoS Pathog. 2017, 13, e1006526. [Google Scholar] [CrossRef] [PubMed]
- Totani, T.; Nishiuchi, Y.; Tateishi, Y.; Yoshida, Y.; Kitanaka, H.; Niki, M.; Kaneko, Y.; Matsumoto, S. Effects of nutritional and ambient oxygen condition on biofilm formation in Mycobacterium avium subsp. hominissuis via altered glycolipid expression. Sci. Rep. 2017, 7, 41775. [Google Scholar]
- Wright, C.C.; Hsu, F.F.; Arnett, E.; Dunaj, J.L.; Davidson, P.M.; Pacheco, S.A.; Harriff, M.J.; Lewinsohn, D.M.; Schlesinger, L.S.; Purdy, G.E. The Mycobacterium tuberculosis MmpL11 Cell Wall Lipid Transporter Is Important for Biofilm Formation, Intracellular Growth, and Nonreplicating Persistence. Infect. Immun. 2017, 85, e00131-17. [Google Scholar] [CrossRef]
- Schoonmaker, M.K.; Bishai, W.R.; Lamichhane, G. Nonclassical transpeptidases of Mycobacterium tuberculosis alter cell size, morphology, the cytosolic matrix, protein localization, virulence, and resistance to -Lactams. J. Bacteriol. 2014, 196, 1394–1402. [Google Scholar] [CrossRef]
- Secanella-Fandos, S.; Noguera-Ortega, E.; Olivares, F.; Luquin, M.; Julián, E. Killed but metabolically active Mycobacterium bovis bacillus calmette-guérin retains the antitumor ability of live bacillus calmette-Guérin. J. Urol. 2014, 191, 1422–1428. [Google Scholar] [CrossRef]
- Secanella-Fandos, S.; Luquin, M.; Julián, E. Connaught and russian strains showed the highest direct antitumor effects of different bacillus calmette-guérin substrains. J. Urol. 2013, 189, 711–718. [Google Scholar] [CrossRef]
- Kim, T.H. High-viability lyophilized Bacille Calmette-Guerin vaccine produced by deep-culture technique. Appl. Environ. Microbiol. 1977, 34, 495–499. [Google Scholar] [CrossRef]
- Himedia. Sautons Fluid Medium Base; Atlas, R.M., Ed.; HiMedia Laboratories: Mumbai, Maharashtra, India, 1996. [Google Scholar]
- Ojha, A.K.; Baughn, A.D.; Sambandan, D.; Hsu, T.; Trivelli, X.; Guerardel, Y.; Alahari, A.; Kremer, L.; Jacobs, W.R.; Hatfull, G.F. Growth of Mycobacterium tuberculosis biofilms containing free mycolic acids and harbouring drug-tolerant bacteria. Mol. Microbiol. 2008, 69, 164–174. [Google Scholar] [CrossRef] [PubMed]
- De Bruyn, J.; Bosmans, R.; Nyabenda, J.; Van Vooren, J.P. Effect of zinc deficiency on the appearance of two immunodominant protein antigens (32 kDa and 65 kDa) in culture filtrates of mycobacterium. J. Gen. Microbiol. 1989, 135, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Lyon, R.H.; Rogers, P.; Hall, W.H.; Lichtein, H.C. Inducible glutamate transport in Mycobacteria and its relation to glutamate oxidation. J. Bacteriol. 1967, 94, 92–100. [Google Scholar] [CrossRef]
- Bowles, J.A.; Segal, W. Kinetics of utilization of organic compounds in the growth of Mycobacterium tuberculosis. J. Bacteriol. 1965, 90, 157–15763. [Google Scholar] [CrossRef] [PubMed]
- Aoyagi, T.; Mizuno, D. Cultivation of mycobacteria rocked in non-protein medium containing a high concentration of tween 80. J. Gen. Microbiol. 1959, 20, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Agapova, A.; Serafini, A.; Petridis, M.; Hunt, D.M.; Garza-Garcia, A.; Sohaskey, C.D.; De Carvalho, L.P.S. Flexible nitrogen utilisation by the metabolic generalist pathogen Mycobacterium tuberculosis. eLife 2019, 8, e41129. [Google Scholar] [CrossRef]
- Chen, J.M.; Alexander, D.C.; Behr, M.A.; Liu, J. Mycobacterium bovis BCG vaccines exhibit defects in alanine and serine catabolism. Infect. Immun. 2003, 71, 708–716. [Google Scholar] [CrossRef]
- Song, H.; Niederweis, M. Uptake of sulfate but not phosphate by Mycobacterium tuberculosis is slower than that for Mycobacterium smegmatis. J. Bacteriol. 2012, 194, 956–964. [Google Scholar] [CrossRef]
- Cook, G.M.; Berney, M.; Gebhard, S.; Heinemann, M.; Cox, R.A.; Danilchanka, O.; Niederweis, M. Physiology of Mycobacteria. Adv. Microb. Physiol. 2009, 55, 81–319. [Google Scholar]
- Gallant, J.L.; Viljoen, A.J.; Van Helden, P.D.; Wiid, I.J.F. Glutamate dehydrogenase is required by Mycobacterium bovis BCG for resistance to cellular stress. PLoS ONE 2016, 11, e0147706. [Google Scholar] [CrossRef]
- O’Hare, H.M.; Durán, R.; Cerveñansky, C.; Bellinzoni, M.; Wehenkel, A.M.; Pritsch, O.; Obal, G.; Baumgartner, J.; Vialaret, J.; Johnsson, K.; et al. Regulation of glutamate metabolism by protein kinases in mycobacteria. Mol. Microbiol. 2008, 70, 1408–1423. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, L.P.S.; Fischer, S.M.; Marrero, J.; Nathan, C.; Ehrt, S.; Rhee, K.Y. Metabolomics of Mycobacterium tuberculosis reveals compartmentalized co-catabolism of carbon substrates. Chem. Biol. 2010, 17, 1122–1131. [Google Scholar] [CrossRef] [PubMed]
- Ehrt, S.; Schnappinger, D.; Rhee, K.Y. Metabolic principles of persistence and pathogenicity in Mycobacterium tuberculosis. Nat. Rev. Microbiol. 2018, 16, 496–507. [Google Scholar] [CrossRef] [PubMed]
- Osborn, T.W. Some effects of nutritional components on the morphology of BCG colonies. Dev. Biol. Stand. 1986, 58 Pt A, 79–94. [Google Scholar]
- Bellerose, M.M.; Baek, S.-H.; Huang, C.-C.; Moss, C.E.; Koh, E.-I.; Proulx, M.K.; Smith, C.M.; Baker, R.E.; Lee, J.S.; Eum, S.; et al. Common variants in the glycerol kinase gene reduce tuberculosis drug efficacy. mBio 2019, 10, e00663-19. [Google Scholar] [CrossRef]
- Fakas, S.; Makri, A.; Bellou, S.; Aggelis, G. Pathways to aerobic glycerol catabolism and their regulation. Microb. Convers. Raw Glycerol 2009, 9, 18. [Google Scholar]
- Shi, L.; Sohaskey, C.D.; Pfeiffer, C.; Datta, P.; Parks, M.; McFadden, J.; North, R.J.; Gennaro, M.L. Carbon flux rerouting during Mycobacterium tuberculosis growth arrest. Mol. Microbiol. 2010, 78, 1199–1215. [Google Scholar] [CrossRef]
- Daniel, J.; Deb, C.; Dubey, V.S.; Sirakova, T.D.; Abomoelak, B.; Morbidoni, H.R.; Kolattukudy, P.E. Induction of a novel class of diacylglycerol acyltransferases and triacylglycerol accumulation in Mycobacterium tuberculosis as it goes into a dormancy-like state in culture. J. Bacteriol. 2004, 186, 5017–5030. [Google Scholar] [CrossRef]
- Ojha, A.K.; Trivelli, X.; Guerardel, Y.; Kremer, L.; Hatfull, G.F. Enzymatic hydrolysis of trehalose dimycolate releases free mycolic acids during mycobacterial growth in biofilms. J. Biol. Chem. 2010, 285, 17380–17389. [Google Scholar] [CrossRef]
- Geisel, R.E.; Sakamoto, K.; Russell, D.G.; Rhoades, E.R. In vivo activity of released cell wall lipids of Mycobacterium bovis Bacillus calmette-guérin is due principally to trehalose mycolates. J. Immunol. 2005, 174, 5007–5015. [Google Scholar] [CrossRef]
- Indrigo, J.; Actor, J.K.; Hunter, R.L. Influence of trehalose 6,6′-dimycolate (TDM) during mycobacterial infection of bone marrow macrophages. Microbiology 2002, 148, 1991–1998. [Google Scholar] [CrossRef]
- Secanella-Fandos, S. Funcionalitat dels Micobacteris Ambientals de Creixement ràpid Com a Agents Antitumorals; Universitat Autònoma de Barcelona: Bellaterra, Spain, 2013. [Google Scholar]
- Abou-Zeid, C.; Rook, G.A.W.; Minnikin, D.E.; Parlett, J.H.; Osbornt, T.W.; Grangets, J.M. Effect of the method of preparation of Bacille Calmette-Guérin (BCG) vaccine on the properties of four daughter strains. J. Appl. Bacteriol. 1987, 63, 449–453. [Google Scholar] [PubMed]
- Hanekom, W.A.; Hawn, T.R.; Ginsberg, A.M. 60-Tuberculosis Vaccines, 7th ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Prados-Rosales, R.; Carreño, L.J.; Weinrick, B.; Batista-Gonzalez, A.; Glatman-Freedman, A.; Xu, J.; Chan, J.; Jacobs, W.R.; Porcelli, S.A.; Casadevall, A. The type of growth medium affects the presence of a mycobacterial capsule and is associated with differences in protective efficacy of BCG vaccination against Mycobacterium tuberculosis. J. Infect. Dis. 2016, 214, 426–437. [Google Scholar] [CrossRef] [PubMed]
- Julián, E.; Cama, M.; Martínez, P.; Luquin, M. An ELISA for five glycolipids from the cell wall of Mycobacterium tuberculosis. J. Immunol. Methods 2001, 251, 21–30. [Google Scholar] [CrossRef]
- Leisching, G.; Pietersen, R.D.; Wiid, I.; Baker, B. Virulence, biochemistry, morphology and host-interacting properties of detergent-free cultured mycobacteria: An update. Tuberculosis 2016, 100, 53–60. [Google Scholar] [CrossRef]
- Leisching, G.; Pietersen, R.-D.; Mpongoshe, V.; Van Heerden, C.; Van Helden, P.; Wiid, I.; Baker, B. The host response to a clinical MDR mycobacterial strain cultured in a detergent-free environment: A global transcriptomics approach. PLoS ONE 2016, 11, e0153079. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guallar-Garrido, S.; Campo-Pérez, V.; Sánchez-Chardi, A.; Luquin, M.; Julián, E. Each Mycobacterium Requires a Specific Culture Medium Composition for Triggering an Optimized Immunomodulatory and Antitumoral Effect. Microorganisms 2020, 8, 734. https://doi.org/10.3390/microorganisms8050734
Guallar-Garrido S, Campo-Pérez V, Sánchez-Chardi A, Luquin M, Julián E. Each Mycobacterium Requires a Specific Culture Medium Composition for Triggering an Optimized Immunomodulatory and Antitumoral Effect. Microorganisms. 2020; 8(5):734. https://doi.org/10.3390/microorganisms8050734
Chicago/Turabian StyleGuallar-Garrido, Sandra, Víctor Campo-Pérez, Alejandro Sánchez-Chardi, Marina Luquin, and Esther Julián. 2020. "Each Mycobacterium Requires a Specific Culture Medium Composition for Triggering an Optimized Immunomodulatory and Antitumoral Effect" Microorganisms 8, no. 5: 734. https://doi.org/10.3390/microorganisms8050734
APA StyleGuallar-Garrido, S., Campo-Pérez, V., Sánchez-Chardi, A., Luquin, M., & Julián, E. (2020). Each Mycobacterium Requires a Specific Culture Medium Composition for Triggering an Optimized Immunomodulatory and Antitumoral Effect. Microorganisms, 8(5), 734. https://doi.org/10.3390/microorganisms8050734




