Does Intra-Wound Vancomycin Powder Affect the Action of Intra-Articular Tranexamic Acid in Total Joint Replacement?
Abstract
1. Introduction
2. Materials and Methods
2.1. Per-Operative Care and Rehabilitation
2.2. Statistical Analysis and Sample Size
3. Results
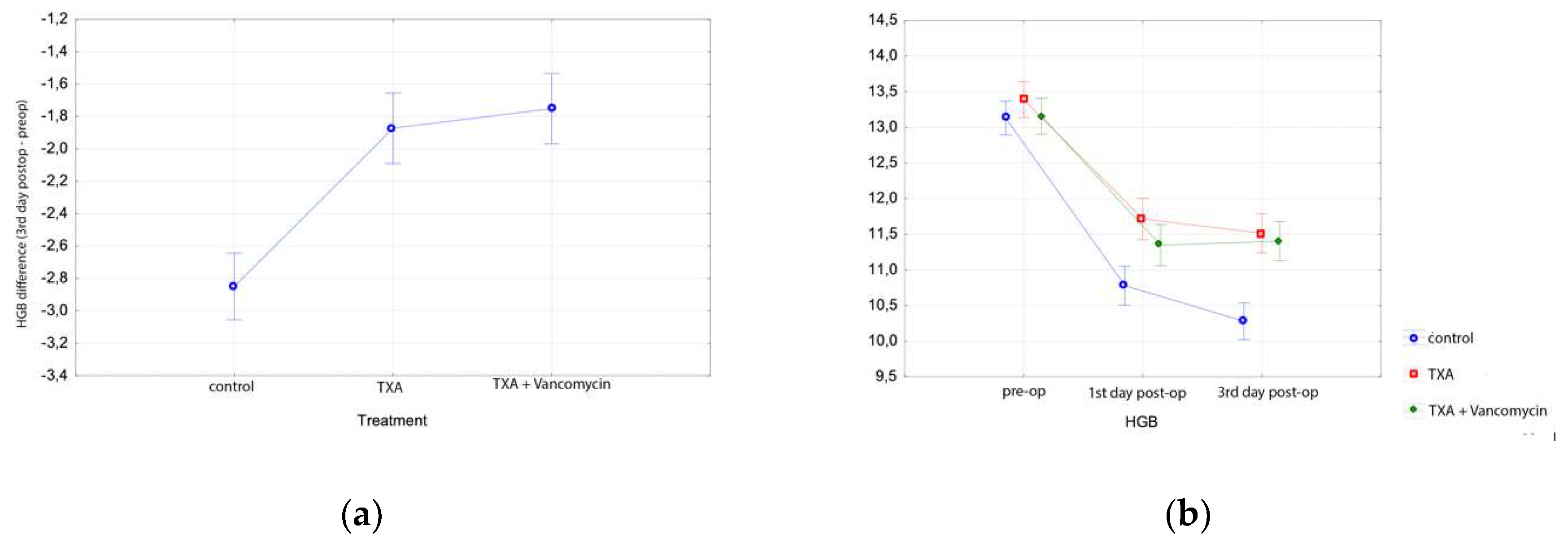
3.1. Total Hip Arthroplasty Results
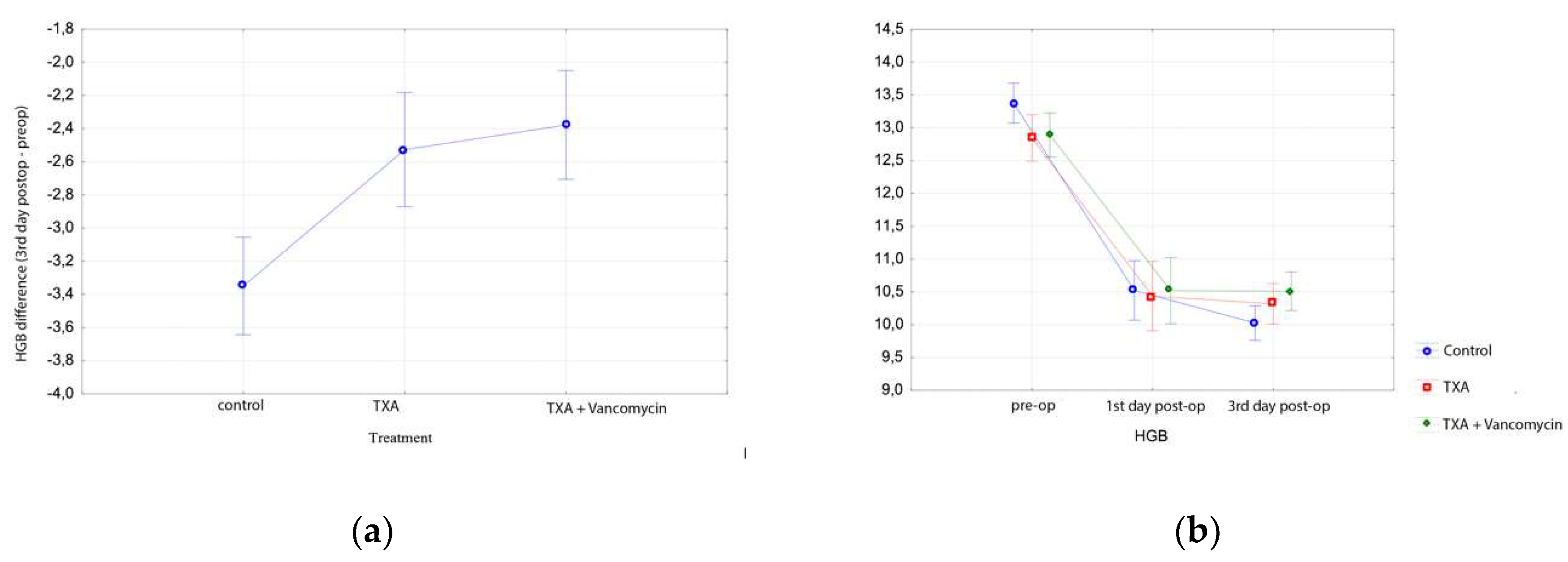
3.2. Total Knee Arthroplasty Results
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Xie, J.; Hu, Q.; Huang, Q.; Ma, J.; Lei, Y.; Pei, F.X. Comparison of intravenous versus topical tranexamic acid in primary total hip and knee arthroplasty: An updated meta-analysis. Thromb. Res. 2017, 153, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Moskal, J.T.; Capps, S.G. Meta-analysis of Intravenous Tranexamic Acid in Primary Total Hip Arthroplasty. Orthopedics 2016, 39, e883–e892. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Du, S.; Sun, Y. Is combined topical and intravenous tranexamic acid superior to single use of tranexamic acid in total joint arthroplasty? A meta-analysis from randomized controlled trials. Medicine 2017, 96, e7609. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Dong, Q.; Zhang, Y.-G. Intravenous versus topical tranexamic acid in primary total hip replacement: A systemic review and meta-analysis. Int. J. Surg. 2016, 32, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Mi, B.; Liu, G.; Zhou, W.; Lv, H.; Liu, Y.; Zha, K.; Wu, Q.; Liu, J. Intra-articular versus intravenous tranexamic acid application in total knee arthroplasty: A meta-analysis of randomized controlled trials. Arch. Orthop. Trauma Surg. 2017, 158, 997–1009. [Google Scholar] [CrossRef]
- Myles, P.S.; Smith, J.A.; Forbes, A.; Silbert, B.; Jayarajah, M.; Painter, T.; Cooper, D.J.; Marasco, S.; McNeil, J.; Bussières, J.S.; et al. Tranexamic Acid in Patients Undergoing Coronary-Artery Surgery. N. Engl. J. Med. 2017, 376, 136–148. [Google Scholar] [CrossRef]
- Lin, Z.; Zou, X. Tranexamic acid-associated seizures: A meta-analysis. Seizure 2016, 36, 70–73. [Google Scholar] [CrossRef]
- Spanyer, J.; Emberton, E.; Patel, J.; Smith, L.S.; Malkani, A.L. Topical Tranexamic Acid in Total Knee Arthroplasty Patients with Increased Thromboembolic Risk. J. Knee Surg. 2016, 30, 474–478. [Google Scholar] [CrossRef]
- Jiménez, J.J.; Iribarren, J.L.; Lorente, L.; Rodriguez, J.M.; Hernández, D.; Nassar, I.; Perez, R.; Brouard, M.; Milena, A.; Martinez, R.; et al. Tranexamic acid attenuates inflammatory response in cardiopulmonary bypass surgery through blockade of fibrinolysis: A case control study followed by a randomized double-blind controlled trial. Crit. Care 2007, 11, R117. [Google Scholar] [CrossRef]
- Loc-Carrillo, C.; Wang, C.; Canden, A.; Burr, M.; Agarwal, J. Local Intramedullary Delivery of Vancomycin Can Prevent the Development of Long Bone Staphylococcus aureus Infection. PLoS ONE 2016, 11, e0160187. [Google Scholar] [CrossRef]
- Iarikov, D.; Demian, H.; Rubin, D.; Alexander, J.; Nambiar, S. Choice and Doses of Antibacterial Agents for Cement Spacers in Treatment of Prosthetic Joint Infections: Review of Published Studies. Clin. Infect. Dis. 2012, 55, 1474–1480. [Google Scholar] [CrossRef] [PubMed]
- Edelstein, A.; Okroj, K.T.; Rogers, T.; Della Valle, C.J.; Sporer, S.M. Systemic Absorption of Antibiotics From Antibiotic-Loaded Cement Spacers for the Treatment of Periprosthetic Joint Infection. J. Arthroplast. 2018, 33, 835–839. [Google Scholar] [CrossRef] [PubMed]
- Chotai, S.; Wright, P.W.; Hale, A.T.; Jones, W.A.; McGirt, M.J.; Patt, J.C.; Devin, C.J. Does Intrawound Vancomycin Application During Spine Surgery Create Vancomycin-Resistant Organism? Neurosurgery 2017, 80, 746–753. [Google Scholar] [CrossRef] [PubMed]
- Devin, C.J.; Chotai, S.; McGirt, M.J.; Vaccaro, A.R.; Youssef, J.A.; Orndorff, D.G.; Arnold, P.M.; Frempong-Boadu, A.; Lieberman, I.H.; Branch, C.; et al. Intrawound Vancomycin Decreases the Risk of Surgical Site Infection After Posterior Spine Surgery. Spine 2018, 43, 65–71. [Google Scholar] [CrossRef]
- Texakalidis, P.; Lu, V.M.; Yolcu, Y.; Kerezoudis, P.; Alvi, M.A.; Parney, I.F.; Fogelson, J.L.; Bydon, M. Impact of Powdered Vancomycin on Preventing Surgical Site Infections in Neurosurgery: A Systematic Review and Meta-analysis. Neurosurgery 2018, 84, 569–580. [Google Scholar] [CrossRef]
- Dailiana, Z.H.; Rigopoulos, N.; Varitimidis, S.; Poultsides, L.; Petinaki, E.; Malizos, K.N. Clinical and epidemiological features of upper-extremity infections caused by Staphylococcus aureus carrying the PVL gene: A four-year study in Greece. Med. Sci. Monit. 2008, 14, 511–514. [Google Scholar]
- Drakos, A.; Raoulis, V.; Karatzios, K.; Doxariotis, N.; Kontogeorgakos, V.; Malizos, K.; Varitimidis, S.E. Efficacy of Local Administration of Tranexamic Acid for Blood Salvage in Patients Undergoing Intertrochanteric Fracture Surgery. J. Orthop. Trauma 2016, 30, 409–414. [Google Scholar] [CrossRef]
- Carson, J.L.; Grossman, B.J.; Kleinman, S.; Tinmouth, A.T.; Marques, M.; Fung, M.; Holcomb, J.B.; Illoh, O.; Kaplan, L.J.; Katz, L.M.; et al. Red Blood Cell Transfusion: A Clinical Practice Guideline From the AABB. Ann. Intern. Med. 2012, 157, 49–58. [Google Scholar] [CrossRef]
- Mangram, A.J.; Horan, T.C.; Pearson, M.L.; Silver, L.C.; Jarvis, W.R. Guideline for Prevention of Surgical Site Infection. Am. J. Infect. Control 1999, 27, 97–134. [Google Scholar] [CrossRef]
- Springer, B.D. The diagnosis of periprosthetic joint infection. J. Arthroplast. 2015, 30, 908–911. [Google Scholar] [CrossRef]
- Parvizi, J.; Gehrke, T.; Chen, A.F. Proceedings of the International Consensus on Periprosthetic Joint Infection. Bone Jt. J. 2013, 95, 1450–1452. [Google Scholar] [CrossRef]
- Martin, J.G.; Cassatt, K.B.; Kincaid-Cinnamon, K.A.; Westendorf, D.S.; Garton, A.S.; Lemke, J.H. Topical Administration of Tranexamic Acid in Primary Total Hip and Total Knee Arthroplasty. J. Arthroplast. 2014, 29, 889–894. [Google Scholar] [CrossRef]
- Alshryda, S.; Mason, J.M.; Sarda, P.; Nargol, A.V.; Cooke, N.; Ahmad, H.; Tang, S.; Logishetty, R.; Vaghela, M.; McPartlin, L.; et al. Topical (Intra-Articular) Tranexamic Acid Reduces Blood Loss and Transfusion Rates Following Total Hip Replacement. JBJS 2013, 95, 1969–1974. [Google Scholar] [CrossRef] [PubMed]
- Yoon, B.-H.; Kim, T.-Y.; Ko, Y.S.; Lee, Y.-K.; Ha, Y.-C.; Koo, K.-H. Optimal use of tranexamic acid for total hip arthroplasty: A network meta-analysis. PLoS ONE 2018, 13, e0206480. [Google Scholar] [CrossRef]
- Fillingham, Y.A.; Ramkumar, D.B.; Jevsevar, D.S.; Yates, A.J.; Shores, P.; Mullen, K.; Bini, S.A.; Clarke, H.D.; Schemitsch, E.; Johnson, R.L.; et al. The Efficacy of Tranexamic Acid in Total Hip Arthroplasty: A Network Meta-analysis. J. Arthroplast. 2018, 33, 3083–3089. [Google Scholar] [CrossRef] [PubMed]
- Fillingham, Y.A.; Ramkumar, D.B.; Jevsevar, D.S.; Yates, A.J.; Shores, P.; Mullen, K.; Bini, S.A.; Clarke, H.D.; Schemitsch, E.; Johnson, R.L.; et al. The Efficacy of Tranexamic Acid in Total Knee Arthroplasty: A Network Meta-Analysis. J. Arthroplast. 2018, 33, 3090–3098. [Google Scholar] [CrossRef] [PubMed]
- National Joint Registry for England, Wales, Northern Ireland and the Isle of Man. 16th Annual Report. Available online: http://www.njrcentre.org.uk (accessed on 4 April 2020).
- Wong, J.; Abrishami, A.; El Beheiry, H.; Canizares, M.; Davey, J.R.; Gandhi, R.; Syed, K.A.; Hasan, S.M.O.; De Silva, Y.; Chung, F. Topical Application of Tranexamic Acid Reduces Postoperative Blood Loss in Total Knee Arthroplasty. JBJS 2010, 92, 2503–2513. [Google Scholar] [CrossRef]
- Alshryda, S.; Mason, J.M.; Vaghela, M.; Sarda, P.; Nargol, A.V.; Maheswaran, S.; Tulloch, C.; Anand, S.; Logishetty, R.; Stothart, B.; et al. Topical (Intra-Articular) Tranexamic Acid Reduces Blood Loss and Transfusion Rates Following Total Knee Replacement. JBJS 2013, 95, 1961–1968. [Google Scholar] [CrossRef]
- Wukich, D.K.; Dikis, J.W.; Monaco, S.J.; Strannigan, K.; Suder, N.C.; Rosario, B.L. Topically Applied Vancomycin Powder Reduces the Rate of Surgical Site Infection in Diabetic Patients Undergoing Foot and Ankle Surgery. Foot Ankle Int. 2015, 36, 1017–1024. [Google Scholar] [CrossRef]
- Pavey, G.J.; Formby, P.M.; Hoyt, B.; Wagner, S.C.; Forsberg, J.A.; Potter, B.K. Intrawound Antibiotic Powder Decreases Frequency of Deep Infection and Severity of Heterotopic Ossification in Combat Lower Extremity Amputations. Clin. Orthop. Relat. Res. 2018, 477, 802–810. [Google Scholar] [CrossRef]
- Qadir, R.; Ochsner, J.L.; Chimento, G.; Meyer, M.S.; Waddell, B.; Zavatsky, J.M. Establishing a Role for Vancomycin Powder Application for Prosthetic Joint Infection Prevention—Results of a Wear Simulation Study. J. Arthroplast. 2014, 29, 1449–1456. [Google Scholar] [CrossRef] [PubMed]
- Heckmann, N.; Mayfield, C.K.; Culvern, C.N.; Oakes, D.A.; Lieberman, J.R.; Della Valle, C.J. Systematic Review and Meta-Analysis of Intrawound Vancomycin in Total Hip and Total Knee Arthroplasty: A Call for a Prospective Randomized Trial. J. Arthroplast. 2019, 34, 1815–1822. [Google Scholar] [CrossRef] [PubMed]
- Cohen, E.M.; Marcaccio, S.; Goodman, A.D.; Lemme, N.J.; Limbird, R. Efficacy and Cost-effectiveness of Topical Vancomycin Powder in Primary Cementless Total Hip Arthroplasty. Orthopedics 2019, 42, e430–e436. [Google Scholar] [CrossRef] [PubMed]
- Yavuz, I.A.; Oken, O.F.; Yildirim, A.O.; Inci, F.; Ceyhan, E.; Gurhan, U. No effect of vancomycin powder to prevent infection in primary total knee arthroplasty: A retrospective review of 976 cases. Knee Surg. Sports Traumatol. Arthrosc. 2019, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Hanada, M.; Nishikino, S.; Hotta, K.; Furuhashi, H.; Hoshino, H.; Matsuyama, Y. Intrawound vancomycin powder increases post-operative wound complications and does not decrease periprosthetic joint infection in primary total and unicompartmental knee arthroplasties. Knee Surg. Sports Traumatol. Arthrosc. 2019, 27, 2322–2327. [Google Scholar] [CrossRef] [PubMed]
| Number of Patients | Gender (Females/Males) (n) | Age (Mean ± SD) | |
|---|---|---|---|
| Total knee replacements | |||
| TXA (Group A) | 83 | 69/14 | 71.3 ± 5.7 |
| TXA + vancomycin (Group B) | 83 | 66/17 | 70.3 ± 7.6 |
| Control (Group C) | 93 | 76/17 | 70.5 ± 5.5 |
| Chi square test, p = 0.565 | ANOVA test, p = 0.556 | ||
| Total hip replacements | |||
| TXA (Group A) | 65 | 42/23 | 64 ± 12.5 |
| TXA + vancomycin (Group B) | 59 | 35/24 | 62.1 ± 11.9 |
| Control (Group C) | 85 | 52/33 | 62.5 ± 11.3 |
| Chi square test, p = 0.332 | ANOVA test, p = 0.631 |
| Hgb Preop (gr/dL) (Mean, SD) | Hgb 1st Day Post-op (gr/dL) (Mean, SD) | Hgb 3rd Day Post-op (gr/dL) (Mean, SD) | Hct Preop (%) (Mean, SD) | Hct 1st Day Post-op (%) (Mean, SD) | Hct 3rd Day Post-op (%) (Mean, SD) | |
|---|---|---|---|---|---|---|
| THA | ||||||
| Group A | 12.8 ± 1.0 | 10.4 ± 1.3 | 10.3 ± 1.2 | 38.6 ± 2.8 | 31.2 ± 3.9 | 31.0 ± 3.7 |
| Group B | 12.9 ± 1.2 | 10.5 ± 1.5 | 10.5 ± 1.3 | 38.9 ± 3.5 | 30.9 ± 4.0 | 32.2 ± 2.3 |
| Group C | 13.3 ± 1.6 | 10.5 ± 2.7 | 10.0 ± 1.1 | 40.5 ± 3.5 | 30.3 ± 4.0 | 33.8 ± 3.6 |
| TKA | ||||||
| Group A | 13.4 ± 1.0 | 11.7 ± 1.2 | 11.5 ± 1.4 | 40.3 ± 2.8 | 35.0 ± 3.1 | 34.5 ± 3.6 |
| Group B | 13.2 ± 1.0 | 11.3 ± 1.3 | 11.4 ± 1.2 | 39.5 ± 2.8 | 34.0 ± 3.5 | 34.2 ± 3.5 |
| Group C | 13.1 ± 1.4 | 10.8 ± 1.5 | 10.3 ± 1.2 | 39.4 ± 3.7 | 32.4 ± 4.2 | 30.8 ± 3.4 |
| Transfusions | Delayed Wound Healing | Superficial Infections | Deep Infections | DVTs | |
|---|---|---|---|---|---|
| THA | |||||
| Group A (n = 65) | 5 | 0 | 0 | 0 | 0 |
| Group B (n = 59) | 7 | 0 | 0 | 1 | 0 |
| Group C (n = 85) | 14 | 0 | 0 | 1 | 1 |
| TKA | |||||
| Group A (n = 83) | 1 | 4 | 1 | 1 | 0 |
| Group B (n = 83) | 1 | 1 | 0 | 1 | 0 |
| Group C (n = 93) | 9 | 1 | 0 | 1 | 0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koutalos, A.A.; Drakos, A.; Fyllos, A.; Doxariotis, N.; Varitimidis, S.; Malizos, K.N. Does Intra-Wound Vancomycin Powder Affect the Action of Intra-Articular Tranexamic Acid in Total Joint Replacement? Microorganisms 2020, 8, 671. https://doi.org/10.3390/microorganisms8050671
Koutalos AA, Drakos A, Fyllos A, Doxariotis N, Varitimidis S, Malizos KN. Does Intra-Wound Vancomycin Powder Affect the Action of Intra-Articular Tranexamic Acid in Total Joint Replacement? Microorganisms. 2020; 8(5):671. https://doi.org/10.3390/microorganisms8050671
Chicago/Turabian StyleKoutalos, Antonios A., Athanasios Drakos, Apostolos Fyllos, Nikos Doxariotis, Sokratis Varitimidis, and Konstantinos N. Malizos. 2020. "Does Intra-Wound Vancomycin Powder Affect the Action of Intra-Articular Tranexamic Acid in Total Joint Replacement?" Microorganisms 8, no. 5: 671. https://doi.org/10.3390/microorganisms8050671
APA StyleKoutalos, A. A., Drakos, A., Fyllos, A., Doxariotis, N., Varitimidis, S., & Malizos, K. N. (2020). Does Intra-Wound Vancomycin Powder Affect the Action of Intra-Articular Tranexamic Acid in Total Joint Replacement? Microorganisms, 8(5), 671. https://doi.org/10.3390/microorganisms8050671





