Mycobacterium tuberculosis YrbE3A Promotes Host Innate Immune Response by Targeting NF-κB/JNK Signaling
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacteria and Antibodies
2.2. Mice and Mammalian Cell Lines
2.3. Construction of Recombinant Strains and YrbE3A Protein Expression
2.4. Macrophage Infection and CFU Counting
2.5. ELISA and Quantitative RT-PCR Analysis to Detect Cytokine Expression
2.6. Localization of the YrbE3A Protein
2.7. Western Blot Assays of Molecules Critical to Related Signal Pathways
2.8. Effect of Signaling Pharmacological Inhibition on Cytokine Production
2.9. Mouse Infection
2.10. Histopathological and Immunohistochemical Examination
2.11. The Multiplex Immunoassay for Inflammatory Cytokines in Lungs
2.12. Statistics
3. Results
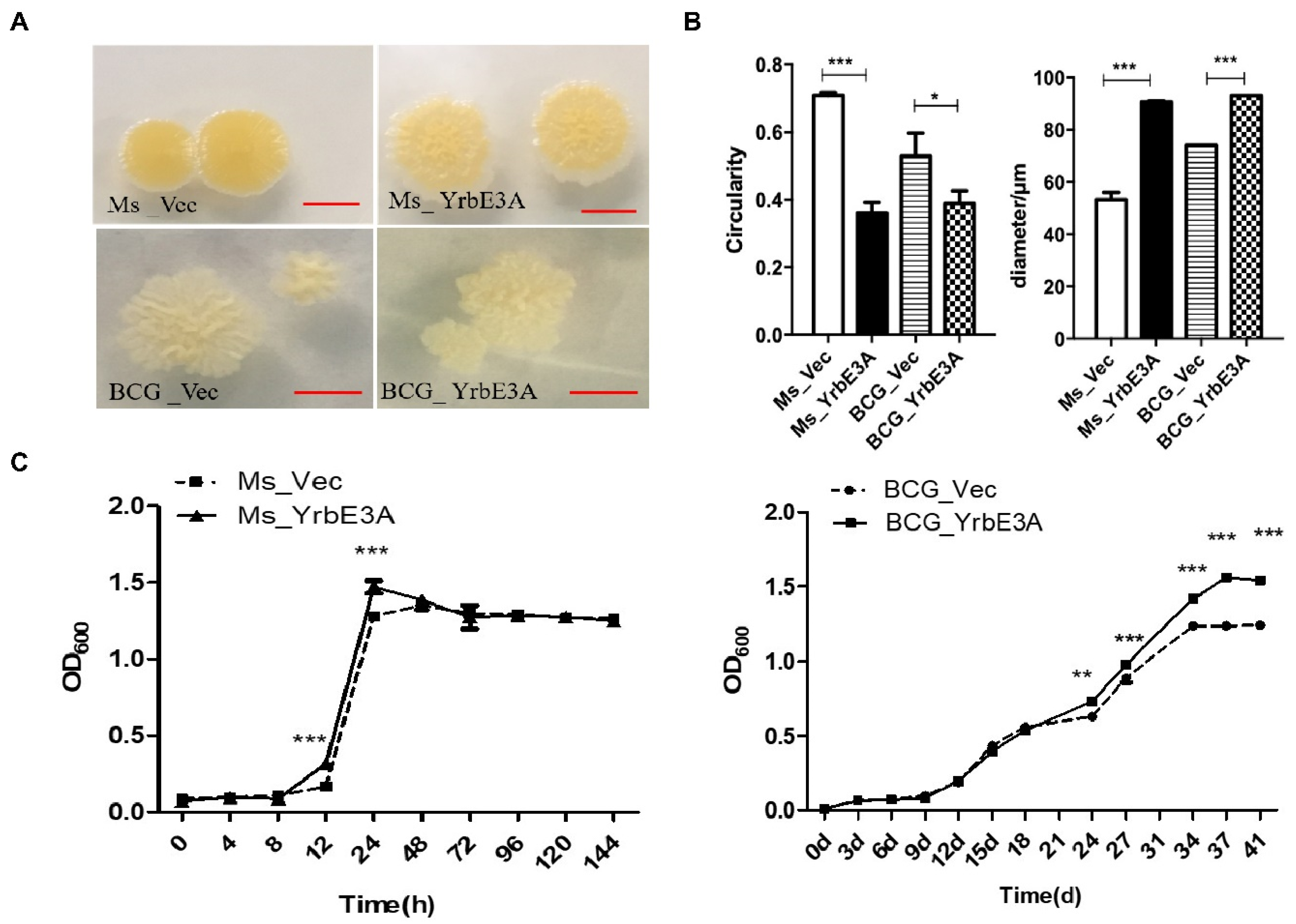
3.1. YrbE3A Expression and Growth Characterization of Ms_YrbE3A and BCG_YrbE3A Strains
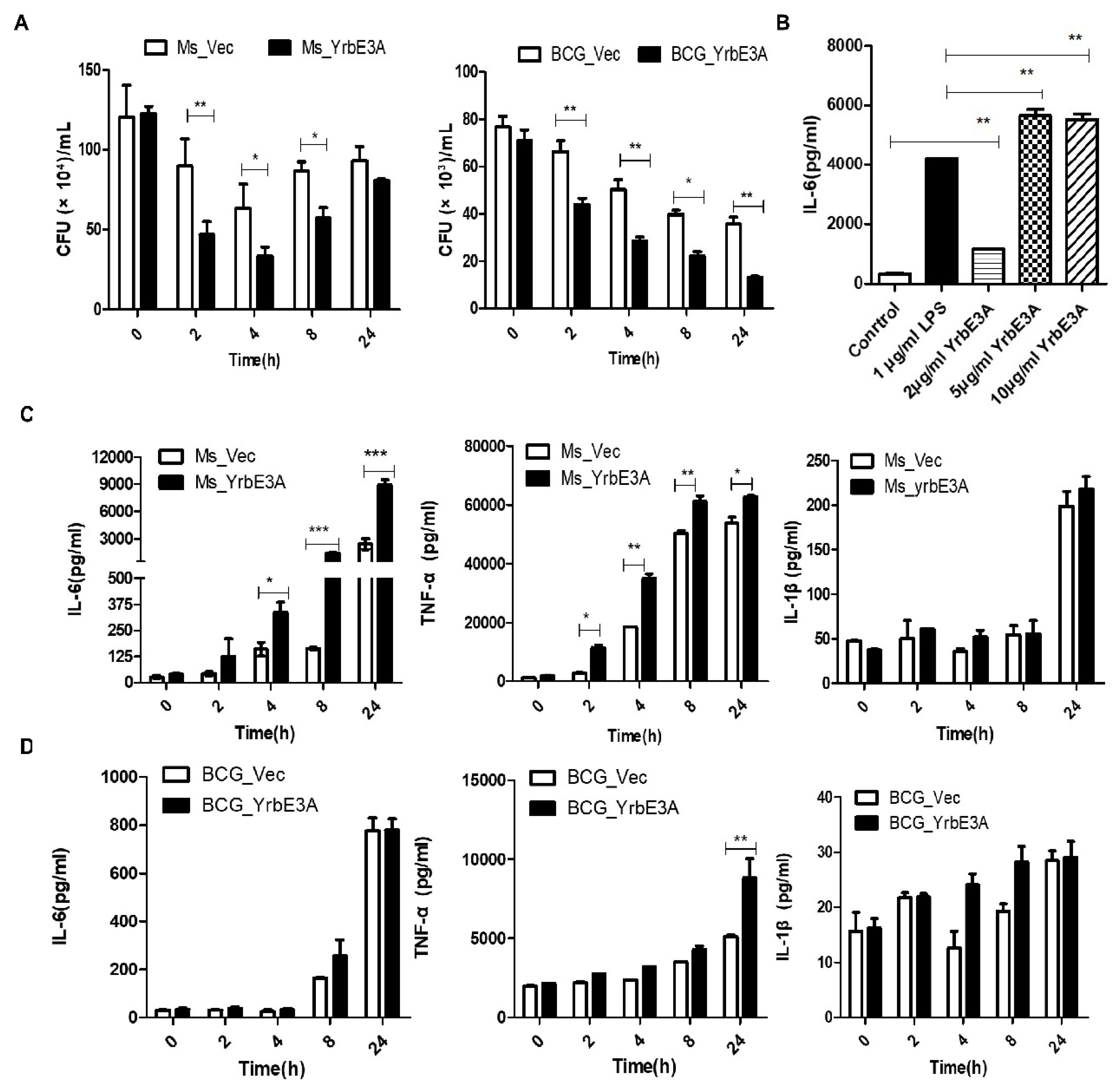
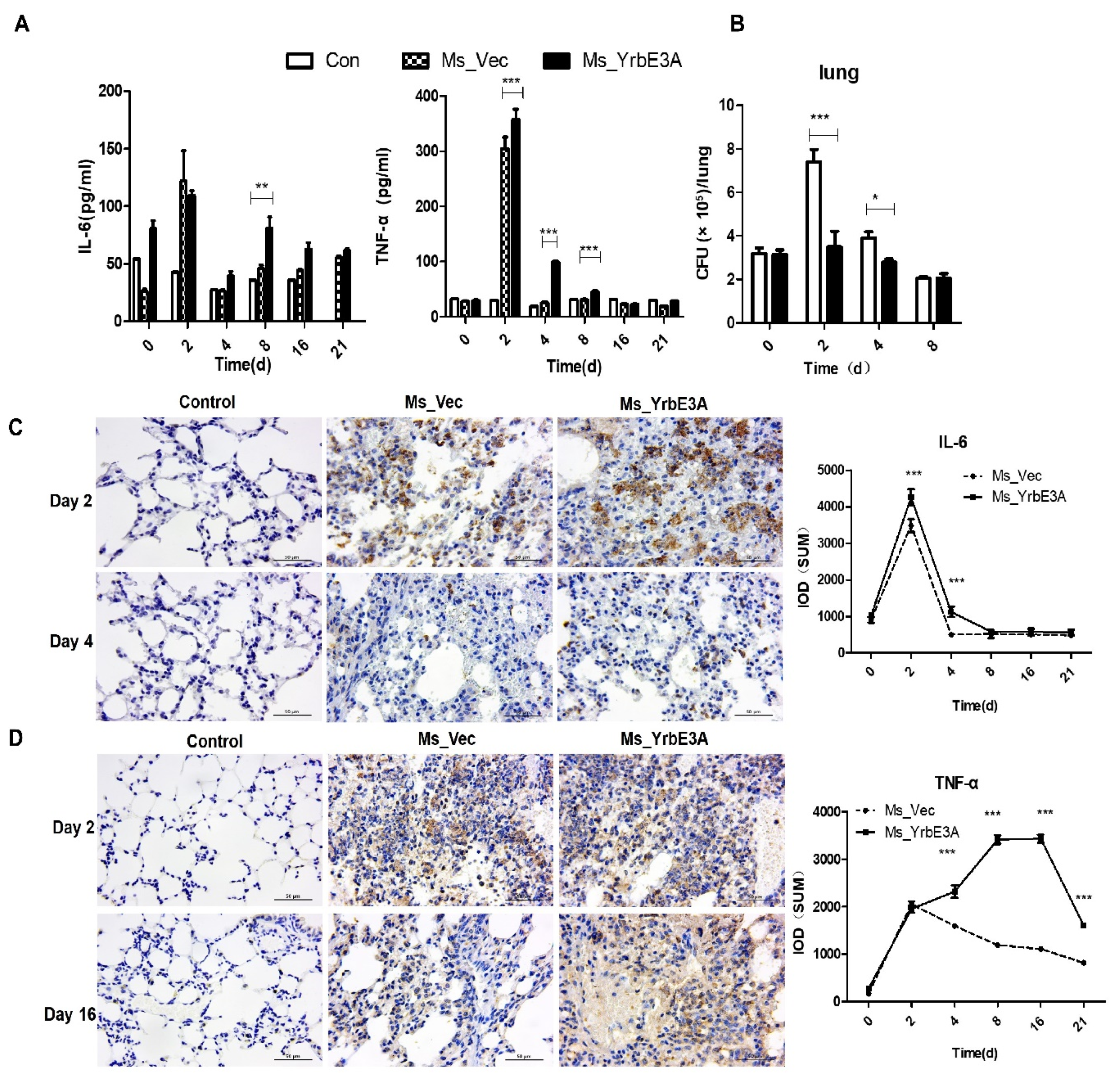
3.2. YrbE3A Reduced Mycobacterial Intracellular Survival and Induced Cytokine Production
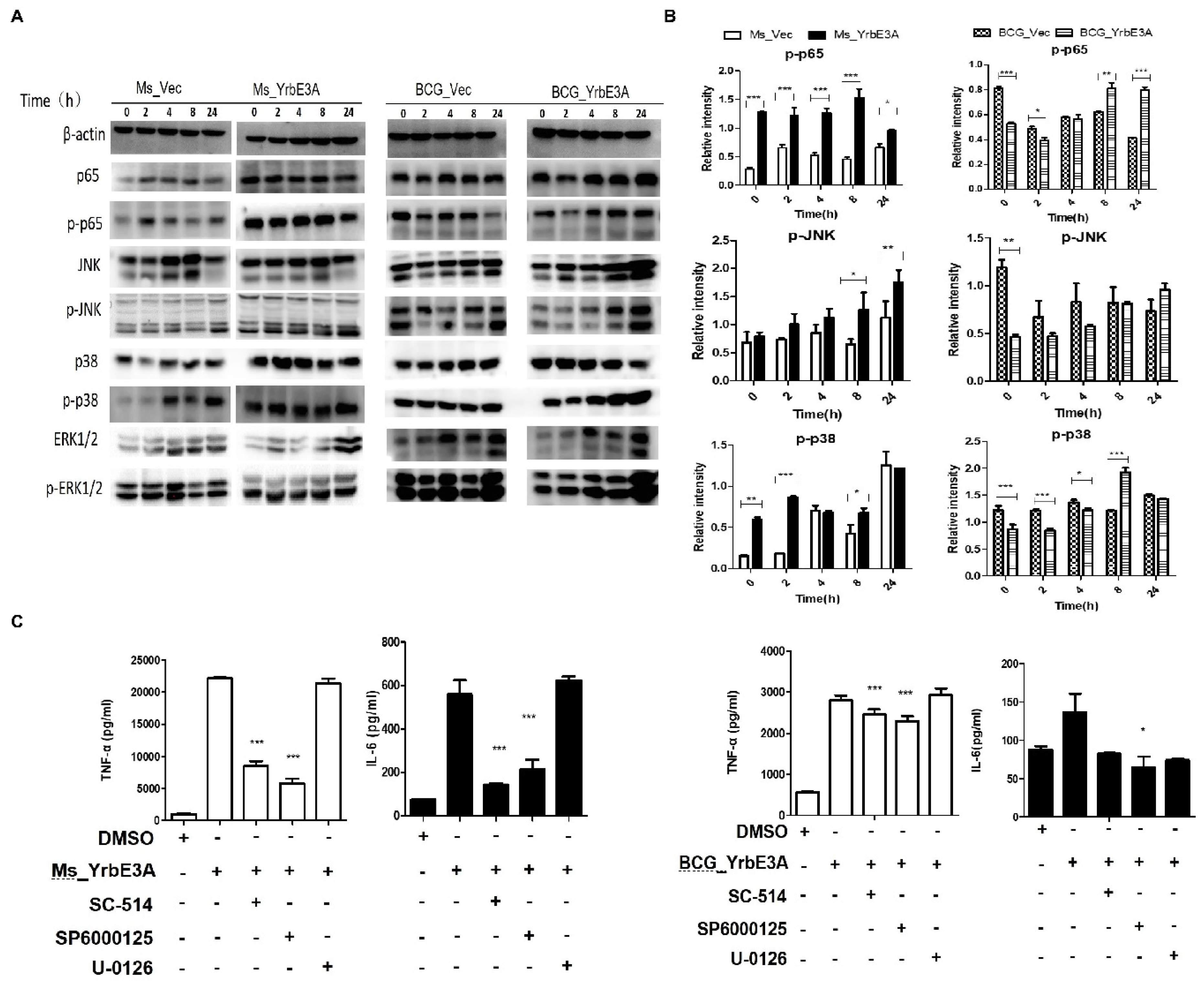
3.3. MAPK-JNK/p38 and NF-κB p65 Signaling Were Involved in the YrbE3A-Mediated Induction of Cytokines
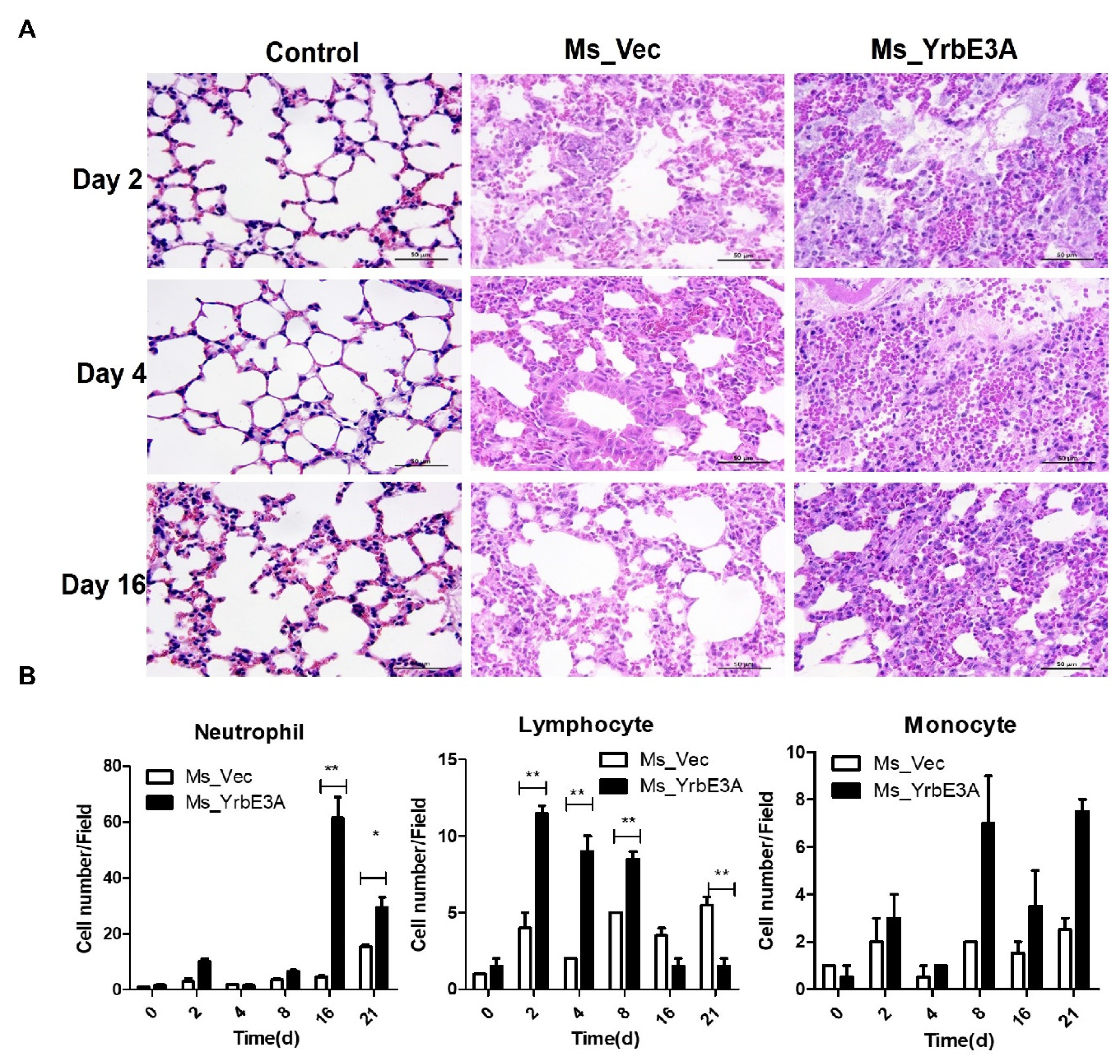
3.4. YrbE3A Exacerbated Histopathology during Ms_YrbE3A Infection
4. Discussion
4.1. The Character of Yrb3EA Protein
4.2. How Yrb3EA Affects Cytokine Expression in Vitro and in Vivo
4.3. How the MAPK and NF-κB Signaling Pathways Are Involved in Cytokine Expression
4.4. The Overexpression of YrbE3A Inhibits the Survival of Mycobacteria in Vitro and in Vivo
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| T | Tuberculosis |
| M.tb | Mycobacterium tuberculosis |
| M.bovis | Mycobacterium bovis |
| BCG | Bacillus Calmette–Guérin |
| IFN-γ | Interferon-γ |
| IL-1β | Interleukin 1β |
| IL-6 | Interleukin 6 |
| TNF-α | Tumor necrosis factor-α |
| IL-10 | Interleukin 10 |
| IL-12p70 | Interleukin 12p70 |
| MCP-1 | Monocyte chemoattractant protein 1 |
| IL-1α | Interleukin 1α |
| ORFs | Open Reading Frame |
| HRP | Horseradish Peroxidase |
| ECL | Enhanced chemiluminescence |
| CFU | Colony-Forming Unit |
| PVDF | Poly vinylidene fluoride |
References
- Fitchett, J.R.; MacPherson, P.; Corbett, E.L. Implementing the End TB Strategy and the intersection with the Sustainable Development Goals, 2016–2030. Trans. R. Soc. Trop. Med. Hyg. 2016, 110, 145–147. [Google Scholar] [CrossRef]
- World Health Organization. Global Tuberculosis Report 2019. World Health Organization 2019. Available online: http://www.who.int/tb/publications/global_report/en/ (accessed on 17 October 2019).
- Deng, W.Y.; Long, Q.X.; Zeng, J.; Li, P.; Yang, W.M.; Chen, X.C.; Xie, J.P. Mycobacterium tuberculosis PE_PGRS41 Enhances the Intracellular Survival of M. smegmatis within Macrophages Via Blocking Innate Immunity and Inhibition of Host Defense. Sci Rep.-Uk 2017, 7, 46716. [Google Scholar] [CrossRef]
- Joshi, S.M.; Pandey, A.K.; Capite, N.; Fortune, S.M.; Rubin, E.J.; Sassetti, C.M. Characterization of mycobacterial virulence genes through genetic interaction mapping. Proc. Natl. Acad. Sci. USA 2006, 103, 11760–11765. [Google Scholar] [CrossRef]
- Lerner, T.R.; Borel, S.; Gutierrez, M.G. The innate immune response in human tuberculosis. Cell Microbiol. 2015, 17, 1277–1285. [Google Scholar] [CrossRef]
- Scriba, T.J.; Penn-Nicholson, A.; Shankar, S.; Hraha, T.; Thompson, E.G.; Sterling, D.; Nemes, E.; Darboe, F.; Suliman, S.; Amon, L.M.; et al. Sequential inflammatory processes define human progression from M. tuberculosis infection to tuberculosis disease. PLoS Pathog. 2017, 13, e1006687. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, N.; Price, S.; Hope, J. BCG vaccination of neonatal calves: Potential roles for innate immune cells in the induction of protective immunity. Comp. Immunol. Microbiol. Infect. Di.s 2012, 35, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Faksri, K.; Xia, E.; Tan, J.H.; Teo, Y.Y.; Ong, R.T.H. In silico region of difference (RD) analysis of Mycobacterium tuberculosis complex from sequence reads using RD-Analyzer. Bmc Genom. 2016, 17. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chen, J.Z.; Zhang, Y.; Diao, N.; Zhang, S.; Wu, J.; Lu, C.Y.; Wang, F.F.; Gao, Y.; Shao, L.Y.; et al. Mycobacterium tuberculosis Region of Difference (RD) 2 Antigen Rv1985c and RD11 Antigen Rv3425 Have the Promising Potential To Distinguish Patients with Active Tuberculosis from M. bovis BCG-Vaccinated Individuals. Clin. Vaccine Immunol. 2013, 20, 69–76. [Google Scholar] [CrossRef][Green Version]
- de la Paz Santangelo, M.; Klepp, L.; Nunez-Garcia, J.; Blanco, F.C.; Soria, M.; Garcia-Pelayo, M.C.; Bianco, M.V.; Cataldi, A.A.; Golby, P.; Jackson, M.; et al. Mce3R, a TetR-type transcriptional repressor, controls the expression of a regulon involved in lipid metabolism in Mycobacterium tuberculosis. Microbiology 2009, 155, 2245–2255. [Google Scholar] [CrossRef]
- Glickman, M.S.; Cox, J.S.; Jacobs, W.R. A novel mycolic acid cyclopropane synthetase is required for cording, persistence, and virulence of Mycobacterium tuberculosis. Mol. Cell 2000, 5, 717–727. [Google Scholar] [CrossRef]
- Russell-Goldman, E.; Xu, J.; Wang, X.; Chan, J.; Tufariello, J.M. A Mycobacterium tuberculosis Rpf double-knockout strain exhibits profound defects in reactivation from chronic tuberculosis and innate immunity phenotypes. Infect. Immun. 2008, 76, 4269–4281. [Google Scholar] [CrossRef] [PubMed]
- Meena, L.S.; Meena, J. Cloning and characterization of a novel PE_PGRS60 protein (Rv3652) of Mycobacterium tuberculosis H37 Rv exhibit fibronectin-binding property. Biotechnol. Appl. Biochem. 2016, 63, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Nzungize, L.; Ali, M.K.; Wang, X.Y.; Huang, X.; Yang, W.M.; Duan, X.K.; Yan, S.Q.; Li, C.Y.; Abdalla, A.E.; Jeyakkumar, P.; et al. Mycobacterium tuberculosis metC (Rv3340) derived hydrogen sulphide conferring bacteria stress survival. J. Drug Target. 2019, 27, 1004–1016. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhao, Q.; Deng, W.; Chen, T.; Liu, M. Mycobacterium tuberculosis Rv3402c Enhances Mycobacterial Survival within Macrophages and Modulates the Host Pro-Inflammatory Cytokines Production via NF-Kappa B/ERK/p38 Signaling. Plos One 2014, 9, e94418. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, L.L.; Zhou, W.D.; Chen, Y.F.; Wang, Z. The surface of the geometric characteristics analysis for rice endosperm starch granules by using Image J. J. Chin. Electron. Microsc. Soc. 2011, 30, 466–471. [Google Scholar]
- Wang, J.; Li, B.X.; Ge, P.P.; Li, J.; Wang, Q.; Gao, G.F.; Qiu, X.B.; Liu, C.H. Mycobacterium tuberculosis suppresses innate immunity by coopting the host ubiquitin system. Nat. Immunol. 2015, 16, 237–245. [Google Scholar] [CrossRef]
- Pang, X.; Samten, B.; Cao, G.; Wang, X.; Tvinnereim, A.R.; Chen, X.L.; Howard, S.T. MprAB regulates the espA operon in Mycobacterium tuberculosis and modulates ESX-1 function and host cytokine response. J. Bacteriol. 2013, 195, 66–75. [Google Scholar] [CrossRef]
- Gupta, S.; Kumar, A.; Jaryal, K.; Kumari, R.; Sharma, A.; Singh, R.K.; Pandey, S.K.; Anupurba, S. Rv1273c, an ABC transporter of Mycobacterium tuberculosis promotes mycobacterial intracellular survival within macrophages via modulating the host cell immune response. Int. J. Biol. Macromol. 2019, 142, 320–331. [Google Scholar] [CrossRef]
- Xu, X.Y.; Lu, X.L.; Dong, X.F.; Luo, Y.P.; Wang, Q.; Liu, X.; Fu, J.; Zhang, Y.; Zhu, B.D.; Ma, X.M. Effects of hMASP-2 on the formation of BCG infection-induced granuloma in the lungs of BALB/c mice. Sci Rep.-Uk 2017, 7. [Google Scholar] [CrossRef]
- Liu, Q.; Wu, H.; Chim, S.M.; Zhou, L.; Zhao, J.; Feng, H.; Wei, Q.; Wang, Q.; Zheng, M.H.; Tan, R.X.; et al. SC-514, a selective inhibitor of IKKbeta attenuates RANKL-induced osteoclastogenesis and NF-kappaB activation. Biochem. Pharmacol. 2013, 86, 1775–1783. [Google Scholar] [CrossRef]
- Bennett, B.L.; Sasaki, D.T.; Murray, B.W.; O’Leary, E.C.; Sakata, S.T.; Xu, W.; Leisten, J.C.; Motiwala, A.; Pierce, S.; Satoh, Y.; et al. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc. Natl. Acad. Sci. USA 2001, 98, 13681–13686. [Google Scholar] [CrossRef] [PubMed]
- Forrellad, M.A.; McNeil, M.; Santangelo Mde, L.; Blanco, F.C.; Garcia, E.; Klepp, L.I.; Huff, J.; Niederweis, M.; Jackson, M.; Bigi, F. Role of the Mce1 transporter in the lipid homeostasis of Mycobacterium tuberculosis. Tuberculosis (Edinb) 2014, 94, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Chandolia, A.; Rathor, N.; Sharma, M.; Saini, N.K.; Sinha, R.; Malhotra, P.; Brahmachari, V.; Bose, M. Functional analysis of mce4A gene of Mycobacterium tuberculosis H37Rv using antisense approach. Microbiol. Res. 2014, 169, 780–787. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Katoch, V.M.; Mohanty, K.K.; Chauhan, D.S. Analysis of expression profile of mce operon genes (mce1, mce2, mce3 operon) in different Mycobacterium tuberculosis isolates at different growth phases. Indian J. Med. Res. 2016, 143, 487–494. [Google Scholar]
- Udgata, A.; Qureshi, R.; Mukhopadhyay, S. Transduction of functionally contrasting signals by two mycobacterial PPE proteins downstream of TLR2 receptors. J. Immunol. 2016, 197, 1776–1787. [Google Scholar] [CrossRef]
- Qureshi, R.; Rameshwaram, N.R.; Battu, M.B.; Mukhopadhyay, S. PPE65 of M. tuberculosis regulate pro-inflammatory signalling through LRR domains of Toll like receptor-2. Biochem. Biophys. Res. Commun. 2019, 508, 152–158. [Google Scholar] [CrossRef]
- Kumar, A.; Bose, M.; Brahmachari, V. Analysis of expression profile of mammalian cell entry (mce) operons of Mycobacterium tuberculosis. Infect. Immun. 2003, 71, 6083–6087. [Google Scholar] [CrossRef]
- Li, J.; Chai, Q.Y.; Zhang, Y.; Li, B.X.; Wang, J.; Qiu, X.B.; Liu, C.H. Mycobacterium tuberculosis Mce3E suppresses host innate immune responses by targeting ERK1/2 signaling. J. Immunol. 2015, 194, 3756–3767. [Google Scholar] [CrossRef]
- Lai, X.; Shen, Y.; Zhou, D.; Sehgal, P.; Shen, L.; Simon, M.; Qiu, L.; Letvin, N.L.; Chen, Z.W. Immune biology of macaque lymphocyte populations during mycobacterial infection. Clin. Exp. Immunol. 2003, 133, 182–192. [Google Scholar] [CrossRef]
- Kim, W.S.; Kim, J.S.; Cha, S.B.; Han, S.J.; Kim, H.; Kwon, K.W.; Kim, S.J.; Eum, S.Y.; Cho, S.N.; Shin, S.J. Virulence-Dependent Alterations in the Kinetics of Immune Cells during Pulmonary Infection by Mycobacterium tuberculosis. Plos One 2015, 10, e0145234. [Google Scholar] [CrossRef]
- Bourigault, M.L.; Segueni, N.; Rose, S.; Court, N.; Vacher, R.; Vasseur, V.; Erard, F.; Le Bert, M.; Garcia, I.; Iwakura, Y.; et al. Relative contribution of IL-1alpha, IL-1beta and TNF to the host response to Mycobacterium tuberculosis and attenuated M. bovis BCG. Immun. Inflamm. Dis. 2013, 1, 47–62. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, U.; Chadha, A.; Gupta, P.; Tiwari, B.; Bhattacharyya, K.; Popli, S.; Raman, R.; Brahamachari, V.; Singh, Y.; Malhotra, P.; et al. Suppression of Toll-like receptor 2-mediated proinflammatory responses by Mycobacterium tuberculosis protein Rv3529c. J. Leukoc. Biol. 2017, 102, 1249–1259. [Google Scholar] [CrossRef]
- Han, Y.; Wu, Z.; Wu, T.; Huang, Y.; Cheng, Z.; Li, X.; Sun, T.; Xie, X.; Zhou, Y.; Du, Z. Tumor-suppressive function of long noncoding RNA MALAT1 in glioma cells by downregulation of MMP2 and inactivation of ERK/MAPK signaling. Cell Death Dis. 2016, 7, e2123. [Google Scholar] [CrossRef] [PubMed]
- Hayden, M.S.; Ghosh, S. Regulation of NF-kappaB by TNF family cytokines. Semin. Immunol. 2014, 26, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Sabio, G.; Davis, R.J. TNF and MAP kinase signalling pathways. Semi.n Immunol. 2014, 26, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Flynn, J.L.; Chan, J. Immunology of tuberculosis. Annu. Rev. Immunol. 2001, 19, 93–129. [Google Scholar] [CrossRef]
- Yao, J.; Du, X.; Chen, S.; Shao, Y.; Deng, K.; Jiang, M.; Liu, J.; Shen, Z.; Chen, X.; Feng, G. Rv2346c enhances mycobacterial survival within macrophages by inhibiting TNF-α and IL-6 production via the p38/miRNA/NF-κB pathway. Emerg. Microbes Infect. 2018, 7, 1–16. [Google Scholar] [CrossRef]
- Shiloh, M.U.; Nathan, C.F. Reactive nitrogen intermediates and the pathogenesis of Salmonella and mycobacteria. Curr. Opin. Microbiol. 2000, 3, 35–42. [Google Scholar] [CrossRef]
- Harris, J.; Hope, J.C.; Lavelle, E.C. Autophagy and the immune response to TB. Transbound. Emerg. Dis. 2009, 56, 248–254. [Google Scholar] [CrossRef]
- Gutierrez, M.G.; Master, S.S.; Singh, S.B.; Taylor, G.A.; Colombo, M.I.; Deretic, V. Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell 2004, 119, 753–766. [Google Scholar] [CrossRef]
| Genes | Direction | Sequences (5′-3′) |
|---|---|---|
| Rv1964 | F-BamH I | GCCAAGACAATTGCGGATCCATGGTAATCGTGGCCGACAAG |
| R-EcoR V | TGGTGGTGGTGGTGGATATCTCAGGACACCATGAATGGGATG | |
| IL-6 | Sense | TGCCTTCTTGGGACTGAT |
| Antisense | CTGGCTTTGTCTTTCTTGTT | |
| TNF-α | Sense | CGATGAGGTCAATCTGCCCA |
| Antisense | CCAGGTCACTGTCCCAGC | |
| IL-1β | Sense | GCTAGGCTGCTGAGGTTTCTT |
| Antisense | TGAAATGCCACCTTTTGACAG | |
| β-actin | Sense | TGCTGTCCCTGTATGCCTCT |
| Antisense | GGTCTTTACGGATGTCAACG |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Zhu, X.; Peng, Y.; Zhu, T.; Liu, H.; Zhu, Y.; Xiong, X.; Chen, X.; Hu, C.; Chen, H.; et al. Mycobacterium tuberculosis YrbE3A Promotes Host Innate Immune Response by Targeting NF-κB/JNK Signaling. Microorganisms 2020, 8, 584. https://doi.org/10.3390/microorganisms8040584
Wang J, Zhu X, Peng Y, Zhu T, Liu H, Zhu Y, Xiong X, Chen X, Hu C, Chen H, et al. Mycobacterium tuberculosis YrbE3A Promotes Host Innate Immune Response by Targeting NF-κB/JNK Signaling. Microorganisms. 2020; 8(4):584. https://doi.org/10.3390/microorganisms8040584
Chicago/Turabian StyleWang, Jieru, Xiaojie Zhu, Yongchong Peng, Tingting Zhu, Han Liu, Yifan Zhu, Xuekai Xiong, Xi Chen, Changmin Hu, Huanchun Chen, and et al. 2020. "Mycobacterium tuberculosis YrbE3A Promotes Host Innate Immune Response by Targeting NF-κB/JNK Signaling" Microorganisms 8, no. 4: 584. https://doi.org/10.3390/microorganisms8040584
APA StyleWang, J., Zhu, X., Peng, Y., Zhu, T., Liu, H., Zhu, Y., Xiong, X., Chen, X., Hu, C., Chen, H., Chen, Y., & Guo, A. (2020). Mycobacterium tuberculosis YrbE3A Promotes Host Innate Immune Response by Targeting NF-κB/JNK Signaling. Microorganisms, 8(4), 584. https://doi.org/10.3390/microorganisms8040584






