Highly Sensitive and Rapid Quantitative Detection of Plasmodium falciparum Using an Image Cytometer
Abstract
1. Introduction
2. Materials and Methods
2.1. Parasite Culture
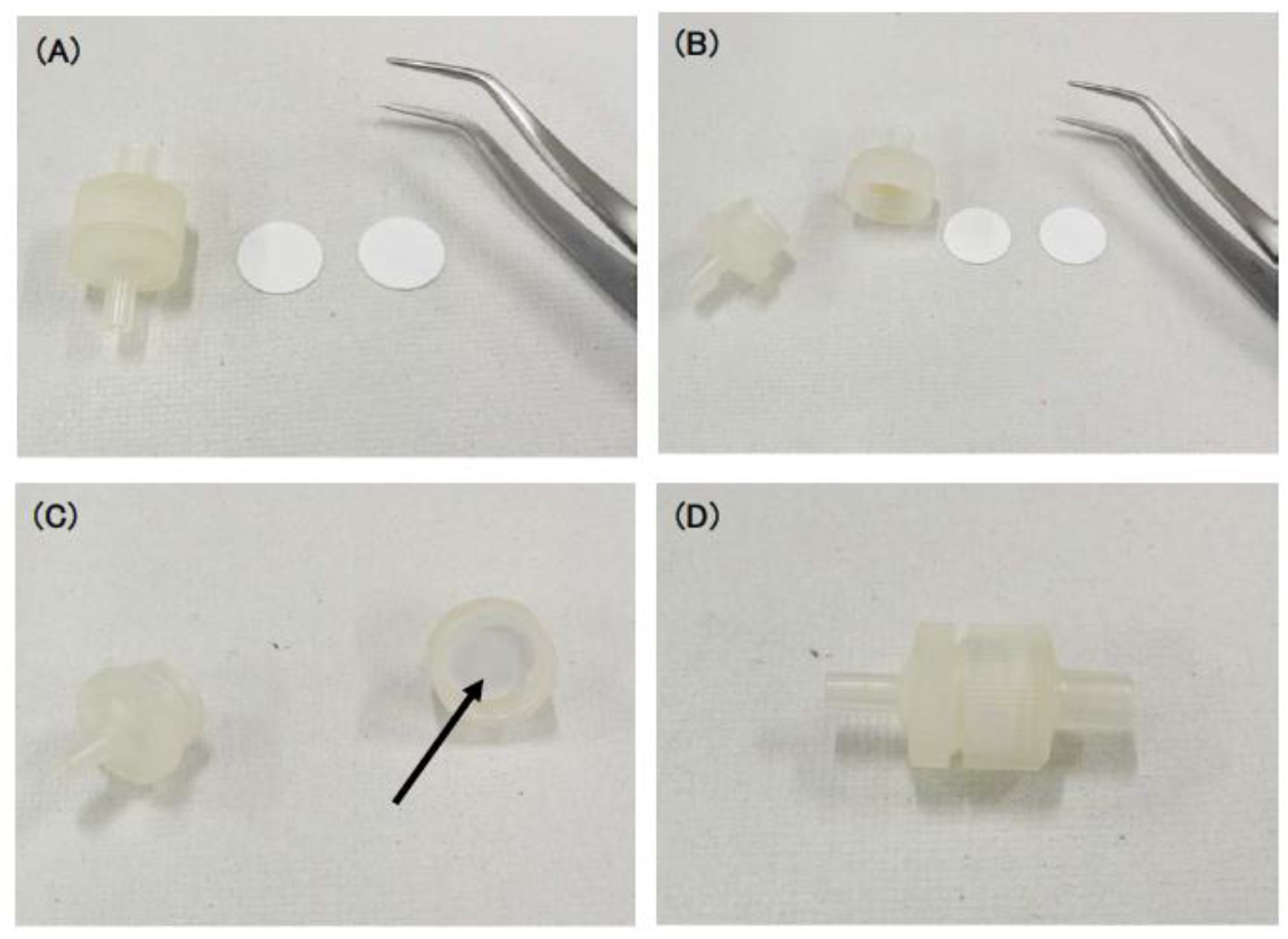
2.2. Preparation of Push Columns
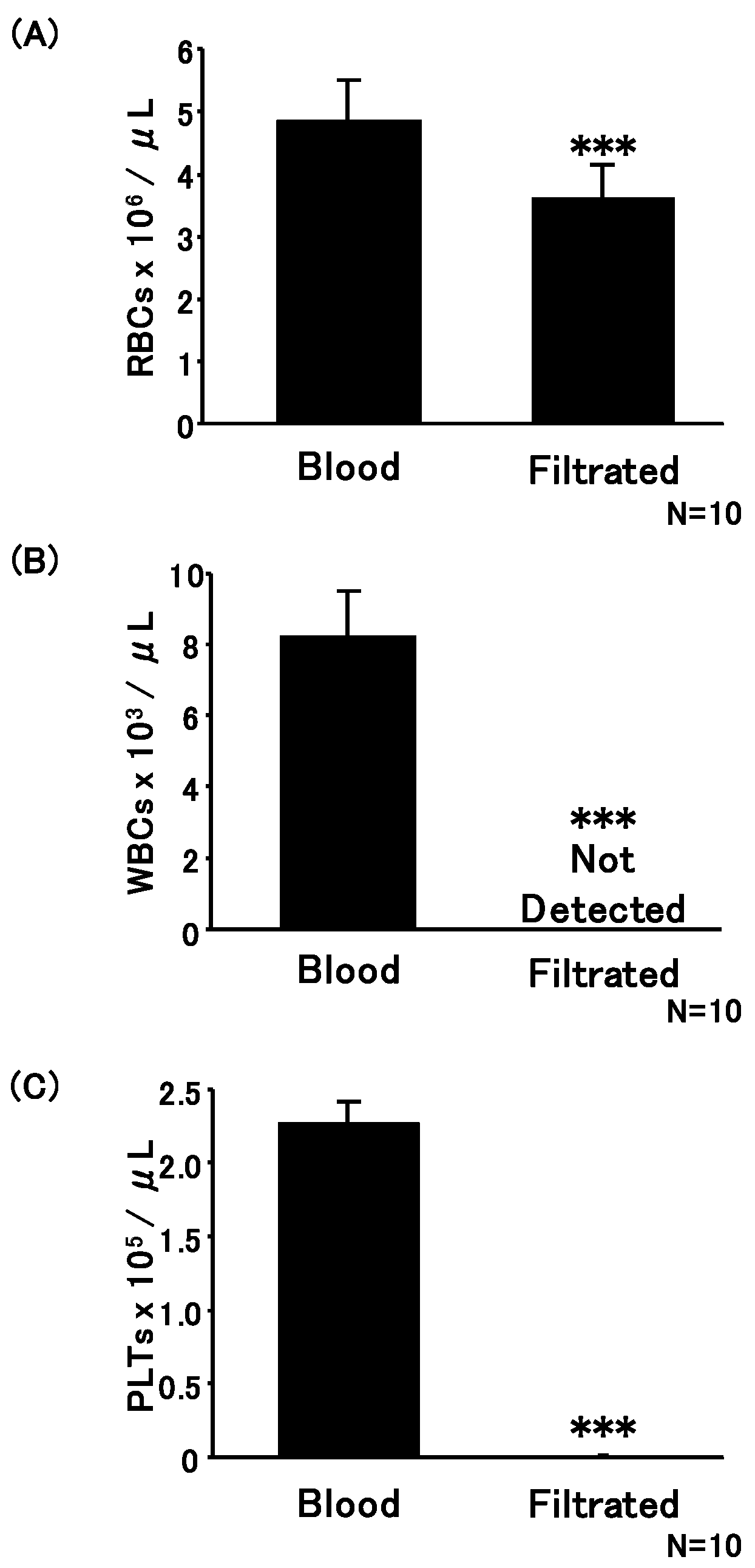
2.3. Erythrocytes, Leucocytes, and Platelet Enumeration
2.4. Detection of Parasite-Infected Erythrocytes Using CQ1
2.5. Statistical Analysis
2.6. Ethics Statement
3. Results and Discussion
3.1. Development of Push Columns for the Purification of Erythrocytes
3.2. Detection of Parasites Using CQ1
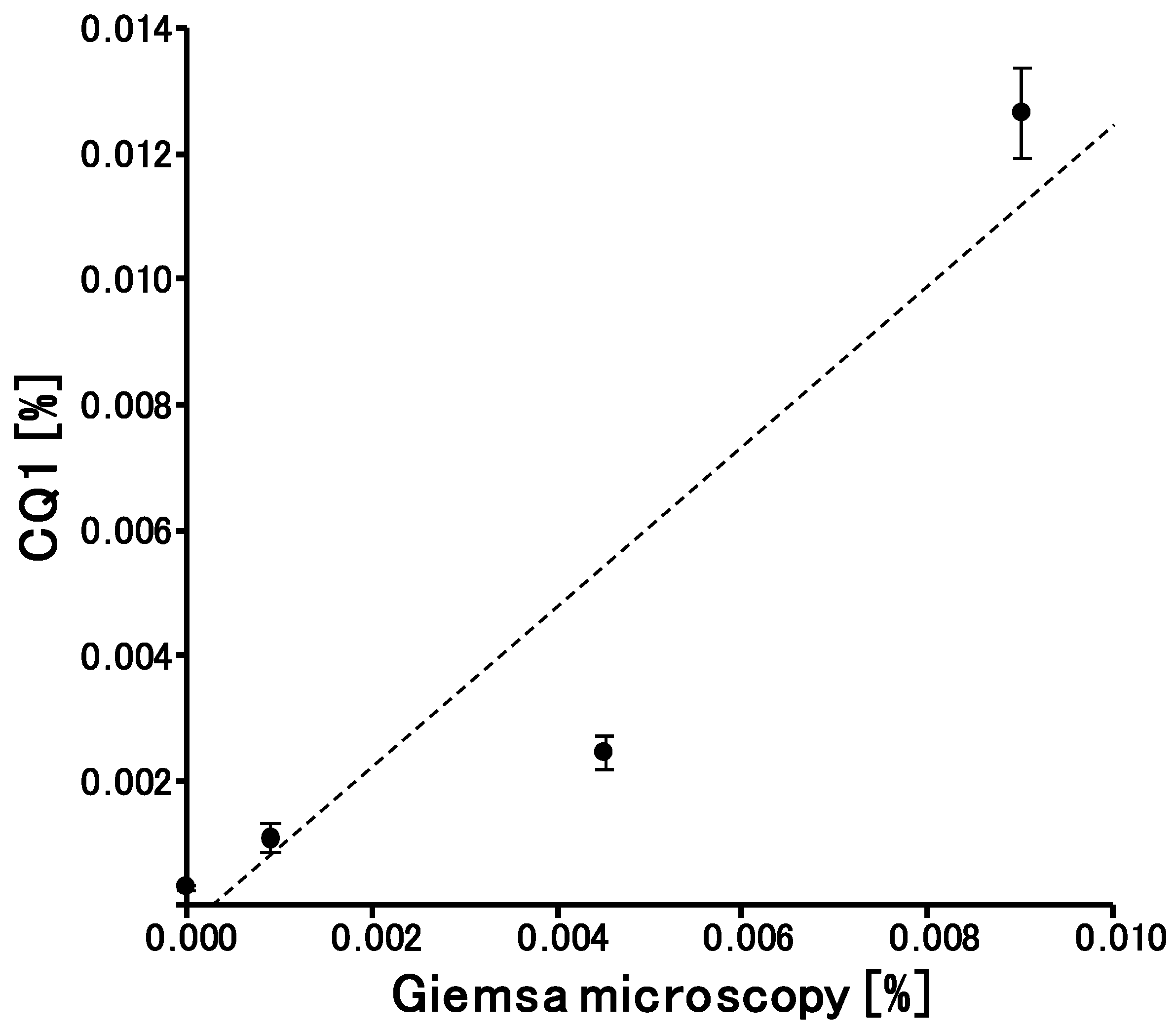
3.3. Quantitively and LOD of CQ1 for Parasitemia Calculation
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO. World Malaria Report 2019; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Ashley, E.A.; Pyae Phyo, A.; Woodrow, C.J. Malaria. Lancet 2018, 391, 1608–1621. [Google Scholar] [CrossRef]
- Ndour, P.A.; Larréché, S.; Mouri, O.; Argy, N.; Gay, F.; Roussel, C.; Jauréguiberry, S.; Perillaud, C.; Langui, D.; Biligui, S.; et al. Measuring the Plasmodium falciparum HRP2 protein in blood from artesunate-treated malaria patients predicts post-artesunate delayed hemolysis. Sci. Transl. Med. 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Slater, H.C.; Ross, A.; Ouédraogo, A.L.; White, L.J.; Nguon, C.; Walker, P.G.; Ngor, P.; Aguas, R.; Silal, S.P.; Dondorp, A.M.; et al. Assessing the impact of next-generation rapid diagnostic tests on Plasmodium falciparum malaria elimination strategies. Nature 2015, 528, S94–S101. [Google Scholar] [CrossRef] [PubMed]
- Slater, H.C.; Ross, A.; Felger, I.; Hofmann, N.E.; Robinson, L.; Cook, J.; Gonçalves, B.P.; Björkman, A.; Ouedraogo, A.L.; Morris, U.; et al. The temporal dynamics and infectiousness of subpatent Plasmodium falciparum infections in relation to parasite density. Nat. Commun. 2019, 10, 1433. [Google Scholar] [CrossRef] [PubMed]
- Cheaveau, J.; Mogollon, D.C.; Mohon, M.A.N.; Golassa, L.; Yewhalaw, D.; Pillai, D.R. Asymptomatic malaria in the clinical and public health context. Expert Rev. Anti. Infect. Ther. 2019, 17, 997–1010. [Google Scholar] [CrossRef] [PubMed]
- Girma, S.; Cheaveau, J.; Mohon, A.N.; Marasinghe, D.; Legese, R.; Balasingam, N.; Abera, A.; Feleke, S.M.; Golassa, L.; Pillai, D.R. Prevalence and Epidemiological Characteristics of Asymptomatic Malaria Based on Ultrasensitive Diagnostics: A Cross-sectional Study. Clin. Infect. Dis. 2019, 69, 1003–1010. [Google Scholar] [CrossRef]
- Yatsushiro, S.; Yamamura, S.; Yamaguchi, Y.; Shinohara, Y.; Tamiya, E.; Horii, T.; Baba, Y.; Kataoka, M. Rapid and highly sensitive detection of malaria-infected erythrocytes using a cell microarray chip. PLoS ONE 2010, 5, e13179. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Yatsushiro, S.; Hashimoto, M.; Kajimoto, K.; Ido, Y.; Abe, K.; Sofue, Y.; Nogami, T.; Hayashi, T.; Nagatomi, K.; et al. Development of a highly sensitive, quantitative, and rapid detection system for Plasmodium falciparum-infected red blood cells using a fluorescent blue-ray optical system. Biosens. Bioelectron. 2019, 132, 375–381. [Google Scholar] [CrossRef]
- Hashimoto, M.; Yokota, K.; Kajimoto, K.; Matsumoto, M.; Tatsumi, A.; Yamamoto, K.; Hyodo, T.; Matsushita, K.; Minakawa, N.; Mita, T.; et al. Quantitative Detection of Plasmodium falciparum Using, LUNA-FL, A Fluorescent Cell Counter. Microorganisms 2020, 8, 1356. [Google Scholar] [CrossRef] [PubMed]
- Yatsushiro, S.; Yamamoto, T.; Yamamura, S.; Abe, K.; Obana, E.; Nogami, T.; Hayashi, T.; Sesei, T.; Oka, H.; Okello-Onen, J.; et al. Application of a cell microarray chip system for accurate, highly sensitive, and rapid diagnosis for malaria in Uganda. Sci. Rep. 2016, 6, 30136. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Hashimoto, M.; Nagatomi, K.; Nogami, T.; Sofue, Y.; Hayashi, T.; Ido, Y.; Yatsushiro, S.; Abe, K.; Kajimoto, K.; et al. Development of a quantitative, portable, and automated fluorescent blue-ray device-based malaria diagnostic equipment with an on-disc SiO2 nanofiber filter. Sci. Rep. 2020, 10, 6585. [Google Scholar] [CrossRef] [PubMed]
- Vashist, S.K.; Luppa, P.B.; Yeo, L.Y.; Ozcan, A.; Luong, J.H.T. Emerging Technologies for Next-Generation Point-of-Care Testing. Trends Biotechnol. 2015, 33, 692–705. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, M.; Bando, M.; Kido, J.I.; Yokota, K.; Mita, T.; Kajimoto, K.; Kataoka, M. Nucleic acid purification from dried blood spot on FTA Elute Card provides template for polymerase chain reaction for highly sensitive Plasmodium detection. Parasitol. Int. 2019, 73, 101941. [Google Scholar] [CrossRef] [PubMed]
- Eshel, Y.; Houri-Yafin, A.; Benkuzari, H.; Lezmy, N.; Soni, M.; Charles, M.; Swaminathan, J.; Solomon, H.; Sampathkumar, P.; Premji, Z.; et al. Evaluation of the Parasight Platform for Malaria Diagnosis. J. Clin. Microbiol. 2017, 55, 768–775. [Google Scholar] [CrossRef] [PubMed]
- Homer, M.J.; Aguilar-Delfin, I.; Telford, S.R., 3rd; Krause, P.J.; Persing, D.H. Babesiosis. Clin. Microbiol. Rev. 2000, 13, 451–469. [Google Scholar] [CrossRef] [PubMed]
- Barrett, M.P.; Croft, S.L. Management of trypanosomiasis and leishmaniasis. Br. Med. Bull. 2012, 104, 175–196. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hashimoto, M.; Yokota, K.; Kajimoto, K.; Matsumoto, M.; Tatsumi, A.; Nakajima, Y.; Mita, T.; Minakawa, N.; Oka, H.; Kataoka, M. Highly Sensitive and Rapid Quantitative Detection of Plasmodium falciparum Using an Image Cytometer. Microorganisms 2020, 8, 1769. https://doi.org/10.3390/microorganisms8111769
Hashimoto M, Yokota K, Kajimoto K, Matsumoto M, Tatsumi A, Nakajima Y, Mita T, Minakawa N, Oka H, Kataoka M. Highly Sensitive and Rapid Quantitative Detection of Plasmodium falciparum Using an Image Cytometer. Microorganisms. 2020; 8(11):1769. https://doi.org/10.3390/microorganisms8111769
Chicago/Turabian StyleHashimoto, Muneaki, Kazumichi Yokota, Kazuaki Kajimoto, Musashi Matsumoto, Atsuro Tatsumi, Yoshihiro Nakajima, Toshihiro Mita, Noboru Minakawa, Hiroaki Oka, and Masatoshi Kataoka. 2020. "Highly Sensitive and Rapid Quantitative Detection of Plasmodium falciparum Using an Image Cytometer" Microorganisms 8, no. 11: 1769. https://doi.org/10.3390/microorganisms8111769
APA StyleHashimoto, M., Yokota, K., Kajimoto, K., Matsumoto, M., Tatsumi, A., Nakajima, Y., Mita, T., Minakawa, N., Oka, H., & Kataoka, M. (2020). Highly Sensitive and Rapid Quantitative Detection of Plasmodium falciparum Using an Image Cytometer. Microorganisms, 8(11), 1769. https://doi.org/10.3390/microorganisms8111769




