1. Introduction
The World Health Organization (WHO) recommends that all suspected malaria cases should have a parasite-based diagnosis, by either a microscopic analysis with Giemsa-stained blood films (Giemsa microscopy) or a rapid diagnosis test (RDT), prior to treatment with artemisinin-based combination therapy (ACT) [
1]. For point-of-care testing (POCT), the gold standard or reference for malaria diagnosis is Giemsa microscopy. Giemsa microscopy with thick films and thin smears provides sensitivity and allows quantitation, respectively [
2]. Accurate diagnosis with Giemsa microscopy is time-consuming and laborious, and it requires skilled microscopists [
3]. RDTs are often used as the first-line investigation. In Africa, the most endemic area, a
Plasmodium falciparum-only test based on the highly expressed histidine-rich protein 2 (HRP2) antigen is often used [
2]. Diagnosis with RDTs is qualitative with high false-positive rates. RDTs can remain positive for several weeks after clearance because of persisting pitted (once-infected) erythrocytes [
4]. It is necessary to develop a malaria diagnosis device, that is quantitative, easy to operate, and not time-consuming, for POCT.
Acridine orange (AO) staining has been optimised to reduce the time required to stain blood films [
5,
6,
7,
8,
9]. Devices for automatic analysis of thick or thin Giemsa-stained blood films have also been developed [
10]. They can be operated with smartphones, and may be useful for POCT; however, skilled technicians are required for the preparation of blood films. Furthermore, the preparation of thick blood films involves more than 1 h in an incubator at 37 to 40 °C, or overnight at room temperature.
In order to perform highly sensitive and quantitative malaria diagnosis, some devices, that can detect fluorescent nuclear-stained parasites in infected erythrocytes by forming a monolayer on microchambers or a microplate [
11,
12,
13,
14], or by using flow cytometers [
15], have been developed. These devices have a microscope with a digital camera or a charge-coupled device camera for the detection of erythrocytes and/or labelled parasites, and hence, they may be unsuitable for POCT except in hospitals located in urban areas. The limitations of these devices include: (1) Unstable power supply in endemic areas, (2) High cost which makes it unaffordable for people in developing countries, and (3) Fragility which makes maintenance difficult.
Recently, we developed a novel diagnosis device by applying a Blu-ray optical system [
16]. The device consists of a disposable compact disk (CD)-like cassettes and a CD player-like image reader. Approximately one million erythrocytes form a monolayer on the cassette, and nuclear-stained parasites are detected with an image reader followed by the automatic determination of the infection rate (parasitemia). However, the device has some limitations. Firstly, although nine samples can be analysed at the same time, parasite counting takes a moderately long time (approximately 40 min). Secondly, computers that are able to process a large amount of image data are required.
In this study, we attempted to develop a novel malaria diagnosis device that is more suitable for POCT in endemic countries. Whole blood of healthy donors spiked with in vitro cultured parasite-infected erythrocytes was prepared, and parasitemia was estimated using a fluorescent cell counter, LUNA-FL (Logos Biosystems, Inc., Gyeonggi-do, Korea). LUNA-FL is a compact and computer-integrated device, and rapid cell counting can be performed by AO staining. Actually, rapid, easy-to-operate, and accurate quantitative malaria detection was possible using LUNA-FL, suggesting that malaria diagnosis can be performed in endemic countries using the device.
2. Materials and Methods
2.1. Parasite Culture
P. falciparum (3D7 strain) was cultured as previously described [
16]. We synchronised the parasites at the ring stage using 5% D-sorbitol and harvested highly synchronous ring-stage parasite-infected erythrocytes (>95%) [
17]. Parasitemia was estimated by microscopic analysis with Giemsa-stained thin blood films. More than 5000 erythrocytes were counted for the estimation of parasitemia [% parasitemia = (parasitised RBCs/total RBCs) × 100].
2.2. Preparation of Spin Columns
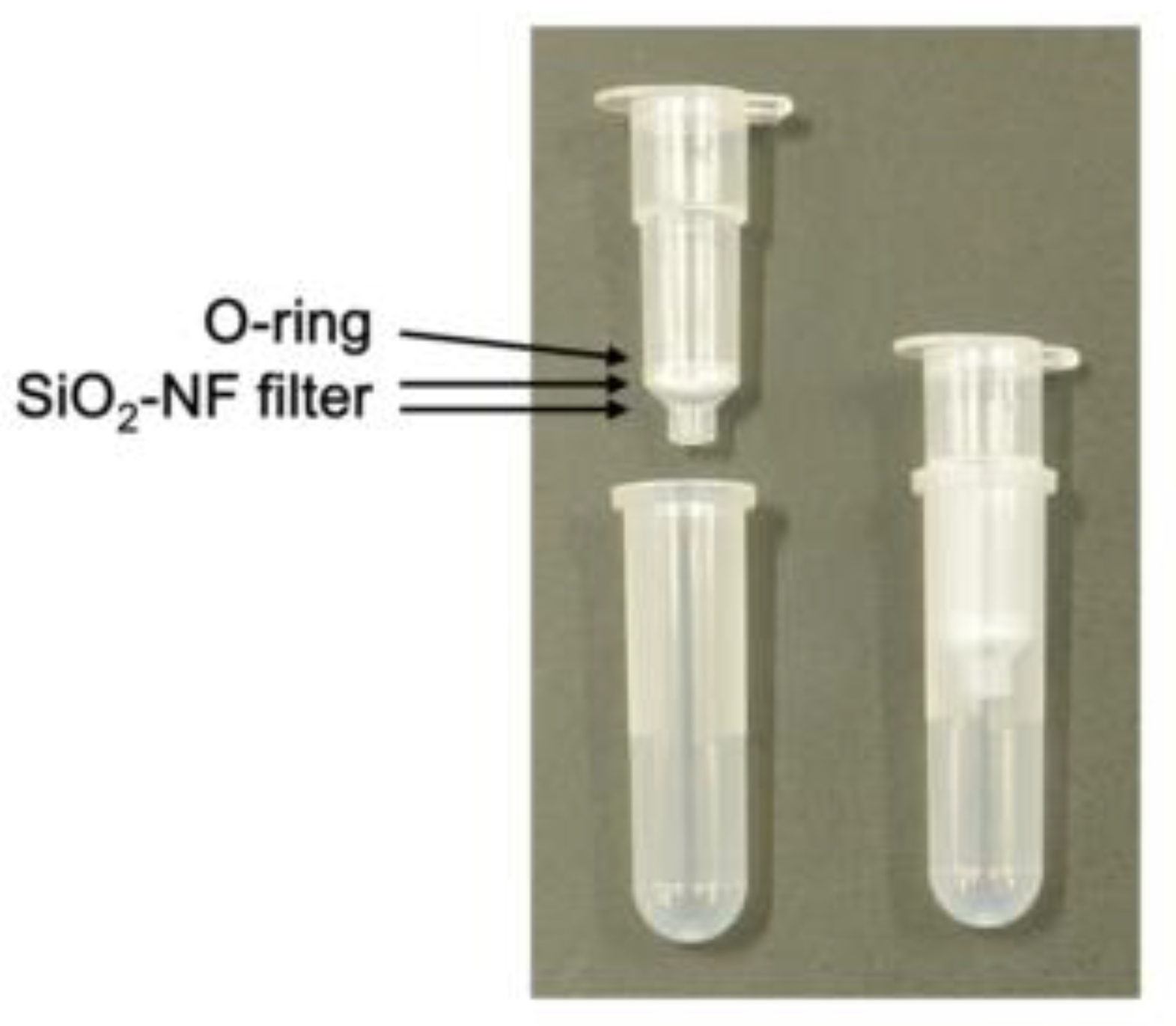
Empty spin columns were purchased from Nippon Gene Co., Ltd. (Tokyo, Japan). SiO2-nanofiber (NF) filters (250 µm thick) (Nitto Denko Corp., Osaka, Japan) were punched at 7 mm diameters. Two sheets of the punched filters were set on the spin column and fixed with an O-ring.
2.3. Erythrocytes, Leucocytes, and Platelet Counting
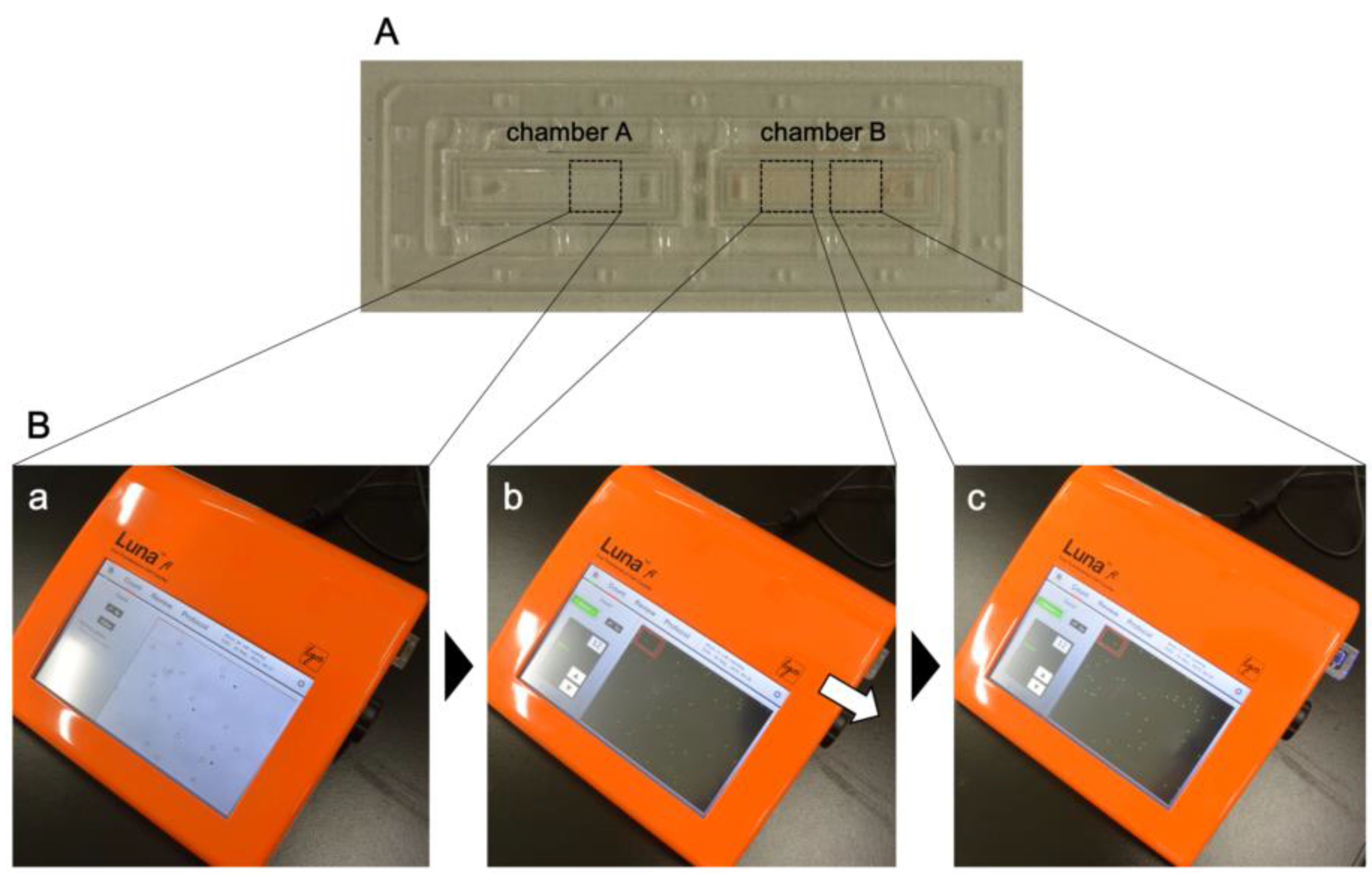
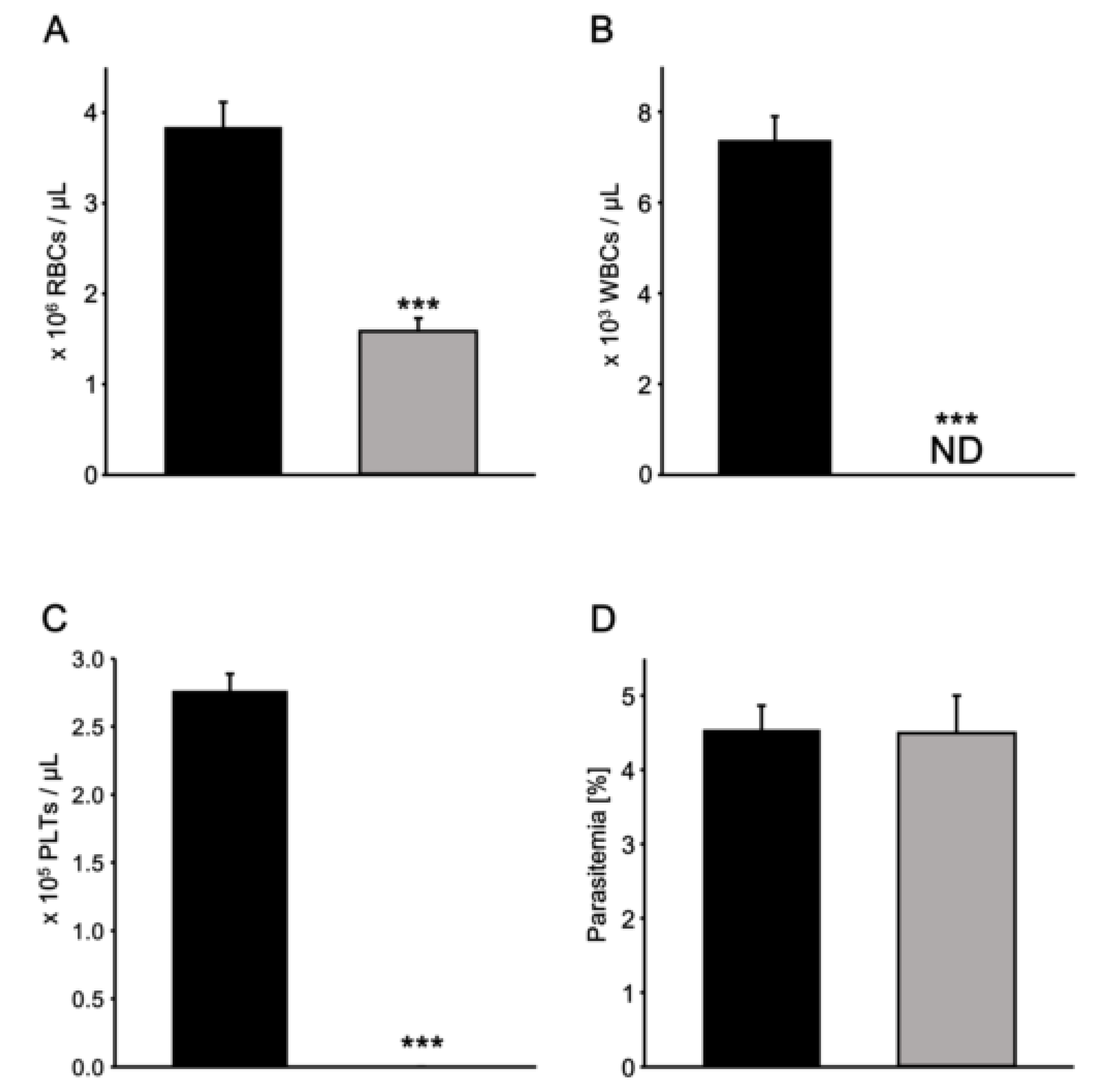
Fresh whole blood samples drawn by finger pricking from Japanese healthy volunteers who have never been infected with malaria parasites were collected in BD Microcontainer blood collection tubes (K2EDTA, Lavender, Becton, Dickinson Co., Franklin Lakes, NJ, USA). The whole blood sample (200 µL) was diluted 25-fold with phosphate buffered salts (PBS) and applied to the spin column followed by centrifugation at 400 g for 30 sec. To count the erythrocytes, the filtered blood sample was diluted 100-fold with PBS, and 10 µL of the aliquot was applied to a photon slide (Logos Biosystems, Inc., Gyeonggi-do, Korea). The number of erythrocytes was counted with LUNA-FL using the “Bright-Field Counting mode” as described in the manual. The leucocytes in the filtered blood samples were stained with AO/PI Cell Viability Kit (Logos Biosystems, Inc.) in order to count the leucocytes and 10 µL of the aliquot was applied to a photon slide. The number of leucocytes was counted with LUNA-FL using the “Fluorescence Cell Counting mode”. The exposure times for the green fluorescence and red fluorescence were set to Levels 5 and 1, respectively. For the specific detection of leucocytes using LUNA-FL at the exposure times, the threshold for both the green fluorescence and the red fluorescence in the protocol menu was set to Level 5, and the size gating was from 5 to 30 µm. Counting of the platelets was performed using a flow cytometer (BD FACS Calibur, Becton, Dickinson Co.) with a fluorescein isothiocyanate (FITC)-labelled antihuman CD61 monoclonal antibody (Beckman Coulter, Inc., Brea, CA, USA). The percentage of platelet in the blood cells was estimated by counting 100,000 blood cells.
2.4. Detection of Parasite-Infected Erythrocytes Using LUNA-FL
To analyse parasite-infected erythrocytes using LUNA-FL, an appropriate volume of purified parasite-infected erythrocyte suspension was added to whole blood to obtain 0.4, 0.2, 0.08, 0.04, and 0.02% parasitemia at 2% haematocrit. Parasitemia was estimated by thin-smear microscopy; each sample (200 µL) was filtered with the spin column. To estimate parasitemia with LUNA-FL, the filtered blood sample was diluted 100-fold with PBS and the number of erythrocytes was counted using the “Bright-Field Counting” mode. To count the parasites, 9 µL of the filtered sample was stained with 1 µL of AO/PI Cell Viability Kit and placed on a photon slide. The number of parasites was counted using the “Fluorescence Cell Counting” mode. The exposure times for green fluorescence and red fluorescence were set to Level 12 and Level 1, respectively. The thresholds of the green fluorescence and the red fluorescence in the protocol menu was set to Level 8 and Level 5, respectively, and the size gating was 1 to 9 µm. A high threshold setting does not mark the signals with low fluorescent intensity as those of the parasite nuclei.
2.5. Statistical Analysis
Statistical analysis was performed with SigmaPlot Version 13 (Systat Software, Inc., San Jose, CA, USA).
2.6. Ethics Statement
Ethics approvals were obtained from the National Institute of Advanced Industrial Science and Technology ethics committee (No. 2018-0204) on 4 December, 2019.
4. Discussion
In this study, a commercially available fluorescent cell counter, LUNA-FL, was used for the determination of parasitemia. Blood samples with appropriate parasitemia were prepared, and whole blood from uninfected volunteers spiked with the parasites cultured in vitro was analysed for the evaluation of the device. By using spin columns with SiO2-NF filters that could almost completely remove leucocytes and platelets from whole blood samples, purified erythrocyte samples were prepared quickly. The samples were stained with AO, and we found that accurate parasite detection could be performed easily using LUNA-FL.
The estimation of parasitemia is crucial for malaria diagnosis. As the severity of malaria and/or the effect of antimalarial drug treatment should be monitored, the calculation of parasitemia is very important for malaria diagnosis. Microscopic analysis with thin or thick blood films remains the gold standard diagnosis method. However, to perform Giemsa microscopy, fully trained and skilled technicians must be employed, and the diagnosis is largely skill- and equipment- (e.g., microscope) dependent [
10]. For Giemsa microscopy, a light microscope with a 100× objective lens and highly paid microscopists are required. Thus, not only the front-end cost, but also the running cost is very high. Considering the cost of maintaining skilled microscopists in medical institutions, the running cost of diagnosis using LUNA-FL may be much lower than that using Giemsa microscopy. The number of erythrocytes that can be counted on thin blood films in a microscope field is few, and hence, the estimation of parasitemia with Giemsa microscopy is time-consuming. Similarly, the preparation of thick blood films is also time-consuming because the erythrocytes on the films have to be haemolysed completely and the films have to be fully dried before analysis. By using LUNA-FL, these issues may be addressed as follows: (1) Blood samples could be applied to photon slides quite easily, and skills for the preparation of blood films are not required. (2) Calculation of parasitemia by automatic erythrocyte and parasite counting using LUNA-FL is easily and quickly performed. Even if technicians are not experts, stable and correct results can be obtained. (3) The main body of LUNA-FL is small (220 × 210 × 90 mm) and light (1.8 kg). Therefore, it can be put in small medical offices. (4) The average time taken for parasitemia calculation is approximately 5 min, which may enable the quick start of malaria treatment. Because LUNA-FL is not manufactured for developing countries, the machine must be plugged in constantly. An adequate power supply is not available in many medical institutions in malaria-endemic countries. In this case, a solar battery may be useful.
RDTs for HRP2 are often used for malarial diagnosis in Africa. However, diagnosis with RDTs is qualitative with high false-positive rates in the most endemic areas. Furthermore,
P. falciparum parasites with a deletion of the
hrp2 gene yield false-negative RDTs [
18]. As diagnosis using LUNA-FL detects the parasites themselves (i.e., not the antigen) and parasite nuclei (i.e., not the
hrp2 gene), LUNA-FL might be more suitable for malaria diagnosis in endemic areas.
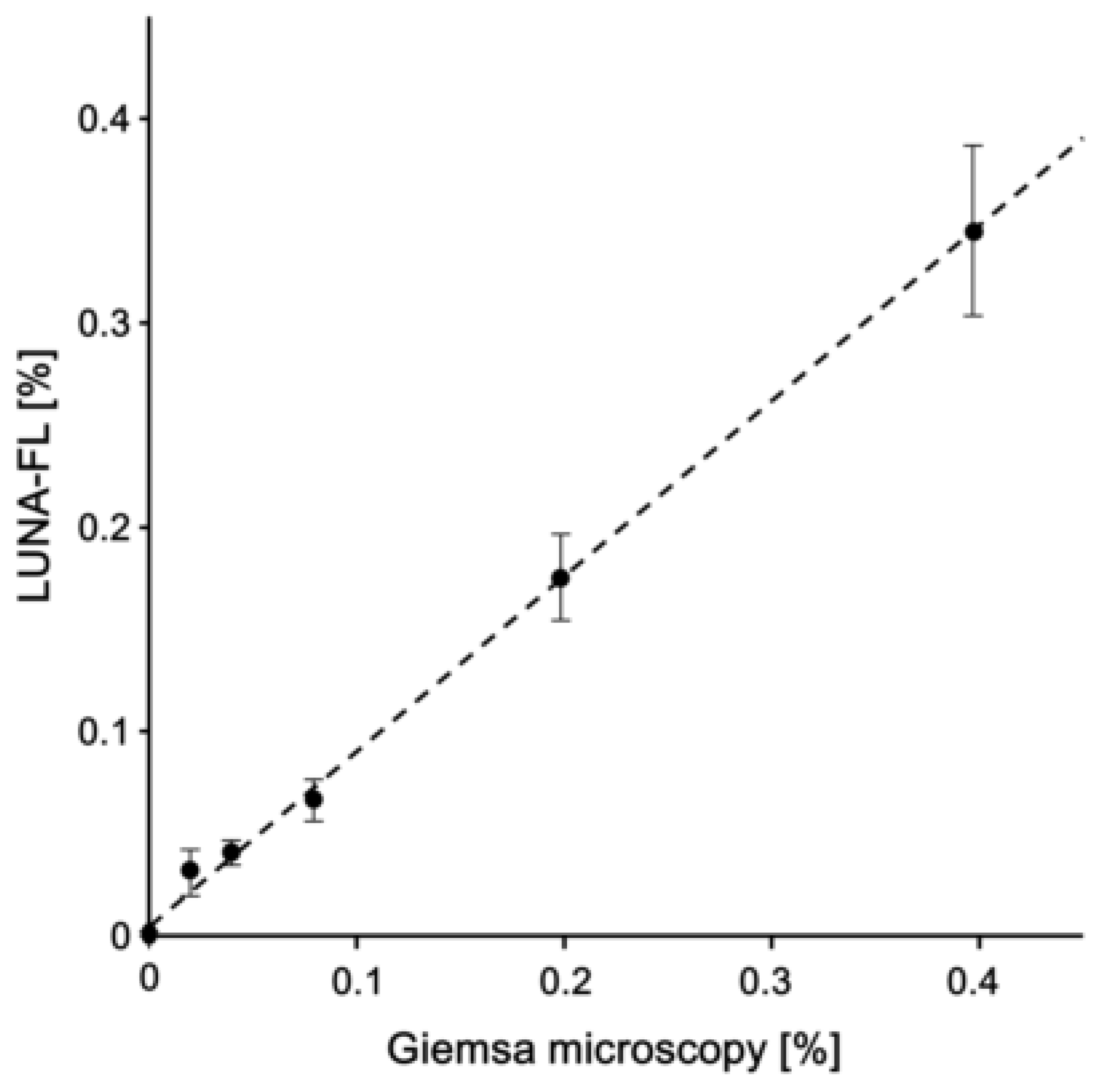
The comparative analysis between LUNA-FL and Giemsa microscopy in
Figure 5 revealed that the equation of the approximation straight line was y = 0.86x + 0.0042. The equation slope value was close to 1, indicating that the underestimation of counted parasites did not occur in the analysis with LUNA-FL. In a previous study, the LOD of Giemsa microscopy with thin blood films by skilled technicians was ~0.004% (~200 parasites/µL of blood) [
19]. The LOD of the analysis with LUNA-FL using the protocol in this study was 0.0043% (215 parasites/µL of blood), and the LOD was equivalent to that of the gold standard method. Two image fields were analysed with a photon slide, and it may be possible to increase the number of analysed erythrocytes by increasing the number of photon slides analysed, but the cost of diagnosis will also increase. Therefore, a device that is able to capture many image fields on a slide should be developed. In addition, it may be necessary to modify the main body of LUNA-FL and/or the slides.
The findings of this study showed that the removal of leucocytes and parasites from whole blood samples could certainly be performed by using spin columns with two sheets of SiO
2-NF filters fixed with O-ring. The fixation of the filters with the O-ring was performed easily and quickly; however, a centrifuge was also required. For malaria diagnosis in facilities that are not equipped, the development of devices that can filter samples without a centrifuge is required. Previously, a push-column-type device that can filter without centrifugation was developed [
11]. The setting of the SiO
2-NF filter to the push-column was performed with glue that hardens upon UV exposure. In our previous study, we observed that the preparation of the spin columns was time-consuming and laborious, and there was an evident difference in the leucocyte and/or platelet removal efficiency of the columns and a possibility that the dregs of the glue contaminated the blood samples (unpublished data). Taken together, the development of a push-column with SiO
2-NF filters fixed with an O-ring may be important.
Two kinds of fluorescent signals (green and red) can be detected using LUNA-FL to distinguish between live and dead cells. The counting of dead cells is not required in malaria diagnosis, and hence, the exposure time for red fluorescent signals was set not to detect any cell and parasite. If LUNA-FL can be modified to develop a device that can detect only a green fluorescent signal, the cost or the time required for malaria diagnosis should be significantly reduced.
The cost of one assay was approximately two dollars. Because the photon slides are manufactured from acryl, initial costs may be very low. The slides are mainly manufactured for and sold to researchers in developed countries. We expect the selling price could be further decreased if the slides were manufactured in Africa, which may result in decreased labour and transportation costs. Furthermore, because it took only approximately 1 min to capture and analyse a field image, and three field images were required to be analysed in this protocol, it took approximately 5 min for the measurement of parasitemia. During that period of time, we were able to analyse an average of 61,690 erythrocytes, indicating that malaria diagnosis using LUNA-FL may be useful for POCT in endemic areas.