Production of Plant-Associated Volatiles by Select Model and Industrially Important Streptomyces spp.
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Growth Conditions
2.2. Volatile Organic Compound Collection and Sampling
2.3. Volatile Organic Compound Analysis and Identification
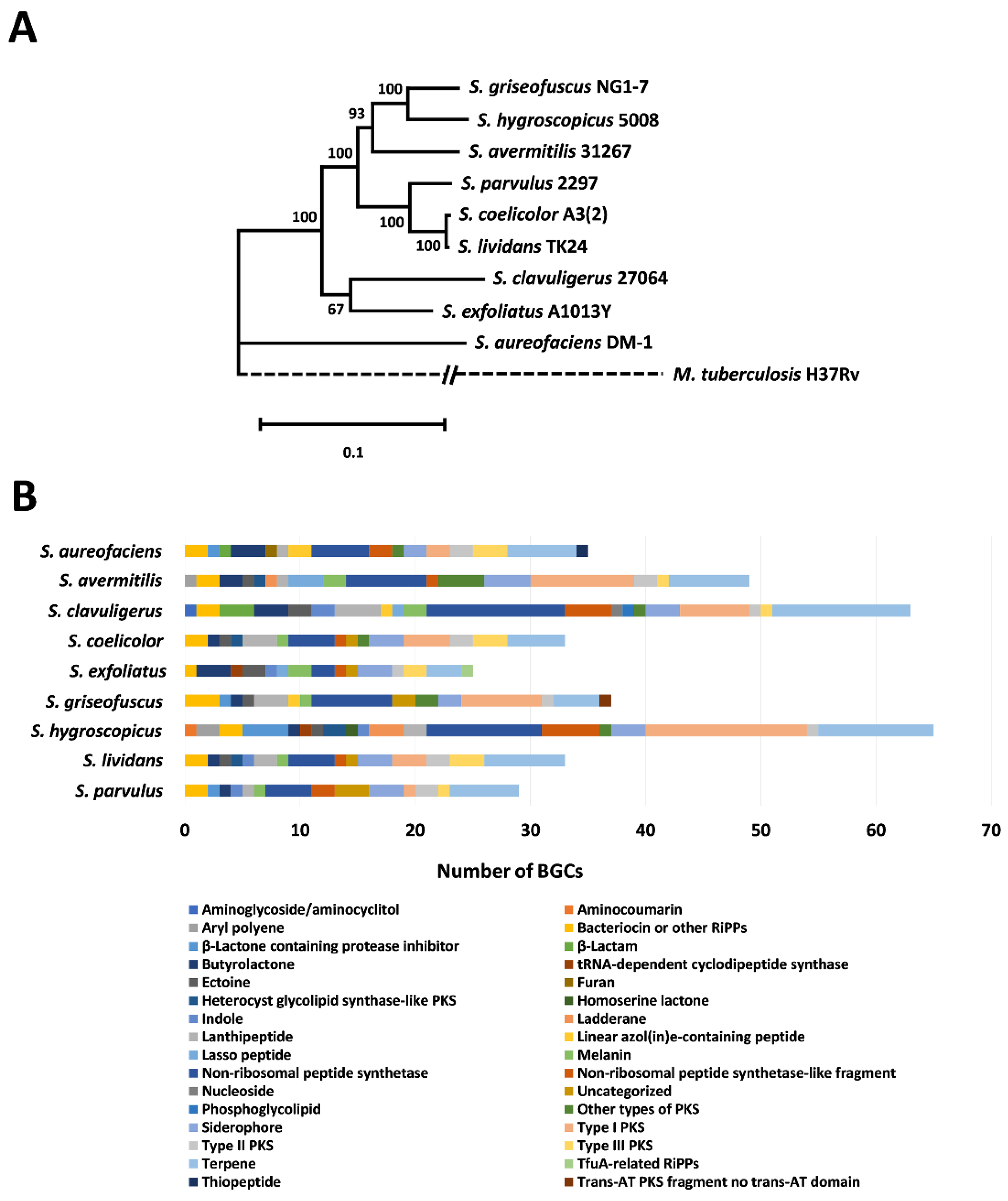
2.4. Streptomyces Phylogenetic and BGC Analysis
3. Results and Discussion
3.1. Relative Phylogeny and Biosynthetic Potential of Select Streptomyces spp.
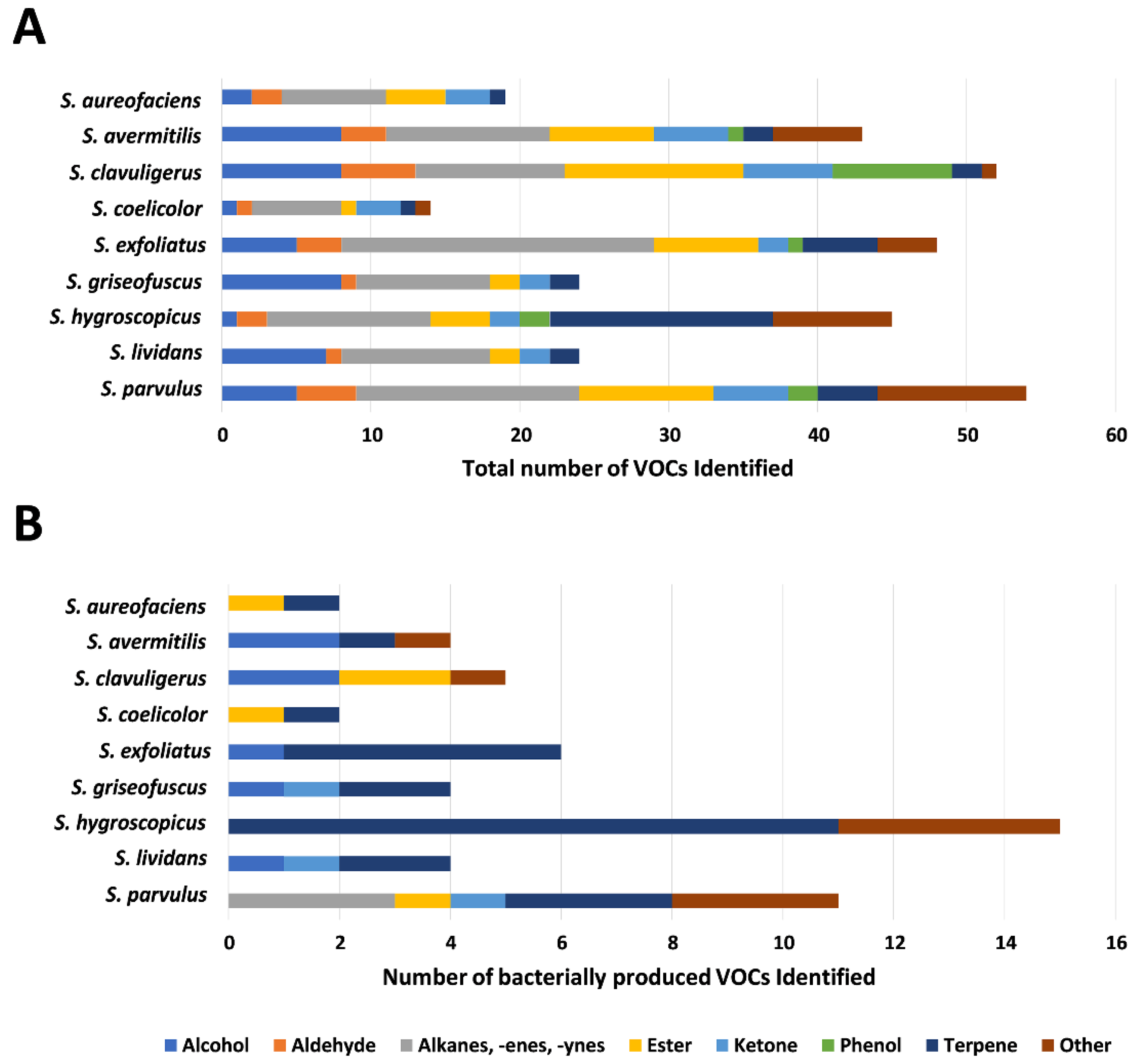
3.2. Overview of VOC Production by Select Streptomyces spp.
3.3. Identification of Previously Reported Streptomyces-Specific VOCs
3.4. Identification of VOCs Previously Unreported in Streptomyces spp.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schmidt, R.; Cordovez, V.; De Boer, W.; Raaijmakers, J.; Garbeva, P. Volatile affairs in microbial interactions. ISME J. 2015, 9, 2329–2335. [Google Scholar] [CrossRef]
- Vivaldo, G.; Masi, E.; Taiti, C.; Caldarelli, G.; Mancuso, S. The network of plants volatile organic compounds. Sci. Rep. 2017, 7, 1–18. [Google Scholar] [CrossRef]
- Ni, J.; Robarge, W.P.; Xiao, C.; Heber, A.J. Volatile organic compounds at swine facilities: A critical review. Chemosphere 2012, 89, 769–788. [Google Scholar] [CrossRef]
- Curran, A.M.; Rabin, S.I.; Prada, P.A.; Furton, K.G. Comparison of the volatile organic compounds present in human odor using SPME-GC/MS. J. Chem. Ecol. 2005, 31, 1607–1619. [Google Scholar] [CrossRef]
- Schnurer, J.; Olsson, J.; Borjesson, T. Fungal volatiles as indicators of food and feeds spoilage. Fungal Genet. Biol. 1999, 27, 209–217. [Google Scholar] [CrossRef]
- Schulz, S.; Dickschat, J.S. Bacterial volatiles: The smell of small organisms. Nat. Prod. Rep. 2007, 24, 814–842. [Google Scholar] [CrossRef]
- Raza, W.; Ling, N.; Liu, D.; Wei, Z.; Huang, Q.; Shen, Q. Volatile organic compounds produced by Pseudomonas fluorescens WR-1 restrict the growth and virulence traits of Ralstonia solanacearum. Microbiol. Res. 2016, 192, 103–113. [Google Scholar] [CrossRef]
- Audrain, B.; Farag, M.A.; Ryu, C.; Ghigo, J. Role of bacterial volatile compounds in bacterial biology. FEMS Microbiol. Rev. 2015, 39, 222–233. [Google Scholar] [CrossRef]
- Schulz-bohm, K.; Geisen, S.; Wubs, E.R.J.; Song, C.; Boer, W.; De Garbeva, P. The prey’s scent—Volatile organic compound mediated interactions between soil bacteria and their protist predators. ISME J. 2017, 11, 817–820. [Google Scholar] [CrossRef]
- Lin, X.; Cane, D.E. Biosynthesis of the sesquiterpene antibiotic albaflavenone in Streptomyces coelicolor. Mechanism and stereochemistry of the enzymatic formation of epi-isozizaene. J. Am. Chem. Soc. 2009, 131, 6332–6333. [Google Scholar] [CrossRef]
- Zhao, B.; Lin, X.; Lei, L.; Lamb, D.C.; Kelly, S.L.; Waterman, M.R.; Cane, D.E. Biosynthesis of the sesquiterpene antibiotic albaflavenone in Streptomyces coelicolor A3 (2). J. Biol. Chem. 2008, 283, 8183–8189. [Google Scholar] [CrossRef]
- Takamatsu, S.; Lin, X.; Nara, A.; Komatsu, M.; Cane, D.E.; Ikeda, H. Characterization of a silent sesquiterpenoid biosynthetic pathway in Streptomyces avermitilis controlling epi-isozizaene albaflavenone biosynthesis and isolation of a new oxidized epi-isozizaene metabolite. Microb. Biotechnol. 2011, 4, 184–191. [Google Scholar] [CrossRef]
- Korpi, A.; Jarnberg, J.; Pasanen, A.-L. Microbial volatile organic compounds. Crit. Rev. Toxicol. 2009, 39, 139–193. [Google Scholar] [CrossRef]
- Rabe, P.; Citron, C.A.; Dickschat, J.S. Volatile terpenes from actinomycetes: A biosynthetic study correlating chemical analyses to genome data. Chembiochem 2013, 14, 2345–2354. [Google Scholar] [CrossRef]
- Li, R.; Chou, W.K.W.; Himmelberger, J.A.; Litwin, K.M.; Harris, G.G.; Cane, D.E.; Christianson, D.W. Reprogramming the chemodiversity of terpenoid cyclization by remolding the active site contour of epi-isozizaene synthase. Biochemistry 2014, 53, 1155–1168. [Google Scholar] [CrossRef]
- Nie, Y.; Chi, C.; Fang, H.; Liang, J.; Lu, S.; Lai, G.; Tang, Y.; Wu, X. Diverse alkane hydroxylase genes in microorganisms and environments. Sci. Rep. 2014, 4, 1–11. [Google Scholar] [CrossRef]
- Choi, Y.J.; Lee, S.Y. Microbial production of short-chain alkanes. Nature 2013, 502, 571–574. [Google Scholar] [CrossRef]
- Citron, C.A.; Barra, L.; Wink, J.; Dickschat, J.S. Volatiles from nineteen recently genome sequenced actinomycetes. Org. Biomol. Chem. 2015, 13, 2673–2683. [Google Scholar] [CrossRef]
- Govindarajan, M.; Rajeswary, M.; Benelli, G. Chemical composition, toxicity and non-target effects of Pinus kesiya essential oil: An eco-friendly and novel larvicide against malaria, dengue and lymphatic filariasis mosquito vectors. Ecotoxicol. Environ. Saf. 2016, 129, 85–90. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, A. A floral fragrance, methyl benzoate, is an efficient green pesticide. Sci. Rep. 2017, 7, 42168. [Google Scholar] [CrossRef]
- Whiley, H.; Gaskin, S.; Schroder, T.; Ross, K. Antifungal properties of essential oils for improvement of indoor air quality: A review. Rev. Environ. Health 2018, 33, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Chen, Q.; Liu, Y.; Zhou, X.; Wang, X. Isolation and biological activities of decanal, linalool, valencene, and octanal from sweet orange oil. J. Food Sci. 2012, 77, C1156–C1161. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Mo, M.; Zhou, J.; Zou, C.; Zhang, K. Evaluation and identification of potential organic nematicidal volatiles from soil bacteria. Soil Biol. Biochem. 2007, 39, 2567–2575. [Google Scholar] [CrossRef]
- Jin, N.; Lu, X.; Wang, X.; Liu, Q.; Peng, D.; Jian, H. The effect of combined application of Streptomyces rubrogriseus HDZ-9-47 with soil biofumigation on soil microbial and nematode communities. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Gupta, C.; Garg, A.P.; Prakash, D. A biotechnological approach to microbial based perfumes and flavours. J. Microbiol. Exp. 2015, 2, 11–18. [Google Scholar] [CrossRef]
- Seipke, R.F.; Kaltenpoth, M.; Hutchings, M.I. Streptomyces as symbionts: An emerging and widespread theme? FEMS Microbiol. Rev. 2012, 36, 862–876. [Google Scholar] [CrossRef] [PubMed]
- Barka, E.A.; Vatsa, P.; Sanchez, L.; Gaveau-Vaillant, N.; Jacquard, C.; Klenk, H.-P.; Clement, C.; Ouhdouch, Y.; Van Wezel, G.P. Taxonomy, physiology, and natural products of Actinobacteria. Am. Soc. Microbiol. 2015, 80, 1–43. [Google Scholar] [CrossRef]
- Tidjani, A.R.; Lorenzi, J.N.; Toussaint, M.; Van Dijk, E.; Naquin, D.; Lespinet, O.; Bontemps, C.; Leblond, P. Massive gene flux drives genome diversity between sympatric Streptomyces conspecifics. MBio 2019, 10, e01533-19. [Google Scholar] [CrossRef]
- Antony-Babu, S.; Stien, D.; Eparvier, V.; Parrot, D.; Tomasi, S.; Suzuki, M.T. Multiple Streptomyces species with distinct secondary metabolomes have identical 16S rRNA gene sequences. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef]
- Thomas, C.M.; Nielsen, K.M. Mechanisms of, and barriers to, horizontal gene transfer between bacteria. Nat. Rev. Microbiol. 2005, 3, 711–722. [Google Scholar] [CrossRef]
- Citron, C.A.; Rabe, P.; Dickschat, J.S. The scent of bacteria: Headspace analysis for the discovery of natural products. J. Nat. Prod. 2012, 75, 1765–1776. [Google Scholar] [CrossRef]
- Becher, P.G.; Verschut, V.; Bibb, M.J.; Bush, M.J.; Molnár, B.P.; Barane, E.; Al-Bassam, M.M.; Chandra, G.; Song, L.; Challis, G.L.; et al. Developmentally regulated volatiles geosmin and 2-methylisoborneol attract a soil arthropod to Streptomyces bacteria promoting spore dispersal. Nat. Microbiol. 2020, 5, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Avalos, M.; Garbeva, P.; Raaijmakers, J.M.; van Wezel, G.P. Production of ammonia as a low-cost and long-distance antibiotic strategy by Streptomyces species. ISME J. 2019, 14, 569–583. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.E.; Pham, C.A.; Zambri, M.P.; McKillip, J.; Carlson, E.E.; Elliot, M.A. Streptomyces volatile compounds influence exploration and microbial community dynamics by altering iron availability. MBio 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.E.; Ho, L.; Rees, C.A.; Hill, J.E.; Nodwell, J.R.; Elliot, M.A. Streptomyces exploration is triggered by fungal interactions and volatile signals. Elife 2017, 6, e21738. [Google Scholar] [CrossRef]
- Huang, H.; Ren, L.; Li, H.; Schmidt, A.; Gershenzon, J.; Lu, Y.; Cheng, D. The nesting preference of an invasive ant is associated with the cues produced by actinobacteria in soil. PLOS Pathog. 2020, 16, e1008800. [Google Scholar] [CrossRef] [PubMed]
- Ho, L.; Daniel-Ivad, M.; Jeedigunta, S.; Li, J.; Iliadi, K.; Boulianne, G.; Hurd, T.; Smibert, C.; Nodwell, J. Chemical entrapment and killing of insects by bacteria. Nat. Commun. 2020, 11, 4608. [Google Scholar] [CrossRef]
- Blin, K.; Shaw, S.; Steinke, K.; Villebro, R.; Ziemert, N.; Lee, S.Y.; Medema, M.H.; Weber, T. antiSMASH 5.0: Updates to the secondary metabolite genome mining pipeline. Nucleic Acids Res. 2019, 47, W81–W87. [Google Scholar] [CrossRef]
- Baltz, R.H. Gifted microbes for genome mining and natural product discovery. J. Ind. Microbiol. Biotechnol. 2017, 44, 573–588. [Google Scholar] [CrossRef]
- Garbeva, P.; Hordijk, C.; Gerards, S.; Boer, W. De Volatile-mediated interactions between phylogenetically different soil bacteria. Front. Microbiol. 2014, 5, 289. [Google Scholar] [CrossRef]
- Gomez-Escribano, J.P.; Bibb, M.J. Engineering Streptomyces coelicolor for heterologous expression of secondary metabolite gene clusters. Microb. Biotechnol. 2011, 4, 207–215. [Google Scholar] [CrossRef]
- Ikeda, H.; Kotaki, H.; Tanaka, H.; Omura, S. Involvement of glucose catabolism in avermectin production by Streptomyces avermitilis. Antimicrob. Agents Chemother. 1988, 32, 282–284. [Google Scholar] [CrossRef]
- Kieser, T.; Bibb, M.J.; Buttner, M.J.; Chater, K.F.; Hopwood, D.A. Practical Streptomyces Genetics. A Laboratory Manual; John Innes Foundation: Norwich, UK, 2000. [Google Scholar]
- Cane, D.E.; He, X.; Kobayashi, S.; Ōmura, S.; Ikeda, H. Molecular cloning, expression, and mechanistic study of the germacradienol/geosmin synthase. J. Antibiot. 2006, 59, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Light, M.; Shutler, D.; Cutler, G.C.; Hillier, N.K. Varroa destructor mite electrophysiological responses to honey bee (Apis mellifera) colony volatiles. Exp. Appl. Acarol. 2020, 81, 495–514. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Rong, X.; Liu, N.; Ruan, J.; Huang, Y. Multilocus sequence analysis of Streptomyces griseus isolates delineating intraspecific diversity in terms of both taxonomy and biosynthetic potential. Antonie Van Leeuwenhoek 2010, 98, 237–248. [Google Scholar] [CrossRef]
- Labeda, D.P.; Doroghazi, J.R.; Ju, K.S.; Metcalf, W.W. Taxonomic evaluation of Streptomyces albus and related species using multilocus sequence analysis and proposals to emend the description of Streptomyces albus and describe Streptomyces pathocidini sp. nov. Int. J. Syst. Evol. Microbiol. 2014, 64, 894–900. [Google Scholar] [CrossRef]
- Guo, Y.P.; Zheng, W.; Rong, X.Y.; Huang, Y. A multilocus phylogeny of the Streptomyces griseus 16S rRNA gene clade: Use of multilocus sequence analysis for streptomycete systematics. Int. J. Syst. Evol. Microbiol. 2008, 58, 149–159. [Google Scholar] [CrossRef]
- Labeda, D.P. Multilocus sequence analysis of phytopathogenic species of the genus Streptomyces. Int. J. Syst. Evol. Microbiol. 2011, 61, 2525–2531. [Google Scholar] [CrossRef]
- Lapaz, M.I.; Huguet-Tapia, J.C.; Siri, M.I.; Verdier, E.; Loria, R.; Pianzzola, M.J. Genotypic and phenotypic characterization of Streptomyces species causing potato common scab in Uruguay. Plant Dis. 2017, 101, 1362–1372. [Google Scholar] [CrossRef]
- Rong, X.; Guo, Y.; Huang, Y. Proposal to reclassify the Streptomyces albidoflavus clade on the basis of multilocus sequence analysis and DNA-DNA hybridization, and taxonomic elucidation of Streptomyces griseus subsp. solvifaciens. Syst. Appl. Microbiol. 2009, 32, 314–322. [Google Scholar] [CrossRef]
- Yamada, Y.; Kuzuyama, T.; Komatsu, M.; Shin-ya, K.; Omura, S.; Cane, D.E.; Ikeda, H. Terpene synthases are widely distributed in bacteria. Proc. Natl. Acad. Sci. USA 2015, 112, 857–862. [Google Scholar] [CrossRef]
- Martín-Sánchez, L.; Singh, K.S.; Avalos, M.; Van Wezel, G.P.; Dickschat, J.S.; Garbeva, P. Phylogenomic analyses and distribution of terpene synthases among Streptomyces. Beilstein J. Org. Chem. 2019, 15, 1181–1193. [Google Scholar] [CrossRef]
- Cane, D.E.; Ikeda, H. Exploration and mining of the bacterial terpenome. Acc. Chem. Res. 2012, 45, 463–472. [Google Scholar] [CrossRef]
- Nicault, M.; Tidjani, A.R.; Gauthier, A.; Dumarcay, S.; Gelhaye, E.; Bontemps, C.; Leblond, P. Mining the biosynthetic potential for specialized metabolism of a Streptomyces soil community. Antibiotics 2020, 9, 271. [Google Scholar] [CrossRef]
- Belknap, K.C.; Park, C.J.; Barth, B.M.; Andam, C.P. Genome mining of biosynthetic and chemotherapeutic gene clusters in Streptomyces bacteria. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef]
- Schöller, C.E.G.; Gürtler, H.; Pedersen, R.; Molin, S.; Wilkins, K. Volatile metabolites from actinomycetes. J. Agric. Food Chem. 2002, 50, 2615–2621. [Google Scholar] [CrossRef]
- Martens, T.; Brinkhoff, T.; Simon, M.; Schulz, S. Volatiles released by a Streptomyces species isolated from the North Sea. Chem. Biodivers. 2005, 2, 837–865. [Google Scholar]
- Liu, H.; An, K.; Su, S.; Yu, Y.; Wu, J.; Xiao, G.; Xu, Y. Aromatic characterization of mangoes (Mangifera indica L.) Using solid phase extraction coupled with gas chromatography-mass spectrometry and olfactometry and sensory analyses. Foods 2020, 9, 75. [Google Scholar] [CrossRef]
- Andreu-Coll, L.; Noguera-Artiaga, L.; Carbonell-Barrachina, Á.A.; Legua, P.; Hernández, F. Volatile composition of prickly pear fruit pulp from six Spanish cultivars. J. Food Sci. 2020, 85, 358–363. [Google Scholar] [CrossRef]
- Chen, J.; Lü, J.; He, Z.; Zhang, F.; Zhang, S.; Zhang, H. Investigations into the production of volatile compounds in Korla fragrant pears (Pyrus sinkiangensis Yu). Food Chem. 2020, 302, 125337. [Google Scholar] [CrossRef]
- Li, X.; Li, B.; Cai, S.; Zhang, Y.; Xu, M.; Zhang, C.; Yuan, B.; Xing, K.; Qin, S. Identification of rhizospheric actinomycete Streptomyces lavendulae sps-33 and the inhibitory effect of its volatile organic compounds against Ceratocystis fimbriata in postharvest sweet potato (Ipomoea batatas (L.) Lam.). Microorganisms 2020, 8, 319. [Google Scholar] [CrossRef]
- Lyu, A.; Yang, L.; Wu, M.; Zhang, J.; Li, G. High efficacy of the volatile organic compounds of Streptomyces yanglinensis 3-10 in suppression of Aspergillus contamination on peanut kernels. Front. Microbiol. 2020, 11, 142. [Google Scholar] [CrossRef]
- Hirata, H.; Ohnishi, T.; Watanabe, N. Biosynthesis of floral scent 2-phenylethanol in rose flowers. Biosci. Biotechnol. Biochem. 2016, 80, 1865–1873. [Google Scholar] [CrossRef]
- Yue, X.; Li, X.; Chen, X.; Ashraf, M.A.; Liu, Z.; Bi, H.; Zheng, D.; Zhao, Y.; Peng, W. Molecules and functions of Cornus officinalis bark volatiles. Emirates J. Food Agric. 2018, 30, 828–838. [Google Scholar]
- Jayaseelan, C.; Gandhi, P.R.; Rajasree, S.R.R.; Suman, T.Y.; Mary, R.R. Toxicity studies of nanofabricated palladium against filariasis and malaria vectors. Environ. Sci. Pollut. Res. 2018, 25, 324–332. [Google Scholar] [CrossRef]
- Mitra, P.; Das, S.; Debnath, R.; Mobarak, S.H.; Barik, A. Identification of Lathyrus sativus plant volatiles causing behavioral preference of Aphis craccivora. Pest Manag. Sci. 2020. [Google Scholar] [CrossRef]
- Rodríguez-López, V.; Aguirre-Crespo, F.; Salazar, L.; Estrada-Soto, S. Identification of fatty acid esters and hydrocarbon derivatives from Cyrtocarpa procera Kunth by GC-MS. Nat. Prod. Res. 2006, 20, 1–7. [Google Scholar] [CrossRef]
- Duan, Y.; Petzold, M.; Saleem-Batcha, R.; Teufel, R. Bacterial tropone natural products and derivatives: Overview on the biosynthesis, bioactivities, ecological role and biotechnological potential. ChemBioChem 2020, 21, 2384–2407. [Google Scholar] [CrossRef]
- Zhao, J.; Zhao, J. Plant troponoids: Chemistry, biological activity, and biosynthesis. Curr. Med. Chem. 2007, 14, 2597–2621. [Google Scholar] [CrossRef]
- Miyazato, H.; Nakamura, M.; Hashimoto, S.; Hayashi, S. Identification of the odour-active cyclic diketone cis-2,6-dimethyl-1,4- cyclohexanedione in roasted Arabica coffee brew. Food Chem. 2013, 138, 2346–2355. [Google Scholar] [CrossRef]
- Komatsu, M.; Tsuda, M.; Omura, S.; Oikawa, H.; Ikeda, H. Identification and functional analysis of genes controlling biosynthesis of 2-methylisoborneol. Proc. Natl. Acad. Sci. USA 2008, 105, 7422–7427. [Google Scholar] [CrossRef]
- Chou, W.K.W.; Gould, C.A.; Cane, D.E. Incubation of 2-methylisoborneol synthase with the intermediate analog 2-methylneryl diphosphate. J. Antibiot. 2017, 70, 625–631. [Google Scholar] [CrossRef]
- D’Ambrosio, M.; Ciocarlan, A.; Colombo, E.; Guerriero, A.; Pizza, C.; Sangiovanni, E.; Dell’Agli, M. Structure and cytotoxic activity of sesquiterpene glycoside esters from Calendula officinalis L.: Studies on the conformation of viridiflorol. Phytochemistry 2015, 117, 1–9. [Google Scholar] [CrossRef]
- Li, Q.; Ning, P.; Zheng, L.; Huang, J.; Li, G.; Hsiang, T. Fumigant activity of volatiles of Streptomyces globisporus JK-1 against Penicillium italicum on Citrus microcarpa. Postharvest Biol. Technol. 2010, 58, 157–165. [Google Scholar] [CrossRef]
- Ramírez, M.; Ortiz, M.I.; Guerenstein, P.; Molina, J. Novel repellents for the blood-sucking insects Rhodnius prolixus and Triatoma infestans, vectors of Chagas disease. Parasites Vectors 2020, 13, 142. [Google Scholar] [CrossRef]
- Alves, T.J.S.; Murcia, A.; Wanumen, A.C.; Wanderley-Teixeira, V.; Teixeira, Á.A.C.; Ortiz, A.; Medina, P. Composition and toxicity of a mixture of essential oils against Mediterranean fruit fly, Ceratitis capitata (Wiedemann) (Diptera: Tephritidae). J. Econ. Entomol. 2019, 112, 164–172. [Google Scholar] [CrossRef]
- Joycharat, N.; Thammavong, S.; Voravuthikunchai, S.P.; Plodpai, P.; Mitsuwan, W.; Limsuwan, S.; Subhadhirasakul, S. Chemical constituents and antimicrobial properties of the essential oil and ethanol extract from the stem of Aglaia odorata Lour. Nat. Prod. Res. 2014, 28, 2169–2172. [Google Scholar] [CrossRef]
- Gelmini, F.; Beretta, G.; Anselmi, C.; Centini, M.; Magni, P.; Ruscica, M.; Cavalchini, A.; Maffei Facino, R. GC-MS profiling of the phytochemical constituents of the oleoresin from Copaifera langsdorffii Desf. and a preliminary in vivo evaluation of its antipsoriatic effect. Int. J. Pharm. 2013, 440, 170–178. [Google Scholar] [CrossRef]
- Asquith, E.; Evans, C.; Dunstan, R.H.; Geary, P.; Cole, B. Distribution, abundance and activity of geosmin and 2-methylisoborneol-producing Streptomyces in drinking water reservoirs. Water Res. 2018, 145, 30–38. [Google Scholar] [CrossRef]
- Schrader, K.K.; Harries, M.D.; Page, P.N. Temperature effects on biomass, geosmin, and 2-methylisoborneol production and cellular activity by Nocardia spp. and Streptomyces spp. isolated from rainbow trout recirculating aquaculture systems. J. Ind. Microbiol. Biotechnol. 2015, 42, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Sinha, A.; Phillips-Salemka, S.; Niraula, T.A.; Short, K.A.; Niraula, N.P. The complete genomic sequence of Streptomyces spectabilis NRRL-2792 and identification of secondary metabolite biosynthetic gene clusters. J. Ind. Microbiol. Biotechnol. 2019, 46, 1217–1223. [Google Scholar] [CrossRef] [PubMed]
- Tsuneki, H.; Ma, E.L.; Kobayashi, S.; Sekizaki, N.; Maekawa, K.; Sasaoka, T.; Wang, M.W.; Kimura, I. Antiangiogenic activity of β-eudesmol in vitro and in vivo. Eur. J. Pharmacol. 2005, 512, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Harada, H.; Yamasaki, K.; Okamoto, S.; Hirase, S.; Tanaka, Y.; Misawa, N.; Utsumi, R. Isolation and functional characterization of a β-eudesmol synthase, a new sesquiterpene synthase from Zingiber zerumbet Smith. FEBS Lett. 2008, 582, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Edwards, C.G.; Fellman, J.K.; Scott Mattinson, D.; Navazio, J. Biosynthetic origin of geosmin in red beets (Beta vulgaris L.). J. Agric. Food Chem. 2003, 51, 1026–1029. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.A. Volatile Compounds from Actinobacteria as Mediators of Microbial Interactions. Ph.D. Thesis, Leiden University, Leiden, The Netherlands, 2019. [Google Scholar]
- Muturi, E.J.; Selling, G.W.; Doll, K.M.; Hay, W.T.; Ramirez, J.L. Leptospermum scoparium essential oil is a promising source of mosquito larvicide and its toxicity is enhanced by a biobased emulsifier. PLoS ONE 2020, 15, e0229076. [Google Scholar] [CrossRef]
- Da Trindade, R.; Almeida, L.; Xavier, L.; Lins, A.L.; Andrade, E.H.; Maia, J.G.; Mello, A.; Setzer, W.N.; Ramos, A.; Da Silva, J.K. Arbuscular mycorrhizal fungi colonization promotes changes in the volatile compounds and enzymatic activity of lipoxygenase and phenylalanine ammonia lyase in Piper nigrum L. “Bragantina”. Plants 2019, 8, 442. [Google Scholar] [CrossRef]
- Zhang, K.; Yao, L. The anxiolytic effect of Juniperus virginiana L. essential oil and determination of its active constituents. Physiol. Behav. 2018, 189, 50–58. [Google Scholar] [CrossRef]
- Yuan, Y.; Sun, Y.; Zhao, Y.; Liu, C.; Chen, X.; Li, F.; Bao, J. Identification of floral scent profiles in bearded irises. Molecules 2019, 24, 1773. [Google Scholar] [CrossRef]
- Thusoo, S.; Gupta, S.; Sudan, R.; Kour, J.; Bhagat, S.; Hussain, R.; Bhagat, M. Antioxidant activity of essential oil and extracts of Valeriana jatamansi Roots. Biomed Res. Int. 2014, 2014, 614187. [Google Scholar] [CrossRef]
- Ahmad, J.; Bagheri, R.; Bashir, H.; Affan Baig, M.; Al-Huqail, A.; Ibrahim, M.M.; Irfan Qureshi, M. Organ-specific phytochemical profiling and antioxidant analysis of Parthenium hysterophorus L. Biomed Res. Int. 2018, 2018, 9535232. [Google Scholar] [CrossRef] [PubMed]
- Braña, A.F.; Rodríguez, M.; Pahari, P.; Rohr, J.; García, L.A.; Blanco, G. Activation and silencing of secondary metabolites in Streptomyces albus and Streptomyces lividans after transformation with cosmids containing the thienamycin gene cluster from Streptomyces cattleya. Arch. Microbiol. 2014, 196, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.L.; He, Q.; Chu, S.S.; Wang, C.F.; Du, S.S.; Zhi Wei, Z.W. Essential oil composition and larvicidal activity of Saussurea lappa roots against the mosquito Aedes albopictus (Diptera: Culicidae). Parasitol. Res. 2012, 110, 2125–2130. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; He, X.; Cane, D.E. Biosynthesis of the earthy odorant geosmin by a bifunctional Streptomyces coelicolor enzyme. Nat. Chem. Biol. 2007, 3, 711–715. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, C.; Bottoni, M.; Ascrizzi, R.; Santagostini, L.; Papini, A.; Flamini, G.; Fico, G. A novel study approach on Scutellaria altissima L. cultivated at the Ghirardi Botanic Garden (Lombardy, Italy). Plant Biol. 2020. [Google Scholar] [CrossRef]
- Lamine, M.; Gargouri, M.; Mliki, A. Identification of the NaCl-responsive metabolites in Citrus roots: A lipidomic and volatomic signature. Plant Signal. Behav. 2020, 15, 1777376. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Z.; Qiao, X.; Li, Z.; Li, F.; Chen, M.; Wang, Y.; Huang, Y.; Cui, H. Antifungal activity of volatile organic compounds from Streptomyces alboflavus TD-1. FEMS Microbiol. Lett. 2013, 341, 45–51. [Google Scholar] [CrossRef]
- Eshiet, E.R.; Zhu, J.; Anderson, T.A.; Smith, E.E. Chemical characterization of Brickellia cavanillesii (Asteraceae) using gas chromatographic methods. Food Sci. Nutr. 2014, 2, 105–113. [Google Scholar] [CrossRef]
- Chen, X.Y.; Dou, Y.X.; Luo, D.D.; Zhang, Z.B.; Li, C.L.; Zeng, H.F.; Su, Z.R.; Xie, J.H.; Lai, X.P.; Li, Y.C. β-Patchoulene from patchouli oil protects against LPS-induced acute lung injury via suppressing NF-κB and activating Nrf2 pathways. Int. Immunopharmacol. 2017, 50, 270–278. [Google Scholar] [CrossRef]
- Pavithra, P.S.; Mehta, A.; Verma, R.S. Synergistic interaction of β-caryophyllene with aromadendrene oxide 2 and phytol induces apoptosis on skin epidermoid cancer cells. Phytomedicine 2018, 47, 121–134. [Google Scholar] [CrossRef]
- Peña-Montes, D.J.; Huerta-Cervantes, M.; Ríos-Silva, M.; Trujillo, X.; Huerta, M.; Noriega-Cisneros, R.; Salgado-Garciglia, R.; Saavedra-Molina, A. Protective effect of the hexanic extract of Eryngium carlinae inflorescences in vitro, in yeast, and in streptozotocin-induced diabetic male rats. Antioxidants 2019, 8, 73. [Google Scholar] [CrossRef]
- Alapati, K.; Handanahal, S.S. Characterization of cholesterol oxidase from a marine Streptomyces sp. and its cytotoxicity. Process Biochem. 2020, 89, 175–185. [Google Scholar] [CrossRef]
- Fonseca, D.F.S.; Salvador, Â.C.; Santos, S.A.O.; Vilela, C.; Freire, C.S.R.; Silvestre, A.J.D.; Rocha, S.M. Bioactive phytochemicals from wild Arbutus unedo L. berries from different locations in Portugal: Quantification of lipophilic components. Int. J. Mol. Sci. 2015, 16, 14194–14209. [Google Scholar] [CrossRef]
- Grogan, D.W.; Cronan, J.E. Cyclopropane ring formation in membrane lipids of bacteria. Microbiol. Mol. Biol. Rev. 1997, 61, 429–441. [Google Scholar] [CrossRef]
- Raffo, A.; D’Aloise, A.; Magrì, A.D.; Leclercq, C. Quantification of allyl hexanoate in pineapple beverages and yogurts as a case study to characterise a source of uncertainty in dietary exposure assessment to flavouring substances. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2012, 29, 43–53. [Google Scholar] [CrossRef]
- Eltayeib, A.A.; Um Ismaeel, H. Extraction of Cyperus rotundus rhizomes oil, identification of chemical constituents and evaluation of antimicrobial activity of the oil in North Kordofan state. Int. J. Adv. Res. Chem. Sci. 2014, 1, 18–29. [Google Scholar]
- Song, Y.; Zhang, Y.; Liu, N.; Ye, D.; Gong, X.; Qin, Y.; Liu, Y. Volatile compounds in wild strawberry and their odorants of wild strawberry wines: Effects of different stages of fermentation. Int. J. Food Prop. 2017, 20, S399–S415. [Google Scholar] [CrossRef]
- Bail, S.; Buchbauer, G.; Jirovetz, L.; Denkova, Z.; Slavchev, A.; Stoyanova, A.; Schmidt, E.; Geissler, M. Antimicrobial activities of roman chamomile oil from France and its main compounds. J. Essent. Oil Res. 2009, 21, 283–286. [Google Scholar] [CrossRef]
- Leisso, R.S.; Buchanan, D.A.; Lee, J.; Mattheis, J.P.; Sater, C.; Hanrahan, I.; Watkins, C.B.; Gapper, N.; Johnston, J.W.; Schaffer, R.J.; et al. Chilling-related cell damage of apple ( Malus × domestica Borkh.) fruit cortical tissue impacts antioxidant, lipid and phenolic metabolism. Physiol. Plant. 2015, 153, 204–220. [Google Scholar] [CrossRef]
- Cantrell, C.L.; Zaki, M.A.; Reichley, A.; Sink, M.; Kim, S.J.; Ali, A. Biting deterrency of undecanoic acid and dodecanoic acid ester analogs against Aedes aegypti. Pest Manag. Sci. 2020. [Google Scholar] [CrossRef]
- Lu, Y.; Zhou, Y.; Nakai, S.; Hosomi, M.; Zhang, H.; Kronzucker, H.J.; Shi, W. Stimulation of nitrogen removal in the rhizosphere of aquatic duckweed by root exudate components. Planta 2014, 239, 591–603. [Google Scholar] [CrossRef] [PubMed]
- AbuSara, N.F.; Piercey, B.M.; Moore, M.A.; Shaikh, A.A.; Nothias, L.F.; Srivastava, S.K.; Cruz-Morales, P.; Dorrestein, P.C.; Barona-Gómez, F.; Tahlan, K. Comparative genomics and metabolomics analyses of clavulanic acid-producing Streptomyces species provides insight into specialized metabolism. Front. Microbiol. 2019, 10, 2550. [Google Scholar] [CrossRef] [PubMed]
- Liato, V.; Aïder, M. Geosmin as a source of the earthy-musty smell in fruits, vegetables and water: Origins, impact on foods and water, and review of the removing techniques. Chemosphere 2017, 181, 9–18. [Google Scholar] [CrossRef]
- Stensmyr, M.C.; Dweck, H.K.M.; Farhan, A.; Ibba, I.; Strutz, A.; Mukunda, L.; Linz, J.; Grabe, V.; Steck, K.; Lavista-Llanos, S.; et al. A conserved dedicated olfactory circuit for detecting harmful microbes in Drosophila. Cell 2012, 151, 1345–1357. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, C.; Li, F.; Li, Z.; Chen, M.; Wang, Y.; Qiao, X.; Zhang, H. Fumigant activity of volatiles from Streptomyces alboflavus TD-1 against Fusarium moniliforme Sheldon. J. Microbiol. 2013, 51, 477–483. [Google Scholar] [CrossRef]
- Kuwahara, Y.; Ichiki, Y.; Morita, M.; Tanabe, T.; Asano, Y. Chemical polymorphism in defense secretions during ontogenetic development of the millipede Niponia nodulosa. J. Chem. Ecol. 2014, 41, 15–21. [Google Scholar] [CrossRef]
- Fravel, D.R.; Connick, W.J.; Grimm, C.C.; Lloyd, S.W. Volatile compounds emitted by sclerotia of Sclerotinia minor, Sclerotinia sclerotiorum, and Sclerotium rolfsii. J. Agric. Food Chem. 2002, 50, 3761–3764. [Google Scholar] [CrossRef]
- Abd-ElGawad, A.M.; El Gendy, A.E.-N.G.; Assaeed, A.M.; Al-Rowaily, S.L.; Omer, E.A.; Dar, B.A.; Al-Taisan, W.A.; Elshamy, A.I. Essential oil enriched with oxygenated constituents from invasive plant Argemone ochroleuca exhibited potent phytotoxic effects. Plants 2020, 9, 998. [Google Scholar] [CrossRef]
- Laouer, H.; Meriem, E.K.; Prado, S.; Baldovini, N. An antibacterial and antifungal phenylpropanoid from Carum montanum (Coss. et Dur.) Benth. et Hook. Phyther. Res. 2009, 23, 1726–1730. [Google Scholar] [CrossRef]
- Claeson, A.S.; Sunesson, A.L. Identification using versatile sampling and analytical methods of volatile compounds from Streptomyces albidoflavus grown on four humid building materials and one synthetic medium. Indoor Air Suppl. 2005, 15, 41–47. [Google Scholar] [CrossRef]
- Turner, S.L.; Li, N.; Guda, T.; Githure, J.; Cardé, R.T.; Ray, A. Ultra-prolonged activation of CO2-sensing neurons disorients mosquitoes. Nature 2011, 474, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Xu, B.; Kader, A.; Song, B.; Zhang, Z.; Li, F.; Yang, S. Behavioral responses of Scolytus schevyrewi (Coleoptera: Curculionidae: Scolytinae) to volatiles from apricot tree (Rosales: Rosaceae). Environ. Entomol. 2020, 49, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Pollak, F.C.; Berger, R.G. Geosmin and related volatiles in bioreactor-cultured Streptomyces citreus CBS 109.60. Appl. Environ. Microbiol. 1996, 62, 1295–1299. [Google Scholar] [CrossRef] [PubMed]
- Ziesche, L.; Bruns, H.; Dogs, M.; Wolter, L.; Mann, F.; Wagner-Döbler, I.; Brinkhoff, T.; Schulz, S. Homoserine lactones, methyl oligohydroxybutyrates, and other extracellular metabolites of macroalgae-associated bacteria of the Roseobacter clade: Identification and functions. ChemBioChem 2015, 16, 2094–2107. [Google Scholar] [CrossRef] [PubMed]
- Trust, T.J.; Bartlett, K.H. Antibacterial activity of tropilidine and tropone. Antimicrob. Agents Chemother. 1975, 8, 381–383. [Google Scholar] [CrossRef]
- Li, C.; Ngai, M.H.; Reddy, K.K.; Leong, S.C.Y.; Tong, Y.W.; Chai, C.L.L. A fluorescence-displacement assay using molecularly imprinted polymers for the visual, rapid, and sensitive detection of the algal metabolites, geosmin and 2-methylisoborneol. Anal. Chim. Acta 2019, 1066, 121–130. [Google Scholar] [CrossRef]
- Ômura, H.; Kuwahara, Y.; Tanabe, T. 1-Octen-3-ol together with geosmin: New secretion compounds from a polydesmid millipede, Niponia nodulosa. J. Chem. Ecol. 2002, 28, 2601–2612. [Google Scholar] [CrossRef]
- Bartelt, R.J.; Cossé, A.A.; Zilkowski, B.W.; Weisleder, D.; Momany, F.A. Male-specific sesquiterpenes from Phyllotreta and Aphthona flea beetles. J. Chem. Ecol. 2001, 27, 2397–2423. [Google Scholar] [CrossRef]
- Beran, F.; Jiménez-Alemán, G.H.; Lin, M.Y.; Hsu, Y.C.; Mewis, I.; Srinivasan, R.; Ulrichs, C.; Boland, W.; Hansson, B.S.; Reinecke, A. The aggregation pheromone of Phyllotreta striolata (Coleoptera: Chrysomelidae) revisited. J. Chem. Ecol. 2016, 42, 748–755. [Google Scholar] [CrossRef]
- Aberchane, M.; Fechtal, M.; Chaouch, A. Analysis of Moroccan atlas cedarwood oil (Cedrus atlantica Manetti). J. Essent. Oil Res. 2004, 16, 542–547. [Google Scholar] [CrossRef]
- Kanako, S.; Fumiko, N.; Keiko, U.; Ichiro, Y.; Kazuyuki, A.; Itsu, K. Inhibition of Na+,K+-ATPase activity by β-eudesmol, a major component of atractylodis lanceae rhizoma, due to the interaction with enzyme in the Na·E1 state. Biochem. Pharmacol. 1992, 44, 373–378. [Google Scholar] [CrossRef]
- Ohara, K.; Fukuda, T.; Ishida, Y.; Takahashi, C.; Ohya, R.; Katayama, M.; Uchida, K.; Tominaga, M.; Nagai, K. β-Eudesmol, an oxygenized sesquiterpene, stimulates appetite via TRPA1 and the autonomic nervous system. Sci. Rep. 2017, 7, 15785. [Google Scholar] [CrossRef] [PubMed]
- Pavithra, P.S.; Mehta, A.; Verma, R.S. Aromadendrene oxide 2, induces apoptosis in skin epidermoid cancer cells through ROS mediated mitochondrial pathway. Life Sci. 2018, 197, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Pavithra, P.S.; Mehta, A.; Verma, R.S. Induction of apoptosis by essential oil from P. missionis in skin epidermoid cancer cells. Phytomedicine 2018, 50, 184–195. [Google Scholar] [CrossRef]
- Saito, Y.; Sato, T.; Nomoto, K.; Tsuji, H. Identification of phenol- and p-cresol-producing intestinal bacteria by using media supplemented with tyrosine and its metabolites. FEMS Microbiol. Ecol. 2018, 94, fiy125. [Google Scholar] [CrossRef]
- Windey, K.; de Preter, V.; Verbeke, K. Relevance of protein fermentation to gut health. Mol. Nutr. Food Res. 2012, 56, 184–196. [Google Scholar] [CrossRef]
- Yu, L.; Blaser, M.; Andrei, P.I.; Pierik, A.J.; Selmer, T. 4-Hydroxyphenylacetate decarboxylases: Properties of a novel subclass of glycyl radical enzyme systems. Biochemistry 2006, 45, 9584–9592. [Google Scholar] [CrossRef]
- Pascucci, T.; Colamartino, M.; Fiori, E.; Sacco, R.; Coviello, A.; Ventura, R.; Puglisi-Allegra, S.; Turriziani, L.; Persico, A.M. P-cresol alters brain dopamine metabolism and exacerbates autism-like behaviors in the BTBR mouse. Brain Sci. 2020, 10, 233. [Google Scholar] [CrossRef]
- Hsu, Y.H.; Huang, H.P.; Chang, H.R. The uremic toxin p-cresol promotes the invasion and migration on carcinoma cells via Ras and mTOR signaling. Toxicol. Vitr. 2019, 58, 126–131. [Google Scholar] [CrossRef]
- Isberg, E.; Bray, D.P.; Hillbur, Y.; Ignell, R. Evaluation of host-derived volatiles for trapping Culicoides biting midges (Diptera: Ceratopogonidae). J. Chem. Ecol. 2017, 43, 662–669. [Google Scholar] [CrossRef]
- Antolak, H.; Jeleń, H.; Otlewska, A.; Kręgiel, D. Volatile compounds associated with growth of Asaia bogorensis and Asaia lannensis-unusual spoilage bacteria of functional beverages. Food Res. Int. 2019, 121, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Wang, L.; Gu, R.; Zhao, J.Y.; Hou, X.; Zhu, H. Hydroxy-pentanones production by Bacillus sp. H15-1 and its complete genome sequence. J. Biotechnol. 2017, 259, 26–29. [Google Scholar] [CrossRef] [PubMed]
- Benozzi, E.; Romano, A.; Capozzi, V.; Makhoul, S.; Cappellin, L.; Khomenko, I.; Aprea, E.; Scampicchio, M.; Spano, G.; Märk, T.D.; et al. Monitoring of lactic fermentation driven by different starter cultures via direct injection mass spectrometric analysis of flavour-related volatile compounds. Food Res. Int. 2015, 76, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Neuser, F.; Zorn, H.; Berger, R.G. Generation of odorous acyloins by yeast pyruvate decarboxylases and their occurrence in sherry and soy sauce. J. Agric. Food Chem. 2000, 48, 6191–6195. [Google Scholar] [CrossRef]
- Zaccone, E.J.; Goldsmith, W.T.; Shimko, M.J.; Wells, J.R.; Schwegler-Berry, D.; Willard, P.A.; Case, S.L.; Thompson, J.A.; Fedan, J.S. Diacetyl and 2,3-pentanedione exposure of human cultured airway epithelial cells: Ion transport effects and metabolism of butter flavoring agents. Toxicol. Appl. Pharmacol. 2015, 289, 542–549. [Google Scholar] [CrossRef]
- Sawamura, M.; Song, H.-S.; Choi, H.-S.; Sagawa, K.; Ukeda, H. Characteristic aroma components of Tosa-buntan(Citrus grandis Osbeck forma Tosa) fruit. Food Sci. Technol. Res. 2001, 7, 45–49. [Google Scholar] [CrossRef]
- Cho, D.-B.; Seo, H.-Y.; Kim, K.-S. Analysis of the volatile flavor compounds produced during the growth stages of the shiitake mushrooms (Lentinus edodes). Prev. Nutr. Food Sci. 2003, 8, 306–314. [Google Scholar] [CrossRef]
- Mevy, J.P.; Bessiere, J.M.; Greff, S.; Zombre, G.; Viano, J. Composition of the volatile oil from the leaves of Ximenia americana L. Biochem. Syst. Ecol. 2006, 34, 549–553. [Google Scholar] [CrossRef]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential oils’ chemical characterization and investigation of some biological activities: A critical review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef]
- Miresmailli, S.; Isman, M.B. Botanical insecticides inspired by plant-herbivore chemical interactions. Trends Plant Sci. 2014, 19, 29–35. [Google Scholar] [CrossRef]
- Barbosa-Cornelio, R.; Cantor, F.; Coy-Barrera, E.; Rodríguez, D. Tools in the investigation of volatile semiochemicals on insects: From sampling to statistical analysis. Insects 2019, 10, 241. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Wang, X.; Nie, J.; Niu, G. Regulation of antibiotic production by signaling molecules in Streptomyces. Front. Microbiol. 2019, 10, 2927. [Google Scholar] [CrossRef] [PubMed]
- Khalid, A.; Takagi, H.; Panthee, S.; Muroi, M.; Chappell, J.; Osada, H.; Takahashi, S. Development of a terpenoid-production platform in Streptomyces reveromyceticus SN-593. ACS Synth. Biol. 2017, 6, 2339–2349. [Google Scholar] [CrossRef] [PubMed]
- Scheller, U.; Zimmer, T.; Becher, D.; Schauer, F.; Schunck, W. Oxygenation cascade in conversion of N-alkanes to alpha, omega-dioic acids catalyzed by cytochrome P450 52A3. J. Biol. Chem. 1998, 273, 32528–32534. [Google Scholar] [CrossRef] [PubMed]
- Lehtinen, T.; Efimova, E.; Santala, S.; Santala, V. Improved fatty aldehyde and wax ester production by overexpression of fatty acyl-CoA reductases. Microb. Cell Fact. 2018, 17, 19. [Google Scholar] [CrossRef]
- Dudareva, N.; Negre, F.; Nagegowda, D.A.; Orlova, I. Plant volatiles: Recent advances and future perspectives. CRC Crit. Rev. Plant Sci. 2006, 25, 417–440. [Google Scholar] [CrossRef]
- Viaene, T.; Langendries, S.; Beirinckx, S.; Maes, M.; Goormachtig, S. Streptomyces as a plant’s best friend? FEMS Microbiol. Ecol. 2016, 92, 119. [Google Scholar] [CrossRef]
- Dotson, B.; Verschut, V.; Flardh, K.; Becher, P.; Rasmusson, A. The Streptomyces volatile 3-octanone alters auxin/cytokinin and growth in Arabidopsis thaliana via the gene family KISS ME DEADLY. bioRxiv 2020. [Google Scholar] [CrossRef]
- Wu, Y.; Yuan, J.; E, Y.; Raza, W.; Shen, Q.; Huang, Q. Effects of volatile organic compounds from Streptomyces albulus NJZJSA2 on growth of two fungal pathogens. J. Basic Microbiol. 2015, 55, 1104–1117. [Google Scholar] [CrossRef]
- Logrieco, A.; Mulè, G.; Moretti, A.; Bottalico, A. Toxigenic Fusarium species and mycotoxins associated with maize ear rot in Europe. Eur. J. Plant Pathol. 2002, 108, 597–609. [Google Scholar] [CrossRef]
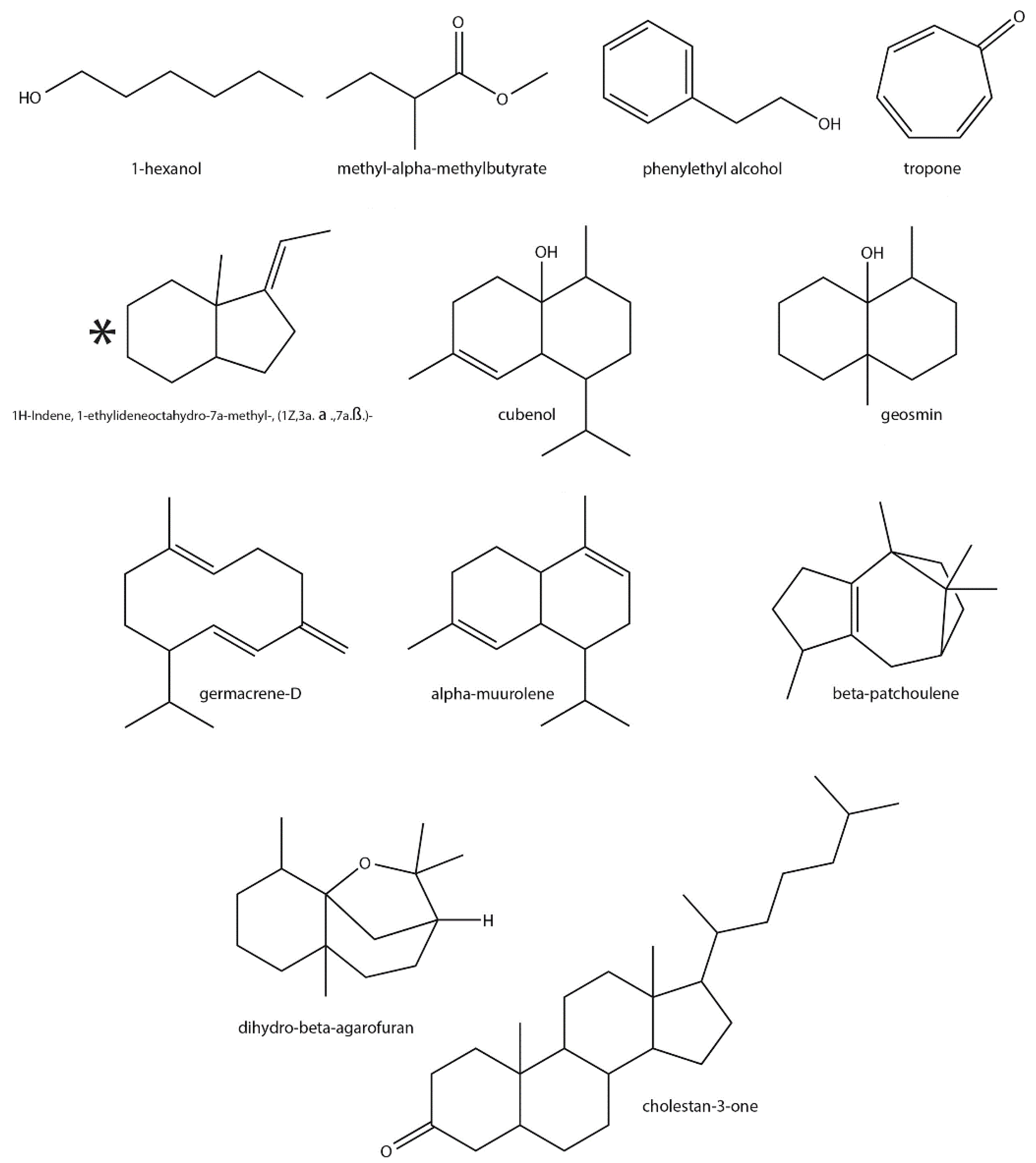
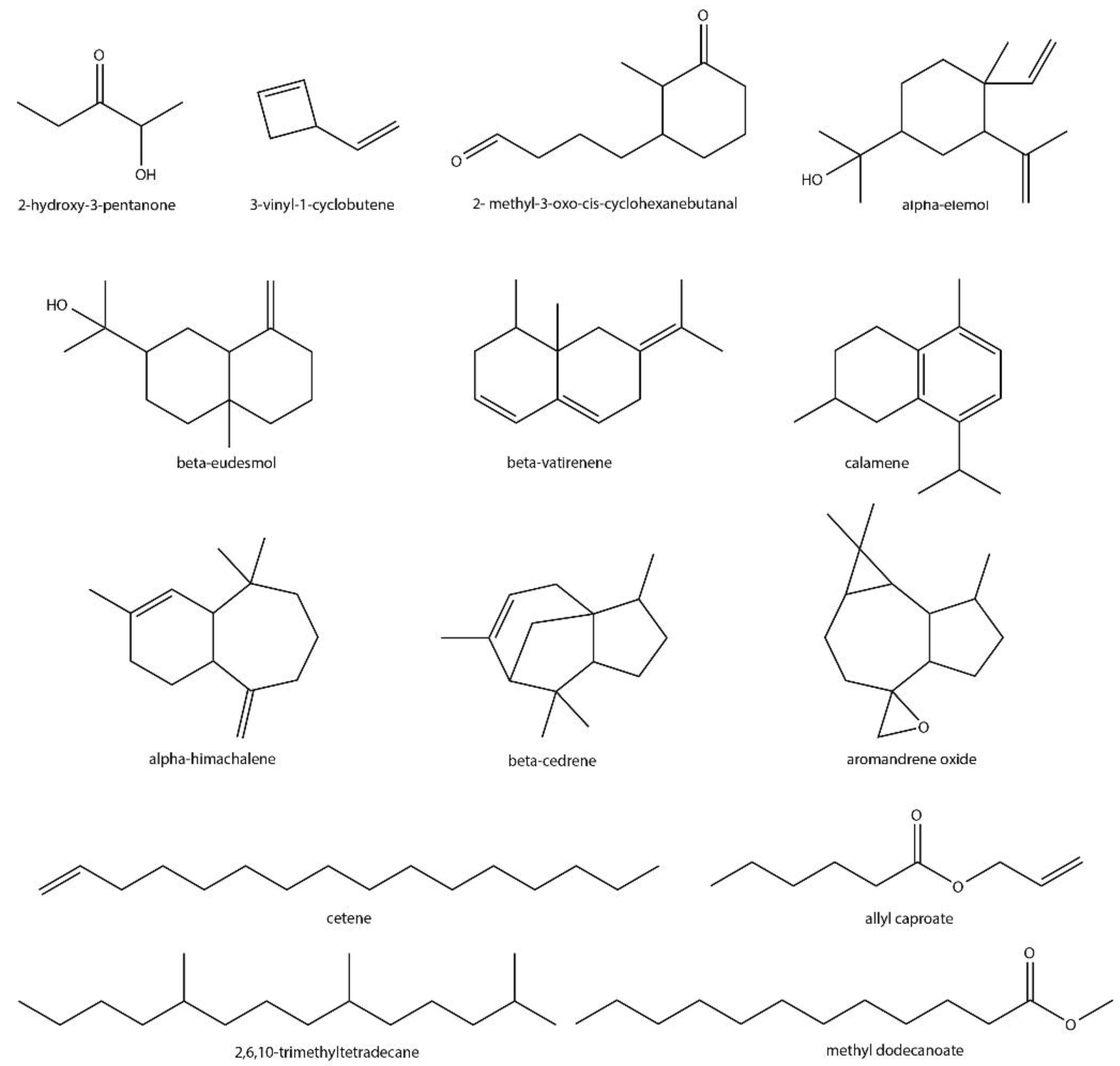
| Chemical Class | VOC ¥ | Functional Group | ID ψ | Detected in ϕ | Previously Reported in Streptomyces | Previously Reported in Plants |
|---|---|---|---|---|---|---|
| Alcohol | 1-Hexanol | Alkane, alcohol | ** | S. ave, S. gri, S. liv | Yes [18,58,59] | Yes [60,61,62] |
| p-Cresol | Aromatic alcohol | * | S. clav | No | No | |
| Phenylethyl alcohol | Aromatic alcohol | *** | S. ave, S. exf | Yes [63,64] | Yes [65] | |
| Hydrocarbon | 3-Vinyl-1-cyclobutene | Alkene | * | S. par | No | Yes [66] |
| Cetene | Alkene | * | S. cla, S. par | No | Yes [67,68] | |
| 2,6,10-trimethyltetradecane | Alkane | * | S. par | No | Yes [69] | |
| Ketone | Tropone | Aromatic ketone | * | S. par | Yes [70] | Yes [71] |
| 2-Hydroxy-3-pentanone | Alcohol, ketone | ** | S. gri, S. liv | No | Yes [72] | |
| Terpenoids | 2-Methyl-2-bornene | Irregular monoterpene | *** | S. exf | Yes [73,74] | No |
| α-Elemol | Sesquiterpene alcohol | * | S. par | No | Yes [75] | |
| α-Muurolene | Sesquiterpene | ** | S. aur | Yes [76] | Yes [63,77] | |
| α-Himachalene | Sesquiterpene | * | S. cla | No | Yes [78,79,80] | |
| 2-MIB | Monoterpene alcohol | *** | S. exf, S. gri, S. liv, S. par | Yes [54,81,82,83] | No | |
| β-Eudesmol | Sesquiterpene alcohol | ** | S. exf, S. hyg | No | Yes [84,85] | |
| Geosmin | Irregular sesquiterpene alcohol | *** | S. ave, S. coe, S. exf, S. gri, S. hyg, S. liv, S. par | Yes [53] | Yes [86] | |
| Cubenol | Sesquiterpene alcohol | ** | S. exf | Yes [87] | Yes [88,89] | |
| β-Cedrene | Sesquiterpene | * | S. hyg | No | Yes [90,91] | |
| β-Vatirenene | Sesquiterpene | * | S. hyg | No | Yes [92,93] | |
| dihydro- β-Agarofuran | Sesquiterpene lactone | * | S. hyg | Yes [94] | Yes [95] | |
| Germacrene-D | Sesquiterpene | ** | S. hyg | Yes [96] | Yes [97,98] | |
| 1H-Indene, 1-ethylideneoctahydro-7a-methyl-, (1Z, 3a. α.,7a.β.)- | Irregular sesquiterpene | * | S. hyg | Yes [99] | Yes [100] † | |
| 1H-Indene, 1-ethylideneoctahydro-7a-methyl-, cis- | Irregular sesquiterpene | * | S. hyg | Yes [76] | Yes [100] † | |
| β-Patchoulene | Sesquiterpene | * | S. hyg | Yes [94] | Yes [101] | |
| Aromadendrene oxide-(2) | Sesquiterpene oxide | * | S. hyg | No | Yes [102] | |
| Calamene | Aromatic sesquiterpene | * | S. hyg | No | Yes [103] | |
| Cholestan-3-one | Triterpenoid ketone | * | S. hyg | Yes [104] | Yes [105] | |
| Other | 2,2,3,3-Tetramethyl-cyclopropanecarboxylic acid, 1-butylhexyl ester | Ester, carboxylic acid | * | S. par | Yes [106] ‡ | No |
| Allyl caproate | Ester, alkene | * | S. hyg | No | Yes [107] | |
| Cyclohexanebutanal, 2-methyl-3-oxo-, cis- | Ketone, aldehyde | * | S. hyg | No | Yes [108] | |
| Methyl α-methylbutyrate | Ester | * | S. coe | Yes [64] | Yes [109,110,111] | |
| Methyl dodecanoate | Ester | ** | S. ave | No | Yes [112,113] | |
| 6-Methyl-cyclodec-5-enol | Enol | * | S. hyg | Yes | No | |
| Isobutyl tetradecyl carbonate | Ester, alkane | * | S. par | No | No |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Z.; McCann, S.; Faraone, N.; Clarke, J.-A.; Hudson, E.A.; Cloonan, K.; Hillier, N.K.; Tahlan, K. Production of Plant-Associated Volatiles by Select Model and Industrially Important Streptomyces spp. Microorganisms 2020, 8, 1767. https://doi.org/10.3390/microorganisms8111767
Cheng Z, McCann S, Faraone N, Clarke J-A, Hudson EA, Cloonan K, Hillier NK, Tahlan K. Production of Plant-Associated Volatiles by Select Model and Industrially Important Streptomyces spp. Microorganisms. 2020; 8(11):1767. https://doi.org/10.3390/microorganisms8111767
Chicago/Turabian StyleCheng, Zhenlong, Sean McCann, Nicoletta Faraone, Jody-Ann Clarke, E. Abbie Hudson, Kevin Cloonan, N. Kirk Hillier, and Kapil Tahlan. 2020. "Production of Plant-Associated Volatiles by Select Model and Industrially Important Streptomyces spp." Microorganisms 8, no. 11: 1767. https://doi.org/10.3390/microorganisms8111767
APA StyleCheng, Z., McCann, S., Faraone, N., Clarke, J.-A., Hudson, E. A., Cloonan, K., Hillier, N. K., & Tahlan, K. (2020). Production of Plant-Associated Volatiles by Select Model and Industrially Important Streptomyces spp. Microorganisms, 8(11), 1767. https://doi.org/10.3390/microorganisms8111767







