Evaluation of Recommended Water Sample Collection Methods and the Impact of Holding Time on Legionella Recovery and Variability from Healthcare Building Water Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Effects of Flushing and Volume Tested on Legionella Recovery
2.2. Effects of Sample Holding Time on Legionella Recovery
2.3. Statistical Analyses
2.3.1. Flushing and Volume Analysis
2.3.2. Sample Holding Time and Location Analysis
3. Results
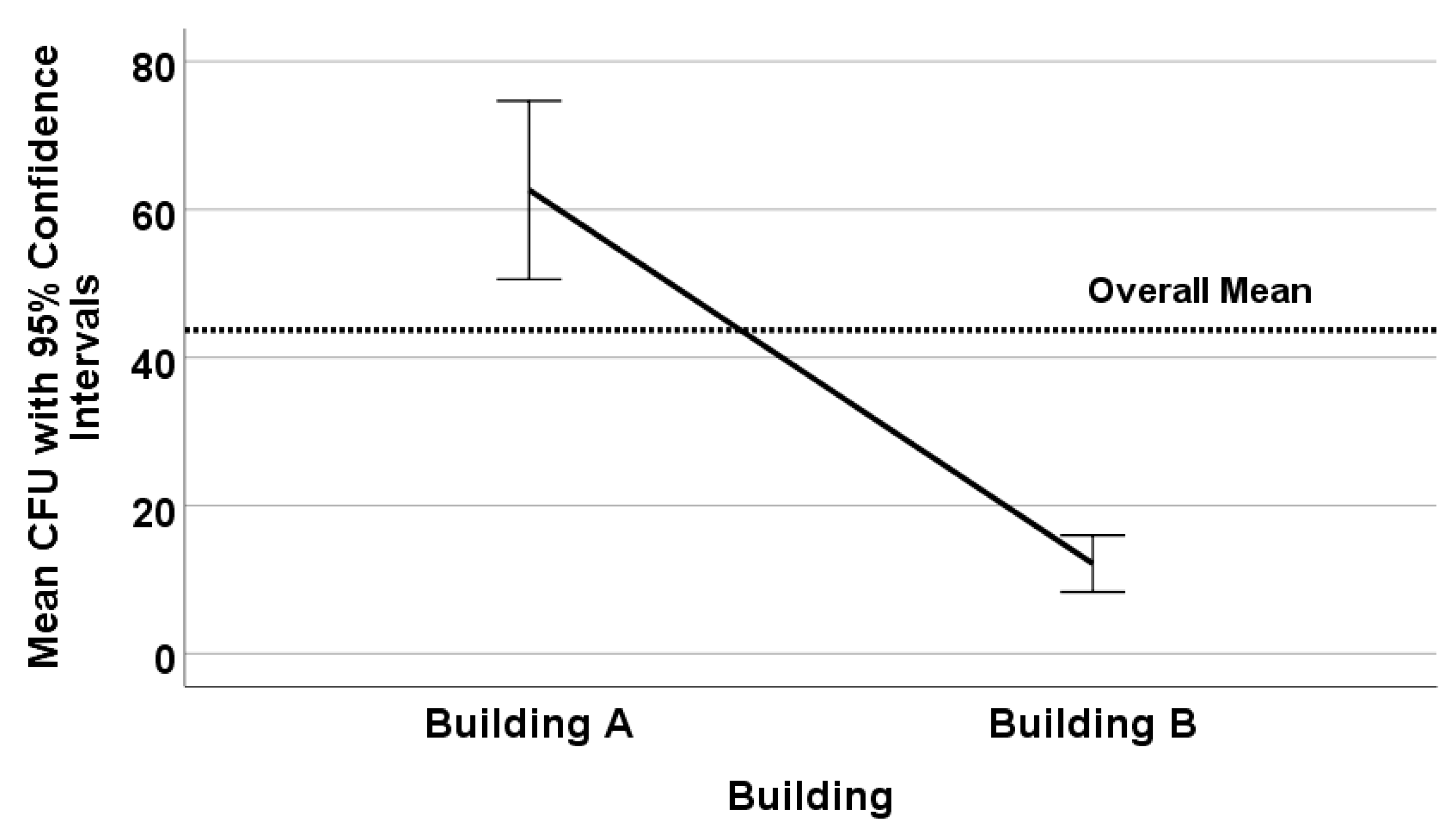
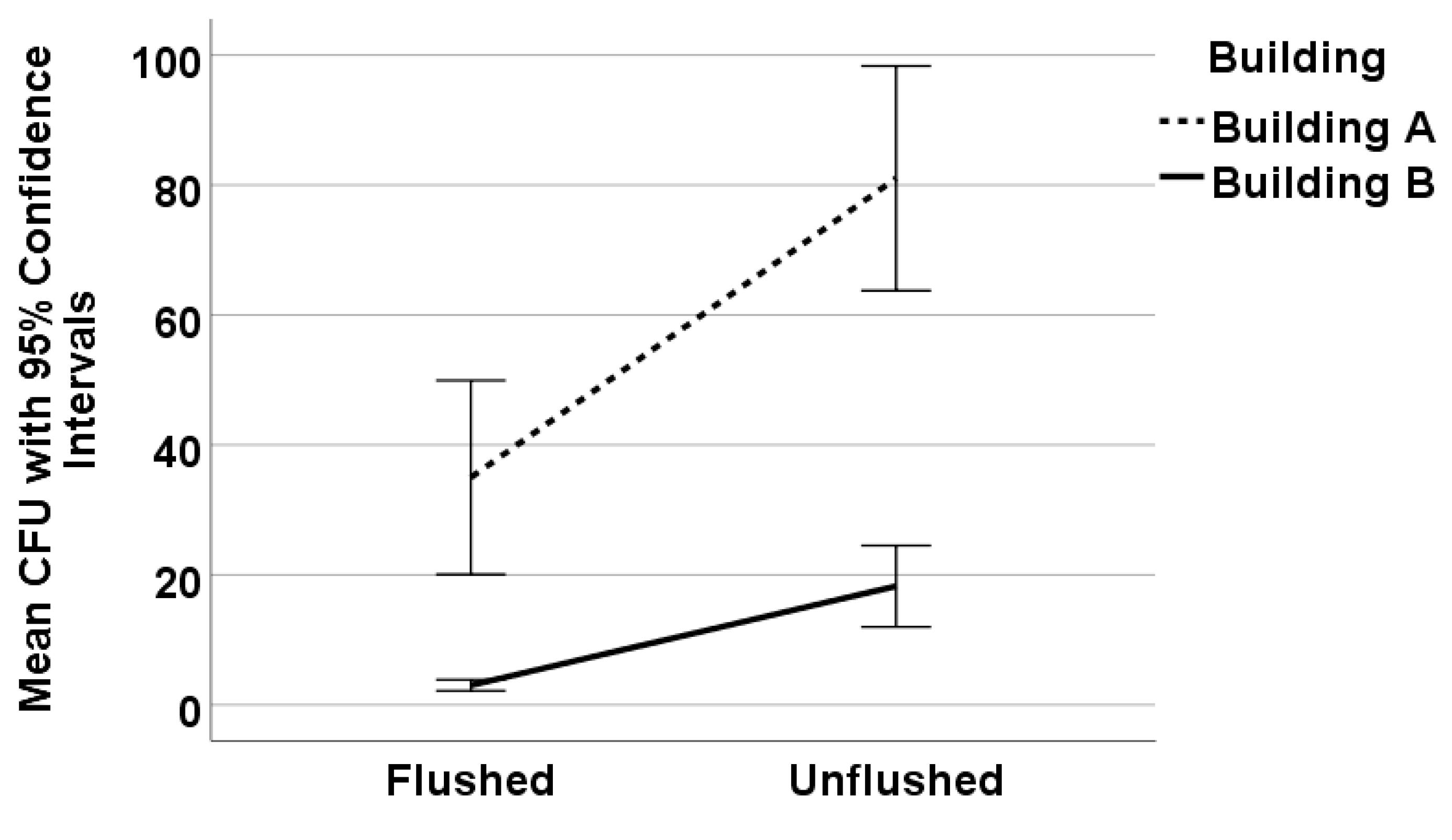
3.1. Effects of Flushing and Volume Tested on Legionella Recovery
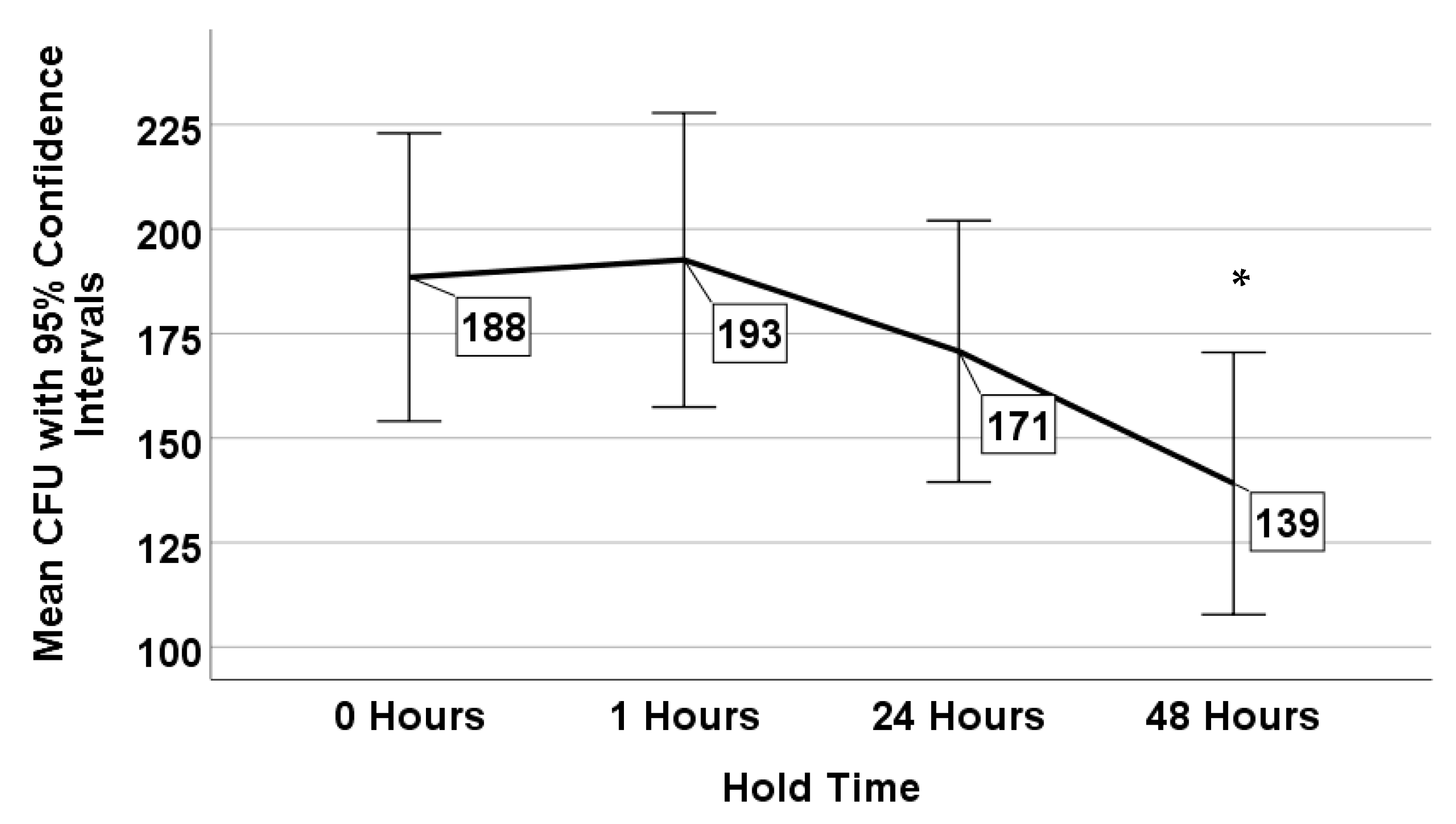
3.2. Effects of Sample Holding Time on Legionella Recovery
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stout, J.E.; Yu, V.L. Legionellosis. N. Engl. J. Med. 1997, 337, 682–687. [Google Scholar] [CrossRef] [PubMed]
- Stout, J.E.; Muder, R.R.; Mietzner, S.; Wagener, M.M.; Perri, M.B.; DeRoos, K.; Goodrich, D.; Arnold, W.; Williamson, T.; Ruark, O.; et al. Role of environmental surveillance in determining the risk of hospital-acquired legionellosis: A national surveillance study with clinical correlations. Infect. Control Hosp. Epidemiol. 2007, 28, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Stout, J.E.; Yu, V.L. Legionella in the Hospital Water Supply: A Plea for Decision Making Based on Evidence-Based Medicine. Infect. Control Hosp. Epidemiol. 2001, 22, 670–672. [Google Scholar] [CrossRef] [PubMed]
- ANSI/ASHRAE Standard 188-2018. Legionellosis: Risk Management for Building Water Systems; American Society of Heating, Refrigerating and Air-Conditioning Engineers: Atlanta, GA, USA, 2018. [Google Scholar]
- Government of New York. Part 4—Protection Against Legionella. In New York Codes, Rules and Regulations; Title 10; Government of New York: New York, NY, USA, 2016. [Google Scholar]
- Kerbel, W.; Krause, J.D.; Shelton, B.G.; Springston, J. (Eds.) Recognition, Evaluation, and Control. of Legionella in Building Water Systems; American Industrial Hygiene Association: Falls Church, VA, USA, 2015. [Google Scholar]
- Clancy, C.M.; Department of Veterans Affairs; Veterans Health Administration. VHA Directive 1061: Prevention of Healthcare-Associated Legionella Disease and Scald Injury from Potable Water Distribution Systems; Department of Veterans Affairs: Washington, DC, USA, 2014. [Google Scholar]
- Centers for Medicare & Medicaid Services (CMS). Requirement to Reduce Legionella Risk in Healthcare Facility Water Systems to Prevent Cases and Outbreaks of Legionnaires’ Disease (LD); S&C 17-30-Hospitals/CAHs/NHs; Department of Health & Human Services: Baltimore, MD, USA, 2017.
- Wang, H.; Bédard, E.; Prévost, M.; Camper, A.K.; Hill, V.R.; Pruden, A. Methodological approaches for monitoring opportunistic pathogens in premise plumbing: A review. Water Res. 2017, 117, 68–86. [Google Scholar] [CrossRef] [PubMed]
- D5952-08(2015). Standard Guide for the Inspection of Water Systems for Legionella and the Investigation of Possible Outbreaks of Legionellosis (Legionnaires’ Disease or Pontiac Fever); ASTM International: West Conshohocken, PA, USA, 2015; Available online: www.astm.org (accessed on 14 May 2020).
- ISO 11731:2017. Water Quality—Enumeration of Legionella; ISO: Geneva, Switzerland, 2017. [Google Scholar]
- Guideline 12-2020. Minimizing the Risk of Legionellosis Associated with Building Water Systems; American Society of Heating, Refrigerating and Air-Conditioning Engineers: Atlanta, GA, USA, 2020. [Google Scholar]
- National Center for Immunization and Respiratory Diseases. CDC Laboratory Guidance for Processing Environmental Samples; U.S. Department of Health and Human Services, Ed.; U.S. Department of Health and Human Services: Atlanta, GA, USA, 2005.
- Borella, P.; Montagna, M.T.; Romano-Spica, V.; Stampi, S.; Stancanelli, G.; Triassi, M.; Neglia, R.; Marchesi, I.; Fantuzzi, G.; Tato, D.; et al. Legionella infection risk from domestic hot water. Emerg. Infect. Dis. 2004, 10, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Flannery, B.; Gelling, L.B.; Vugia, D.J.; Weintraub, J.M.; Salerno, J.J.; Conroy, M.J.; Stevens, V.A.; Rose, C.E.; Moore, M.R.; Fields, B.S.; et al. Reducing Legionella colonization of water systems with monochloramine. Emerg. Infect. Dis. 2006, 12. [Google Scholar] [CrossRef]
- Bargellini, A.; Marchesi, I.; Righi, E.; Ferrari, A.; Cencetti, S.; Borella, P.; Rovesti, S. Parameters predictive of Legionella contamination in hot water systems: Association with trace elements and heterotrophic plate counts. Water Res. 2011, 45, 2315–2321. [Google Scholar] [CrossRef]
- McCoy, W.F.; Downes, E.L.; Leonidas, L.F.; Cain, M.F.; Sherman, D.L.; Chen, K.; Devender, S.; Neville, M.J. Inaccuracy in Legionella tests of building water systems due to sample holding time. Water Res. 2012, 46, 3497–3506. [Google Scholar] [CrossRef]
- Dana Flanders, W.; Kirkland, K.H.; Shelton, B.G. Effects of holding time and measurement error on culturing Legionella in environmental water samples. Water Res. 2014, 62, 293–301. [Google Scholar] [CrossRef][Green Version]
- Nagy, D.J.; Dziewulski, D.M.; Codru, N.; Lauper, U.L. Understanding the distribution of positive Legionella samples in healthcare-premise water systems: Using statistical analysis to determine a distribution for Legionella and to support sample size recommendations. Infect. Control Hosp. Epidemiol. 2020. [Google Scholar] [CrossRef]
- ISO 19458:2006. Water Quality- Sampling for Microbiological Analysis; ISO: Geneva, Switzerland, 2006. [Google Scholar]
- ISO 11731:1998. Water Quality Detection and Enumeration of Legionella; ISO: Geneva, Switzerland, 1998. [Google Scholar]
- Vickers, R.M.; Brown, A.; Garrity, G.M. Dye-containing buffered charcoal yeast extract medium for the differentiation of members of the family Legionellaceae. J. Clin. Microbiol. 1981, 13, 380–382. [Google Scholar] [CrossRef] [PubMed]
- Kozak, N.A.; Lucas, C.E.; Winchell, J.M. Identification of Legionella in the Environment. In Legionella: Methods and Protocols; Buchrieser, C., Hilbi, H., Eds.; Humana Press: Totowa, NJ, USA, 2013; pp. 3–25. [Google Scholar]
- Bédard, E.; Laferrière, C.; Déziel, E.; Prévost, M. Impact of stagnation and sampling volume on water microbial quality monitoring in large buildings. PLoS ONE 2018, 13, e0199429. [Google Scholar] [CrossRef] [PubMed]
- Cristina, M.L.; Spagnolo, A.M.; Casini, B.; Baggiani, A.; Del Giudice, P.; Brusaferro, S.; Poscia, A.; Moscato, U.; Perdelli, F.; Orlando, P. The impact of aerators on water contamination by emerging gram-negative opportunists in at-risk hospital departments. Infect. Control Hosp. Epidemiol. 2014, 35, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Suárez, A.; Dellundé, J.; Salvadó, H.; Cervero-Aragó, S.; Méndez, J.; Canals, O.; Blanco, S.; Arcas, A.; Araujo, R. Microbial and physicochemical parameters associated with Legionella contamination in hot water recirculation systems. Environ. Sci. Pollut Res. Int. 2013, 20, 5534–5544. [Google Scholar] [CrossRef]
- Bédard, E.; Fey, S.; Charron, D.; Lalancette, C.; Cantin, P.; Dolcé, P.; Laferrière, C.; Déziel, E.; Prévost, M. Temperature diagnostic to identify high risk areas and optimize Legionella pneumophila surveillance in hot water distribution systems. Water Res. 2015, 71, 244–256. [Google Scholar] [CrossRef]
- Smith, L.; Carroll, K.; Mottice, S. Comparison of membrane filters for recovery of legionellae from water samples. Appl. Environ. Microbiol. 1993, 59, 344–346. [Google Scholar] [CrossRef]
- Best, M.; Yu, V.L.; Stout, J.; Goetz, A.; Muder, R.R.; Taylor, F. Legionellaceae in the hospital water supply—Epidemiological link with disease and evaluation of a method of control of nosocomial Legionnaires’ disease and Pittsburgh pneumonia. Lancet 1983, 2, 307–310. [Google Scholar] [CrossRef]
- Johnson, J.T.; Yu, V.L.; Best, M.; Vickers, R.M.; Goetz, A.; Wagner, R.; Wicker, H.; Woo, A. Nosocomial legionellosis uncovered in surgical patients with head and neck cancer: Implications for epidemiologic reservoir and mode of transmission. Lancet 1985, 2, 298–300. [Google Scholar] [CrossRef]
- Kool, J.L.; Bergmire-Sweat, D.; Butler, J.C.; Brown, E.W.; Peabody, D.J.; Massi, D.S.; Carpenter, J.C.; Pruckler, J.M.; Benson, R.F.; Fields, B.S. Hospital characteristics associated with colonization of water systems by Legionella and risk of nosocomial Legionnaires’ disease: A cohort study of 15 hospitals. Infect. Control Hosp. Epidemiol. 1999, 20, 798–805. [Google Scholar] [CrossRef]
- Sabria, M.; Modol, J.M.; Garcia-Nunez, M.; Reynaga, E.; Pedro-Botet, M.L.; Gimeno, J.M.; Sopena, N.; Rey-Joly, C. Environmental cultures and hospital-acquired legionnaires’ disease. A 5-year prospective study in 20 hospitals in Catalonia, Spain. Infect. Control Hosp. Epidemiol. 2004, 25, 1072–1076. [Google Scholar] [CrossRef]
- Boccia, S.; Laurenti, P.; Borella, P.; Moscato, U.; Capalbo, G.; Cambieri, A.; Amore, R.; Quaranta, G.; Boninti, F.; Orsini, M.; et al. Prospective 3-year surveillance for nosocomial and environmental Legionella pneumophila: Implications for infection control. Infect. Control Hosp. Epidemiol. 2006, 27, 459–465. [Google Scholar] [CrossRef] [PubMed]

| CDC [13] | ISO 1 [11] | AIHA [6] | ASTM [10] | ASHRAE [12] | |
|---|---|---|---|---|---|
| Collection | Flush hot water for a few minutes until water is warm but not hot | No flush | Immediate and 2-min minimum flush | No flush | No flush to represent outlet conditions; flush to represent system water |
| Volume | 1 L | 1 L | 125 mL–1 L for potable; 125 mL minimum for non-potable | 10–100 mL for non-potable; >1 L for potable | 250 mL for routine monitoring; 1 L for investigations |
| Holding Time or Shipping Recommendations | Not specified; Refrigerate if not processed within 72 h | Hold time 24 h–2 days; transport at 5 ± 3 °C is recommended, ambient temperature is acceptable | Ship overnight in an insulated container without ice | Ship overnight; Process within 48 h; Refrigerate if not processed in 72 h | Ship at ambient temperature within 24 h from collection; if time must exceed 48 h, consult with lab |
| Processing Method | Collection Method | Mean Legionella (CFU/mL) | Standard Error (CFU/mL) | Exp(B) 1 | p-Value | Sample Positivity |
|---|---|---|---|---|---|---|
| Direct | Unflushed | 106.08 | 15.6 | 19.691 | <0.0001 | 70% |
| Direct | Flushed | 40.67 | 9.4 | 7.548 | <0.0001 | 42% |
| Filtered 100 mL | Unflushed | 40.73 | 5.4 | 7.559 | <0.0001 | 90% |
| Filtered 200 mL | Unflushed | 25.67 | 2.9 | 4.806 | <0.0001 | 88% |
| Filtered 1000 mL | Flushed | 5.34 | 0.58 | 1 | n/a | 67% |
| Processing Method | Collection Method | Non-Detect | Low (<10 CFU/mL) | Moderate (10–99 CFU/mL) | High (≥100 CFU/mL) |
|---|---|---|---|---|---|
| Direct | Unflushed | 73 (28%) | 0 (0%) | 124 (33%) | 43 (37%) |
| Direct | Flushed | 139 (54%) | 0 (0%) | 82 (22%) | 19 (16%) |
| Filtered 100 mL | Unflushed | 25 (10%) | 118 (26%) | 69 (18%) | 28 (24%) |
| Filtered 200 mL | Unflushed | 11 (4%) | 140 (31%) | 62 (16%) | 27 (23%) |
| Filtered 1000 mL | Flushed | 10 (4%) | 190 (43%) | 40 (11%) | 0 (0%) |
| Independent Variable | Wald Chi-Square | Degrees of Freedom | p-Value |
|---|---|---|---|
| Sink | 114.660 | 11 | <0.0001 |
| Holding Time | 11.055 | 3 | 0.011 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hirsh, M.B.; Baron, J.L.; Mietzner, S.M.; Rihs, J.D.; Yassin, M.H.; Stout, J.E. Evaluation of Recommended Water Sample Collection Methods and the Impact of Holding Time on Legionella Recovery and Variability from Healthcare Building Water Systems. Microorganisms 2020, 8, 1770. https://doi.org/10.3390/microorganisms8111770
Hirsh MB, Baron JL, Mietzner SM, Rihs JD, Yassin MH, Stout JE. Evaluation of Recommended Water Sample Collection Methods and the Impact of Holding Time on Legionella Recovery and Variability from Healthcare Building Water Systems. Microorganisms. 2020; 8(11):1770. https://doi.org/10.3390/microorganisms8111770
Chicago/Turabian StyleHirsh, Marisa B., Julianne L. Baron, Sue M. Mietzner, John D. Rihs, Mohamed H. Yassin, and Janet E. Stout. 2020. "Evaluation of Recommended Water Sample Collection Methods and the Impact of Holding Time on Legionella Recovery and Variability from Healthcare Building Water Systems" Microorganisms 8, no. 11: 1770. https://doi.org/10.3390/microorganisms8111770
APA StyleHirsh, M. B., Baron, J. L., Mietzner, S. M., Rihs, J. D., Yassin, M. H., & Stout, J. E. (2020). Evaluation of Recommended Water Sample Collection Methods and the Impact of Holding Time on Legionella Recovery and Variability from Healthcare Building Water Systems. Microorganisms, 8(11), 1770. https://doi.org/10.3390/microorganisms8111770





