Screening Fungal Endophytes Derived from Under-Explored Egyptian Marine Habitats for Antimicrobial and Antioxidant Properties in Factionalised Textiles
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Isolation of Endophytic Fungi
2.3. Production and Preparation of Fungal Extracts
2.4. DNA Extraction, Amplification and Sequencing
2.5. Antimicrobial Activity
2.6. Total Antioxidant Capacity (TAC)
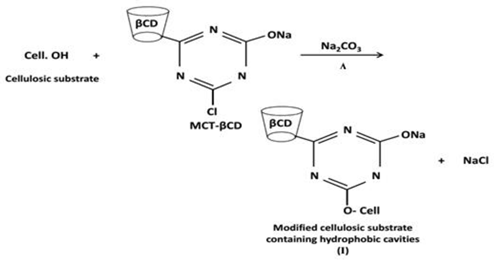
2.7. Grafting of MCT-βCD onto Cellulosic Substrates
2.8. Post-Hosting of Bio-Active Extracts into Hydrophobic Cavities to Impart the Demanded Functionalities
2.9. Methods of Analysis
2.10. In Vitro Cytotoxicity
2.11. Mass Spectral Data Acquisition
2.12. Molecular Networking
3. Results
3.1. Fungal Isolation and Cultivation
3.2. Molecular Identification
3.3. Biological Screening
3.3.1. Antimicrobial Activity
3.3.2. Total Antioxidant Capacity (TAC)
3.4. Functionalization of the Nominated Cellulosic Substrates

3.5. Antimicrobial Properties of the Treated Cellulosic Textiles
3.6. Ultraviolet Protection
3.7. Fungal Extracts Toxicity Study
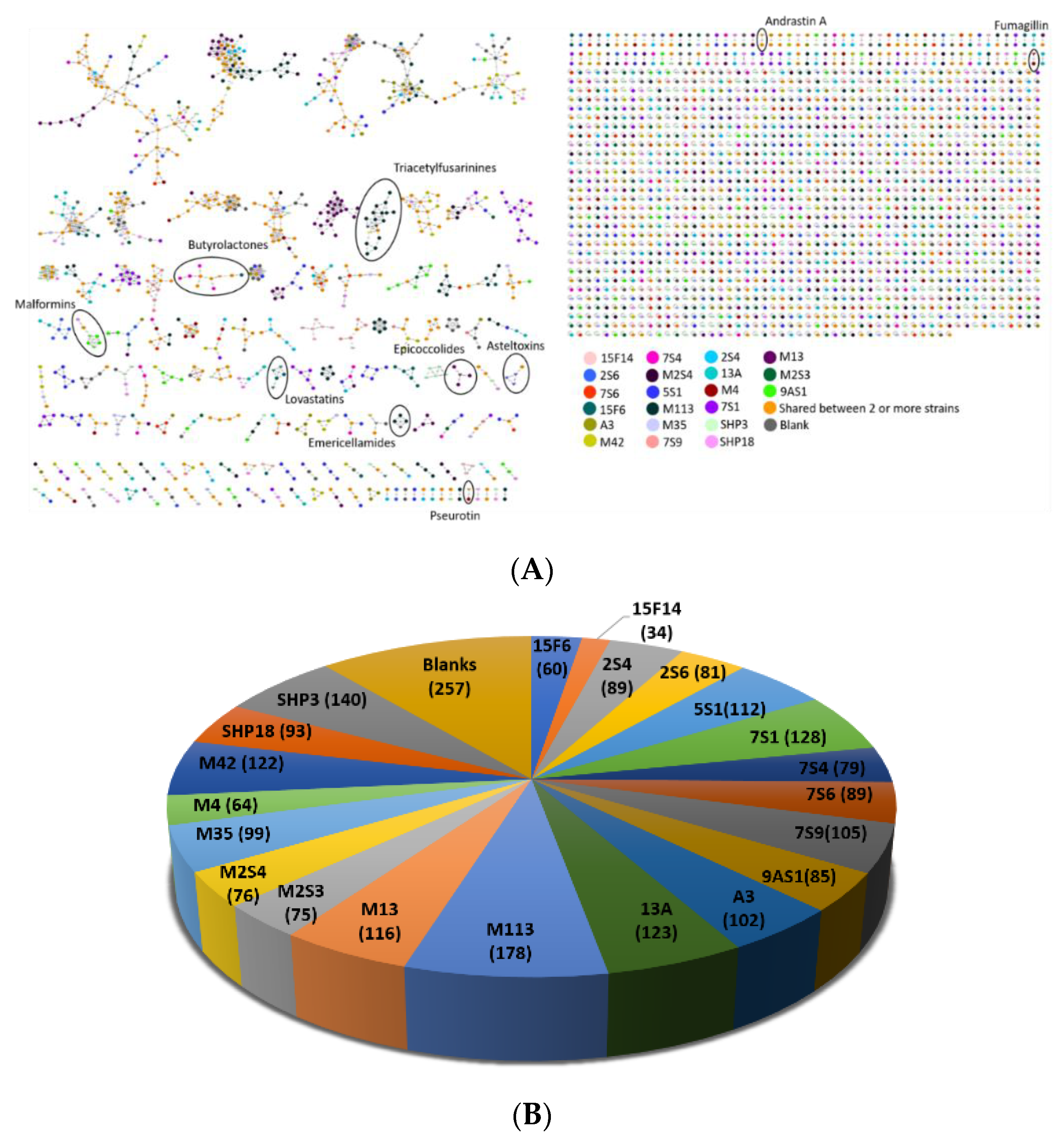
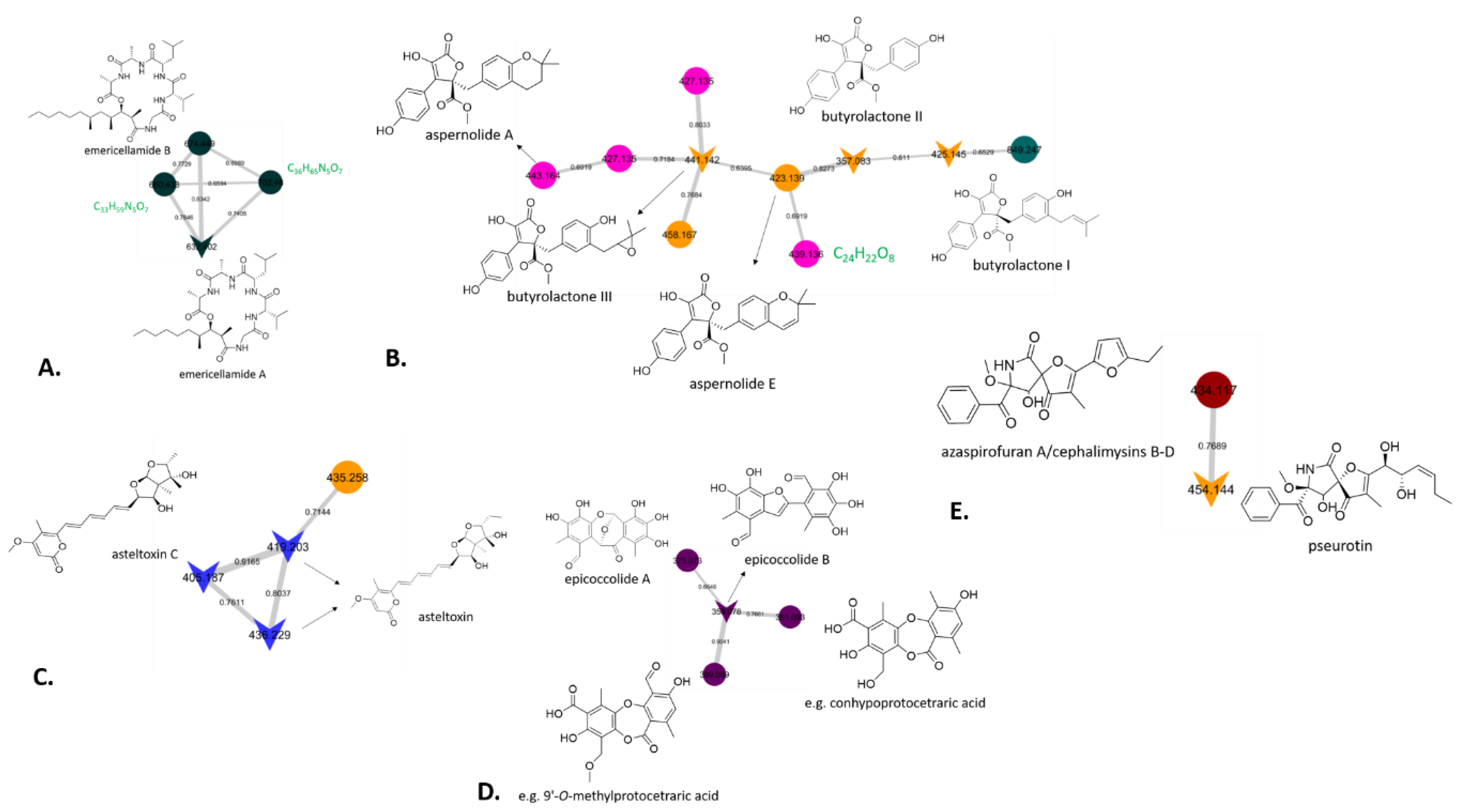
3.8. Chemical Investigation Using a Molecular Network Approach
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aslam, B.; Wang, W.; Arshad, M.I.; Khurshid, M.; Muzammil, S.; Rasool, M.H.; Nisar, M.A.; Alvi, R.F.; Aslam, M.A.; Qamar, M.U.; et al. Antibiotic resistance: A rundown of a global crisis. Infect. Drug Resist. 2018, 11, 1645–1658. [Google Scholar] [CrossRef]
- O’Neil, J. Review on Antibiotic Resisitance. Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations. 2014. Available online: https://amr-review.org/sites/default/files/AMR%20Review%20Paper%20-%20Tackling%20a%20crisis%20for%20the%20health%20and%20wealth%20of%20nations_1.pdf (accessed on 20 September 2020).
- Llor, C.; Bjerrum, L. Antimicrobial resistance: Risk associated with antibiotic overuse and initiatives to reduce the problem. Ther. Adv. drug Saf. 2014, 5, 229–241. [Google Scholar] [CrossRef]
- Salvador, J.A.R.; Moreira, V.M. Natural products action on prostate cancer cell growth, signaling and survival. In Handbook of Prostate Cancer Cell Research: Growth, Signalling, and Survival; Meridith, A.T., Ed.; Nova Science Publishers: New York, NY, USA, 2009; pp. 1–77. [Google Scholar]
- Dzoyem, J.P.; Melong, R.; Tsamo, A.T.; Maffo, T.; Kapche, D.G.W.F.; Ngadjui, B.T.; McGaw, L.J.; Eloff, J.N. Cytotoxicity, antioxidant and antibacterial activity of four compounds produced by an endophytic fungus Epicoccum nigrum associated with Entada abyssinica. Braz. J. Pharmacogn. 2017, 27, 251–253. [Google Scholar] [CrossRef]
- Abdel-Aziz, M.S.; Ghareeb, M.A.; Saad, A.M.; Refahy, L.A.; Hamed, A.A. Chromatographic isolation and structural elucidation of secondary metabolites from the soil-inhabiting fungus Aspergillus fumigatus 3T-EGY. Acta Chromatogr. 2018, 30, 243–249. [Google Scholar] [CrossRef]
- Ghareeb, M.; Hamed, M.; Saad, A.; Abdel-Aziz, M.; Hamed, A.; Refahy, L. Bioactive secondary metabolites from the locally isolated terrestrial fungus, Penicillium sp. SAM16-EGY. Pharmacogn. Res. 2019, 11, 162–170. [Google Scholar] [CrossRef]
- Strobel, G.A. Endophytes as sources of bioactive products. Microbes Infect. 2003, 5, 535–544. [Google Scholar] [CrossRef]
- Rateb, M.E.; Ebel, R. Secondary metabolites of fungi from marine habitats. Nat. Prod. Rep. 2011, 28, 290–344. [Google Scholar] [CrossRef] [PubMed]
- Atiphasaworn, P.; Monggoot, S.; Gentekaki, E.; Brooks, S.; Pripdeevech, P. Antibacterial and Antioxidant Constituents of Extracts of Endophytic Fungi Isolated from Ocimum basilicum var. thyrsiflora Leaves. Curr. Microbiol. 2017, 74, 1185–1193. [Google Scholar] [CrossRef] [PubMed]
- Bhadury, P.; Mohammad, B.T.; Wright, P.C. The current status of natural products from marine fungi and their potential as anti-infective agents. J. Ind. Microbiol. Biotechnol. 2006, 33, 325–337. [Google Scholar] [CrossRef]
- Bugni, T.S.; Ireland, C.M. Marine-derived fungi: A chemically and biologically diverse group of microorganisms. Nat. Prod. Rep. 2004, 21, 143–163. [Google Scholar] [CrossRef] [PubMed]
- Danagoudar, A.; Joshi, C.; Ravi, S.; Rohit Kumar, H.; Ramesh, B. Antioxidant and cytotoxic potential of endophytic fungi isolated from medicinal plant Tragia involucrata L. Pharmacogn. Res. 2018, 10, 188–194. [Google Scholar]
- Uzma, F.; Chowdappa, S. Antimicrobial and antioxidant potential of endophytic fungi isolated from ethnomedicinal plants of Western Ghats, Karnataka. J. Pure Appl. Microbiol. 2017, 11, 1009–1025. [Google Scholar] [CrossRef]
- Yadav, M.; Yadav, A.; Yadav, J.P. In vitro antioxidant activity and total phenolic content of endophytic fungi isolated from Eugenia jambolana Lam. Asian Pac. J. Trop. Med. 2014, 7, S256–S261. [Google Scholar] [CrossRef]
- Wang, M.; Carver, J.J.; Phelan, V.V.; Sanchez, L.M.; Garg, N.; Peng, Y.; Nguyen, D.D.; Watrous, J.; Kapono, C.A.; Luzzatto-Knaan, T.; et al. Sharing and community curation of mass spectrometry data with Global Natural Products Social Molecular Networking. Nat. Biotechnol. 2016, 34, 828–837. [Google Scholar] [CrossRef]
- Ding, C.Y.G.; Pang, L.M.; Liang, Z.X.; Goh, K.K.K.; Glukhov, E.; Gerwick, W.H.; Tan, L.T. MS/MS-Based molecular networking approach for the detection of aplysiatoxin-Related compounds in environmental marine cyanobacteria. Mar. Drugs 2018, 16, 505. [Google Scholar] [CrossRef]
- Yang, J.Y.; Sanchez, L.M.; Rath, C.M.; Liu, X.; Boudreau, P.D.; Bruns, N.; Glukhov, E.; Wodtke, A.; De Felicio, R.; Fenner, A.; et al. Molecular networking as a dereplication strategy. J. Nat. Prod. 2013, 76, 1686–1699. [Google Scholar] [CrossRef]
- Li, F.; Janussen, D.; Peifer, C.; Pérez-Victoria, I.; Tasdemir, D. Targeted isolation of tsitsikammamines from the antarctic deep-sea sponge Latrunculia biformis by molecular networking and anticancer activity. Mar. Drugs 2018, 16, 268. [Google Scholar] [CrossRef]
- de Lima Fávaro, L.C.; de Melo, F.L.; Aguilar-Vildoso, C.I.; Araújo, W.L. Polyphasic Analysis of Intraspecific Diversity in Epicoccum nigrum Warrants Reclassification into Separate Species. PLoS ONE 2011, 6, e14828. [Google Scholar] [CrossRef]
- Henríquez, M.; Vergara, K.; Norambuena, J.; Beiza, A.; Maza, F.; Ubilla, P.; Araya, I.; Chávez, R.; San-Martín, A.; Darias, J.; et al. Diversity of cultivable fungi associated with Antarctic marine sponges and screening for their antimicrobial, antitumoral and antioxidant potential. World J. Microbiol. Biotechnol. 2014, 30, 65–76. [Google Scholar] [CrossRef]
- Visagie, C.M.; Houbraken, J.; Frisvad, J.C.; Hong, S.B.; Klaassen, C.H.W.; Perrone, G.; Seifert, K.A.; Varga, J.; Yaguchi, T.; Samson, R.A. Identification and nomenclature of the genus Penicillium. Stud. Mycol. 2014, 78, 343–371. [Google Scholar] [CrossRef]
- Belbahri, L.; Moralejo, E.; Calmin, G.; Oszako, T.; García, J.A.; Descals, E.; Lefort, F. Phytophthora polonica, a new species isolated from declining Alnus glutinosa stands in Poland. FEMS Microbiol. Lett. 2006, 261, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Felsenstein, J. Evolutionary trees from DNA sequences: A maximum likelihood approach. J. Mol. Evol. 1981, 17, 368–376. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef]
- Hamed, A.A.; Abdel-Aziz, M.S.; Fadel, M.; Ghali, M.F. Antimicrobial, antidermatophytic, and cytotoxic activities from Streptomyces sp. MER4 isolated from Egyptian local environment. Bull. Natl. Res. Cent. 2018, 42, 22. [Google Scholar] [CrossRef]
- Buwa, L.V.; van Staden, J. Antibacterial and antifungal activity of traditional medicinal plants used against venereal diseases in South Africa. J. Ethnopharmacol. 2006, 103, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric Quantitation of Antioxidant Capacity through the Formation of a Phosphomolybdenum Complex: Specific Application to the Determination of Vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Cline, M.S.; Smoot, M.; Cerami, E.; Kuchinsky, A.; Landys, N.; Workman, C.; Christmas, R.; Avila-Campilo, I.; Creech, M.; Gross, B.; et al. Integration of biological networks and gene expression data using cytoscape. Nat. Protoc. 2007, 2, 2366–2382. [Google Scholar] [CrossRef]
- Sobeh, M.; Mahmoud, M.F.; Hasan, R.A.; Abdelfattah, M.A.O.; Sabry, O.M.; Ghareeb, M.A.; El-Shazly, A.M.; Wink, M. Tannin-rich extracts from Lannea stuhlmannii and Lannea humilis (Anacardiaceae) exhibit hepatoprotective activities in vivo via enhancement of the anti-apoptotic protein Bcl-2. Sci. Rep. 2018, 8, 9343. [Google Scholar] [CrossRef]
- Bakchiche, B.; Gherib, A.; Bronze, M.R.; Ghareeb, M.A. Identification, quantification, and antioxidant activity of hydroalcoholic extract of Artemisia campestris from Algeria. Turkish J. Pharm. Sci. 2019, 16, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Prihantini, A.I.; Tachibana, S. Antioxidant compounds produced by Pseudocercospora sp. ESL 02, an endophytic fungus isolated from Elaeocarpus sylvestris. Asian Pac. J. Trop. Biomed. 2017, 7, 110–115. [Google Scholar] [CrossRef]
- Marson Ascêncio, P.G.; Ascêncio, S.D.; Aguiar, A.A.; Fiorini, A.; Pimenta, R.S. Chemical Assessment and Antimicrobial and Antioxidant Activities of Endophytic Fungi Extracts Isolated from Costus spiralis (Jacq.) Roscoe (Costaceae). Evid. Based Complement. Altern. Med. 2014. [Google Scholar] [CrossRef] [PubMed]
- Runeberg, P.A.; Brusentsev, Y.; Rendon, S.M.K.; Eklund, P.C. Oxidative Transformations of Lignans. Molecules 2019, 24, 300. [Google Scholar] [CrossRef]
- Ibrahim, N.A.; Abd El-Ghany, N.A.; Eid, B.M.; Mabrouk, E.M. Green options for imparting antibacterial functionality to cotton fabrics. Int. J. Biol. Macromol. 2018, 111, 526–553. [Google Scholar] [CrossRef]
- Chen, M.; Wang, Y.; Xie, X.; Jiang, H.; Chen, F.; Li, W. Inclusion complex of monochlorotriazine-beta-cyclodextrin and wormwood oil: Preparation, characterization, and finishing on cotton fabric. J. Text. Inst. 2015, 106, 31–38. [Google Scholar] [CrossRef]
- Cusola, O.; Tabary, N.; Belgacem, M.N.; Bras, J. Cyclodextrin functionalization of several cellulosic substrates for prolonged release of antibacterial agents. J. Appl. Polym. Sci. 2013, 129, 604–613. [Google Scholar] [CrossRef]
- Ibrahim, N.A.; El-Zairy, E.M.R.; Eid, B.M. Eco-friendly modification and antibacterial functionalization of viscose fabric. J. Text. Inst. 2017, 108, 1406–1411. [Google Scholar] [CrossRef]
- Ibrahim, N.A.; Abdalla, W.A.; El-Zairy, E.M.R.; Khalil, H.M. Utilization of monochloro-triazine β-cyclodextrin for enhancing printability and functionality of wool. Carbohydr. Polym. 2013, 92, 1520–1529. [Google Scholar] [CrossRef]
- Ibrahim, N.A.; Khalil, H.M.; Eid, B.M.; Tawfik, T.M. Application of MCT-βCD to Modify Cellulose/Wool Blended Fabrics for Upgrading Their Reactive Printability and Antibacterial Functionality. Fibers Polym. 2018, 19, 1655–1662. [Google Scholar] [CrossRef]
- Ibrahim, N.A.; Eid, B.M.; El-Zairy, E.R. Antibacterial functionalization of reactive-cellulosic prints via inclusion of bioactive Neem oil/βCD complex. Carbohydr. Polym. 2011, 86, 1313–1319. [Google Scholar] [CrossRef]
- Ibrahim, N.A.; El-Zairy, E.M.R. Union disperse printing and UV-protecting of wool/polyester blend using a reactive β-cyclodextrin. Carbohydr. Polym. 2009, 76, 244–249. [Google Scholar] [CrossRef]
- Ibrahim, N.A.; E-Zairy, W.R.; Eid, B.M. Novel approach for improving disperse dyeing and UV-protective function of cotton-containing fabrics using MCT-β-CD. Carbohydr. Polym. 2010, 79, 839–846. [Google Scholar] [CrossRef]
- Van Santen, J.A.; Jacob, G.; Singh, A.L.; Aniebok, V.; Balunas, M.J.; Bunsko, D.; Neto, F.C.; Castaño-Espriu, L.; Chang, C.; Clark, T.N.; et al. The Natural Products Atlas: An Open Access Knowledge Base for Microbial Natural Products Discovery. ACS Cent. Sci. 2019, 5, 1824–1833. [Google Scholar] [CrossRef] [PubMed]
- Laatsch, H. AntiBase: The Natural Compound Identifier; Wiley-Vch: Weinheim, Germany, 2017. [Google Scholar]
- Oh, D.C.; Kauffman, C.A.; Jensen, P.R.; Fenical, W. Induced production of emericellamides A and B from the marine-derived fungus Emericella sp. in competing co-culture. J. Nat. Prod. 2007, 70, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Elbanna, A.H.; Khalil, Z.G.; Bernhardt, P.V.; Capon, R.J. Scopularides Revisited: Molecular Networking Guided Exploration of Lipodepsipeptides in Australian Marine Fish Gastrointestinal Tract-Derived Fungi. Mar. Drugs 2019, 17, 475. [Google Scholar] [CrossRef]
- Cazar, M.E.; Schmeda-Hirschmann, G.; Astudillo, L. Antimicrobial butyrolactone I derivatives from the Ecuadorian soil fungus Aspergillus terreus Thorn. var terreus. World J. Microbiol. Biotechnol. 2005, 21, 1067–1075. [Google Scholar] [CrossRef]
- Chen, M.; Wang, K.L.; Liu, M.; She, Z.G.; Wang, C.Y. Bioactive Steroid Derivatives and Butyrolactone Derivatives from a Gorgonian-Derived Aspergillus sp. Chem. Biodivers. 2015, 12, 1398–1406. [Google Scholar] [CrossRef]
- Parvatkar, R.R.; D’Souza, C.; Tripathi, A.; Naik, C.G. Aspernolides A and B, butenolides from a marine-derived fungus Aspergillus terreus. Phytochemistry 2009, 70, 128–132. [Google Scholar] [CrossRef]
- He, F.; Bao, J.; Zhang, X.-Y.; Tu, Z.-C.; Shi, Y.-M.; Qi, S.-H. Asperterrestide A, a Cytotoxic Cyclic Tetrapeptide from the Marine-Derived Fungus Aspergillus terreus SCSGAF0162. J. Nat. Prod. 2013, 76, 1182–1186. [Google Scholar] [CrossRef]
- Kruger, G.J.; Steyn, P.S.; Vleggaar, R.; Rabie, C.J. X-Ray crystal structure of asteltoxin, a novel mycotoxin from Aspergillus stellatus curzi. J. Chem. Soc. Chem. Commun. 1979, 441–442. [Google Scholar] [CrossRef]
- Bao, J.; Zhang, X.Y.; Xu, X.Y.; He, F.; Nong, X.H.; Qi, S.H. New cyclic tetrapeptides and asteltoxins from gorgonian-derived fungus Aspergillus sp. SCSGAF 0076. Tetrahedron 2013, 69, 2113–2117. [Google Scholar] [CrossRef]
- Adachi, H.; Doi, H.; Kasahara, Y.; Sawa, R.; Nakajima, K.; Kubota, Y.; Hosokawa, N.; Tateishi, K.; Nomoto, A. Asteltoxins from the Entomopathogenic Fungus Pochonia bulbillosa 8-H-28. J. Nat. Prod. 2015, 78, 1730–1734. [Google Scholar] [CrossRef] [PubMed]
- Talontsi, F.M.; Dittrich, B.; Schüffler, A.; Sun, H.; Laatsch, H. Epicoccolides: Antimicrobial and antifungal polyketides from an endophytic fungus Epicoccum sp. associated with Theobroma cacao. European J. Org. Chem. 2013, 3174–3180. [Google Scholar] [CrossRef]
- Elix, J.A.; Lumbsch, H.T.; Wardlaw, J.H. Conhypoprotocetraric Acid, a New Lichen β-Orcinol Depsidone. Aust. J. Chem. 1995, 48, 1479–1483. [Google Scholar] [CrossRef]
- Bézivin, C.; Tomasi, S.; Rouaud, I.; Delcros, J.G.; Boustie, J. Cytotoxic activity of compounds from the lichen: Cladonia convoluta. Planta Med. 2004, 70, 874–877. [Google Scholar] [CrossRef]
- Bloch, P.; Tamm, C.; Bollinger, P.; Petcher, T.J.; Weber, H.P. Pseurotin, a New Metabolite of Pseudeurotium ovalis STOLK Having an Unusual Hetero-Spirocyclic System (Preliminary Communication). Helv. Chim. Acta 1976, 59, 133–137. [Google Scholar] [CrossRef]
- Ren, H.; Liu, R.; Chen, L.; Zhu, T.; Zhu, W.M.; Gu, Q.Q. Two new hetero-spirocyclic γ -lactam derivatives from marine sediment-derived fungus Aspergillus sydowi D2-6. Arch. Pharm. Res. 2010, 33, 499. [Google Scholar] [CrossRef]
- Yamada, T.; Kitada, H.; Kajimoto, T.; Numata, A.; Tanaka, R. The relationship between the CD cotton effect and the absolute configuration of FD-838 and its seven stereoisomers. J. Org. Chem. 2010, 75, 4146–4153. [Google Scholar] [CrossRef]
- Borkow, G.; Gabbay, J. Biocidal textiles can help fight nosocomial infections. Med. Hypotheses 2008, 70, 990–994. [Google Scholar] [CrossRef]
- Openshaw, J.J.; Morris, W.M.; Lowry, G.V.; Nazmi, A. Reduction in bacterial contamination of hospital textiles by a novel silver-based laundry treatment. Am. J. Infect. Control 2016, 44, 1705–1708. [Google Scholar] [CrossRef] [PubMed][Green Version]
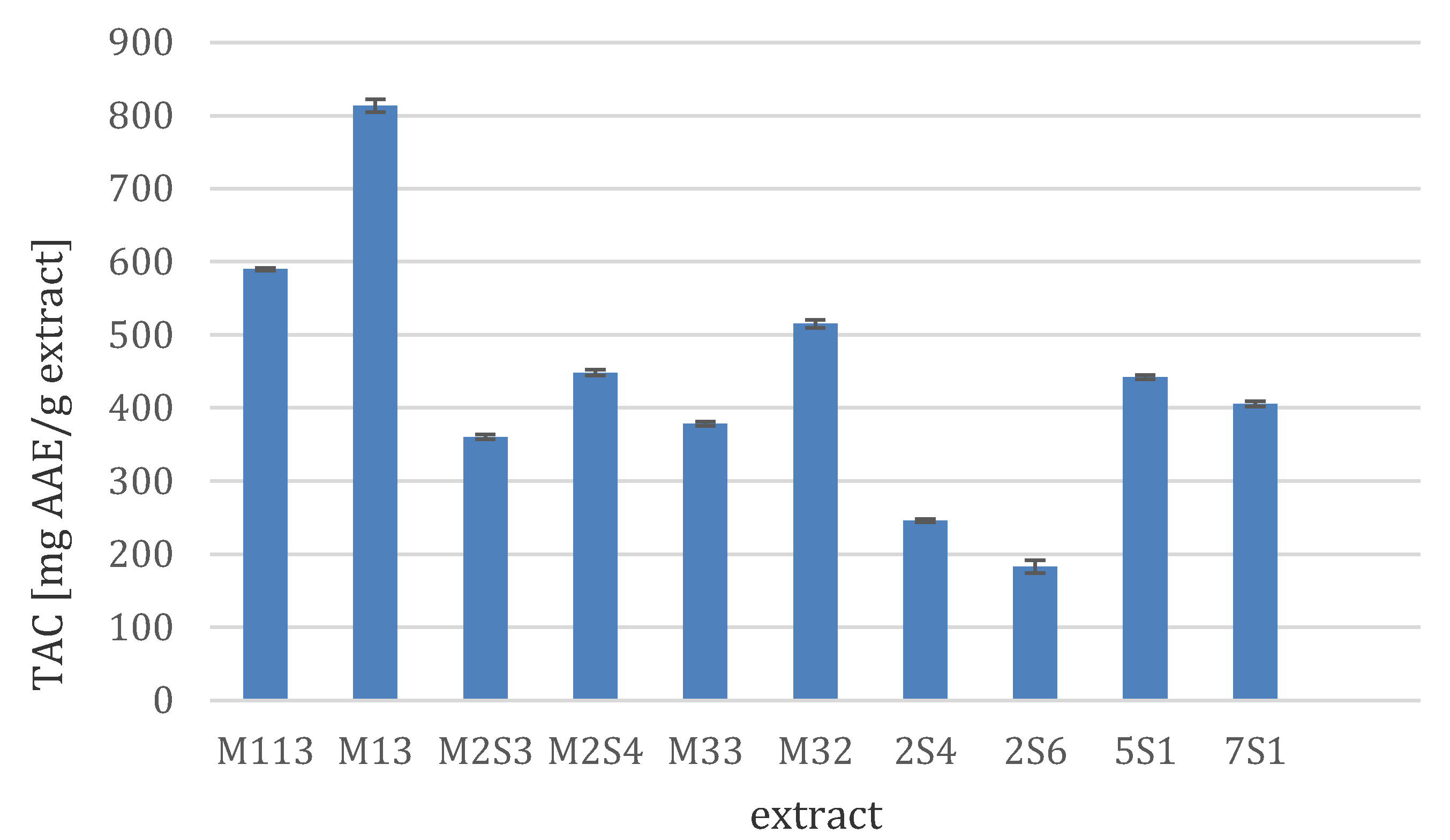
| Strain ID | Target Gene and GenBank Accession Number | ID Assigned in GenBank | Sample Origin | Type | Location | |||
|---|---|---|---|---|---|---|---|---|
| 18S | ITS | β-Tubulin | Calmodulin | |||||
| A3 | - | MN114540 | MT184332 | MT184352 | Aspergillus terreus strain A3 | Crella cyathophora | sponge | Makady Bay South, Hurghada |
| M113 | - | MK262919 | MT184334 | MT184354 | Aspergillus calidoustus strain M113 | Thalassia hemprichii | sea grass | Makady Bay South, Hurghada |
| M13 | - | MK953943 | MT184348 | - | Epicoccum nigrum strain FAS-14 | Thalassia hemprichii | sea grass | Makady Bay South, Hurghada |
| M2S3 | MN328309 | MT152324 | MT184340 | MT184360 | Byssochlamis spectabilis strain M2S3 | Crella cyathophora | sponge | Makady Bay South, Hurghada |
| M2S4 | MN328341 | MT152319 | MT184335 | MT184355 | Aspergillus niger strain M2S4 | Crella cyathophora | sponge | Makady Bay South, Hurghada |
| M35 | - | MK953944 | MT184347 | MT184367 | Penicillium crustosum strain FAS-28 | Siphonochalina siphonella | sponge | Makady Bay South, Hurghada |
| M4 | - | MK953942 | MT184345 | MT184365 | Talaromyces verruculosus strain FAS-10 | Latrunculia magnifica | sponge | Makady Bay South, Hurghada |
| M42 | MN328356 | MT152318 | MT184331 | MT184351 | Aspergillus terreus strain M42 | Latrunculia magnifica | sponge | Makady Bay South, Hurghada |
| SHP3 | - | MN114156 | MT184343 | MT184363 | Cladosporium spinulosum strain SHP3 | Thalassia hemprichii | sea grass | Makady Bay South, Hurghada |
| SHP18 | - | MN114621 | MT184342 | MT184362 | Cladosporium spinulosum strain SHP18 | Thalassia hemprichii | sea grass | Makady Bay South, Hurghada |
| 13A | - | MK248606 | - | - | Alternaria alternata strain 13A | Phragmites australis | plant | Lake El-Bida, Wadi El-Natrun |
| 15F6 | - | MN328763 | MT184330 | MT184350 | Aspergillus terreus strain 15F6 | Hyoscyamus muticus | plant | Lake El-Bida, Wadi El-Natrun |
| 15F14 | MN328048 | MT152320 | MT184336 | MT184356 | Aspergillus fumigatus strain 15F14 | Hyoscyamus muticus | plant | Lake El-Bida, Wadi El-Natrun |
| 2S4 | - | MN115554 | MT184341 | MT184361 | Alternaria alternata strain 2S4 | Juncus acutus | plant | Lake El-Hamra, Wadi El-Natrun |
| 2S6 | - | MN330611 | MT184338 | MT184358 | Aspergillus fumigatus strain 2S6 | Juncus acutus | plant | Lake El-Hamra Wadi El-Natrun |
| 5S1 | - | MN110110 | MT184339 | MT184359 | Aspergillus ochraceopetaliformis strain 5S1 | Panicum turgidum | plant | Lake El-Hamra, Wadi El-Natrun |
| 7S1 | - | MK953941 | MT184346 | MT184366 | Talaromyces verruculosus strain FAS-02 | Tamarix nilotica | plant | Lake El-Hamra, Wadi El-Natrun |
| 7S4 | - | MN114521 | MT184333 | MT184353 | Aspergillus terreus strain 7S4 | Tamarix nilotica | plant | Lake El-Hamra, Wadi El-Natrun |
| 7S6 | MN326853 | MT152321 | MT184337 | MT184357 | Aspergillus fumigatus strain 7S6 | Tamarix nilotica | plant | Lake El-Hamra, Wadi El-Natrun |
| 7S9 | - | MN114217 | MT184344 | MT184364 | Penicillium rubens strain 7S9 | Tamarix nilotica | plant | Lake El-Hamra, Wadi El-Natrun |
| 9AS1 | MN327968 | MT152323 | MT184349 | MT184368 | Sarocladium kiliense strain 9AS1 | Panicum turgidum | plant | Lake El-Hamra, Wadi El-Natrun |
| Sample Code | Staphylococcus aureus MIC (µg/mL) * | Pseudomonas aeruginosa MIC (µg/mL) * | Candida albicans MIC (µg/mL) * | Aspergillus niger MIC (µg/mL) * |
|---|---|---|---|---|
| M1 ° | 250 | 250 | 250 | - |
| M113 | 25.0 ± 1.7 | 22.3 ± 1.5 | 52.3 ± 0.6 | 192.7 ± 3.8 |
| M13 | 20.0 ± 2.0 | 42.0 ± 1.0 | 111.0 ± 1.7 | 122.7 ± 4.6 |
| M23 ° | 250 | 250 | 250 | - |
| M2S3 | 100.3 ± 4.0 | 195.0 ± 6.2 | 177.3 ± 4.5 | - |
| M2S4 | 115.0 ± 2.6 | 102.7 ± 3.8 | 163.3 ± 2.3 | - |
| M32 ° | 250 | 250 | 250 | - |
| M35 | 85.0 ± 2.6 | 101.0 ± 1.7 | 180.7 ± 5.0 | 237.7 ± 2.1 |
| M4 | 172.3 ± 4.0 | 185.0 ± 3.6 | 158.7 ± 4.7 | 210.3 ± 4.2 |
| M42 | 43.0 ± 1.0 | 86.0 ± 2.6 | 92.7 ± 3.8 | - |
| SHP3 ° | - | - | 35.0 ± 1.7 | - |
| SHP18 | 105.0 ± 5.6 | 123.7 ± 0.6 | 203.0 ± 2.6 | - |
| 13A | 45.0 ± 1.0 | 42.0 ± 1.0 | 102.7 ± 3.1 | - |
| 2S4 | 48.0 ± 2.6 | 47.3 ± 3.2 | 87.7 ± 3.8 | 85.0 ± 4.4 |
| 2S6 | 97.7 ± 2.1 | 103.7 ± 2.9 | 209.0 ± 2.0 | - |
| 5S1 | 50.0 ± 2.6 | 46.3 ± 3.5 | 85.3 ± 3.2 | 125.0 ± 0.0 |
| 7S1 | 54.0 ± 1.7 | 51.0 ± 2.0 | 102.7 ± 2.9 | 183.7 ± 5.0 |
| 7S4 | 12.3 ± 1.5 | 13.7 ± 1.2 | 52.3 ± 1.5 | - |
| 7S5 ° | 31.25 | 62.5 | 125 | - |
| 7S6 | 113.7 ± 0.6 | 55.7 ± 1.2 | 175.0 ± 2.6 | - |
| 7S9 | 47.3 ± 3.2 | 41.0 ± 2.6 | 171.0 ± 1.5 | - |
| 9AS1 | 117.7 ± 4.2 | 43.7 ± 3.1 | - | - |
| Cip | 0.078 | 0.156 | - | - |
| Nys | - | - | 5 | 10 |
| Extract (Concentration Tested) | Fabric Type a | Zone of Inhibition (mm) | |||
|---|---|---|---|---|---|
| S. aureus | E. coli | C. albicans | A. niger | ||
| M113 (55 µg/mL) | 1 | 16 | - | 17 | 15 |
| 2 | 18 | - | 18 | 15 | |
| 3 | 19 | - | 19 | 17 | |
| 7S4 (70 µg/mL) | 1 | 13 | - | 14 | 11 |
| 2 | 14 | - | 15 | 12 | |
| 3 | 16 | - | 16 | 14 | |
| 13A (62 µg/mL) | 1 | 12 | 7 | 11 | 10 |
| 2 | 13 | 8 | 12 | 11 | |
| 3 | 14 | 9 | 13 | 12 | |
| modified | 1 | - | - | - | - |
| 2 | - | - | - | - | |
| 3 | - | - | - | - | |
| untreated | 1 | - | - | - | - |
| 2 | - | - | - | - | |
| 3 | - | - | - | - | |
| Extract (Concentration Tested) | Fabric Type a | UPF | Classification |
|---|---|---|---|
| M113 (55 µg/mL) | 1 | 70.8 | excellent |
| 2 | 162.2 | excellent | |
| 3 | 30.7 | Very good | |
| 7S4 (70 µg/mL) | 1 | 33.0 | very good |
| 2 | 77.6 | excellent | |
| 3 | 26.9 | very good | |
| 13A (62 µg/mL) | 1 | 113.9 | excellent |
| 2 | 202.8 | excellent | |
| 3 | 38.3 | very good | |
| modified | 1 | 10.5 | no protection |
| 2 | 15.8 | good | |
| 3 | 8.0 | no protection | |
| untreated | 1 | 7.0 | no protection |
| 2 | 11.2 | no protection | |
| 3 | 5.0 | no protection |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamed, A.A.; Soldatou, S.; Qader, M.M.; Arjunan, S.; Miranda, K.J.; Casolari, F.; Pavesi, C.; Diyaolu, O.A.; Thissera, B.; Eshelli, M.; et al. Screening Fungal Endophytes Derived from Under-Explored Egyptian Marine Habitats for Antimicrobial and Antioxidant Properties in Factionalised Textiles. Microorganisms 2020, 8, 1617. https://doi.org/10.3390/microorganisms8101617
Hamed AA, Soldatou S, Qader MM, Arjunan S, Miranda KJ, Casolari F, Pavesi C, Diyaolu OA, Thissera B, Eshelli M, et al. Screening Fungal Endophytes Derived from Under-Explored Egyptian Marine Habitats for Antimicrobial and Antioxidant Properties in Factionalised Textiles. Microorganisms. 2020; 8(10):1617. https://doi.org/10.3390/microorganisms8101617
Chicago/Turabian StyleHamed, Ahmed A., Sylvia Soldatou, M. Mallique Qader, Subha Arjunan, Kevin Jace Miranda, Federica Casolari, Coralie Pavesi, Oluwatofunmilay A. Diyaolu, Bathini Thissera, Manal Eshelli, and et al. 2020. "Screening Fungal Endophytes Derived from Under-Explored Egyptian Marine Habitats for Antimicrobial and Antioxidant Properties in Factionalised Textiles" Microorganisms 8, no. 10: 1617. https://doi.org/10.3390/microorganisms8101617
APA StyleHamed, A. A., Soldatou, S., Qader, M. M., Arjunan, S., Miranda, K. J., Casolari, F., Pavesi, C., Diyaolu, O. A., Thissera, B., Eshelli, M., Belbahri, L., Luptakova, L., Ibrahim, N. A., Abdel-Aziz, M. S., Eid, B. M., Ghareeb, M. A., Rateb, M. E., & Ebel, R. (2020). Screening Fungal Endophytes Derived from Under-Explored Egyptian Marine Habitats for Antimicrobial and Antioxidant Properties in Factionalised Textiles. Microorganisms, 8(10), 1617. https://doi.org/10.3390/microorganisms8101617








