Protective Effects of Tropical Fruit Processing Coproducts on Probiotic Lactobacillus Strains during Freeze-Drying and Storage
Abstract
1. Introduction
2. Material and Methods
2.1. Preparation of Fruit Processing Coproducts
2.2. Physicochemical Characterization of Fruit Processing Coproducts
2.3. Evaluation of the Protective Effects of Fruit Processing Coproducts on Freeze-Dried Probiotic Lactobacillus Strains
2.3.1. Microorganisms, Inoculum Preparation, and Treatments
2.3.2. Freeze-Drying and Survival of Probiotic Lactobacillus
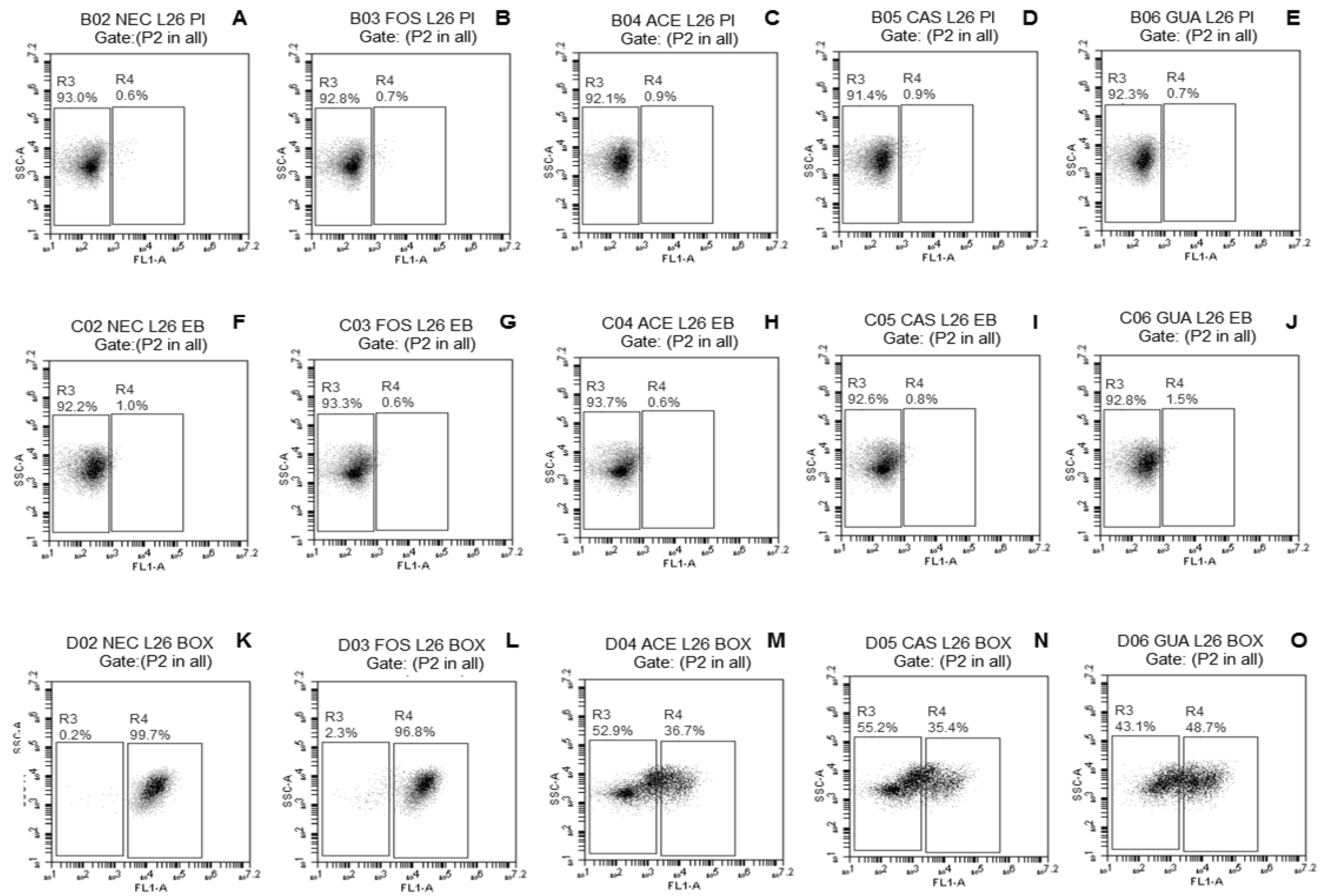
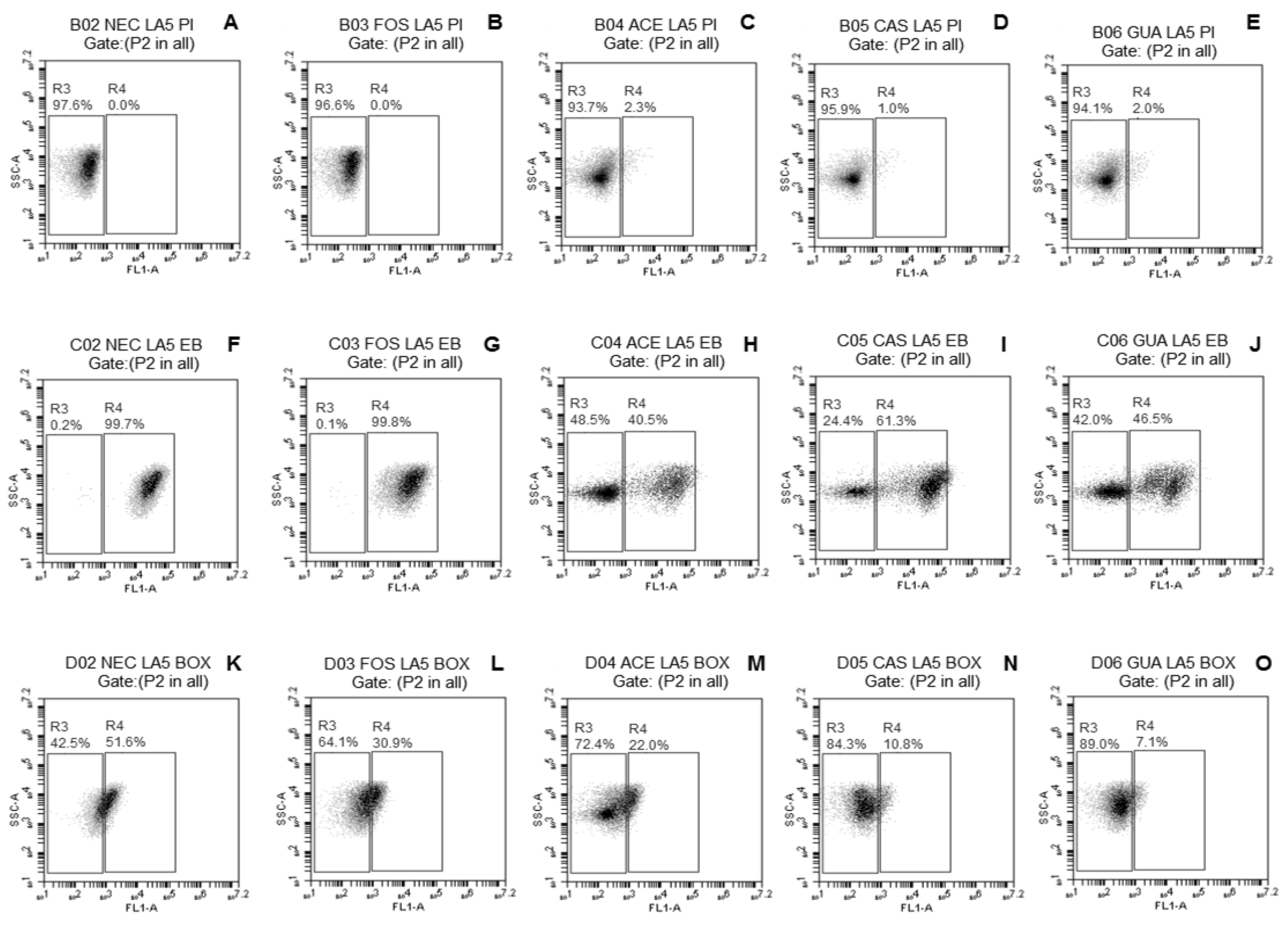
2.3.3. Evaluation of Damage to Membrane Functions of Probiotic Lactobacillus Cells after Freeze-Drying
2.3.4. Enumeration of Viable Cells of Freeze-Dried Probiotic Lactobacillus during Storage
2.4. Statistical Analysis
3. Results and Discussion
3.1. Viable Counts of Probiotic Lactobacillus before and after Freeze-Drying
3.2. Evaluation of Damage to Membrane Functions of Probiotic Lactobacillus Cells after Freeze-Drying
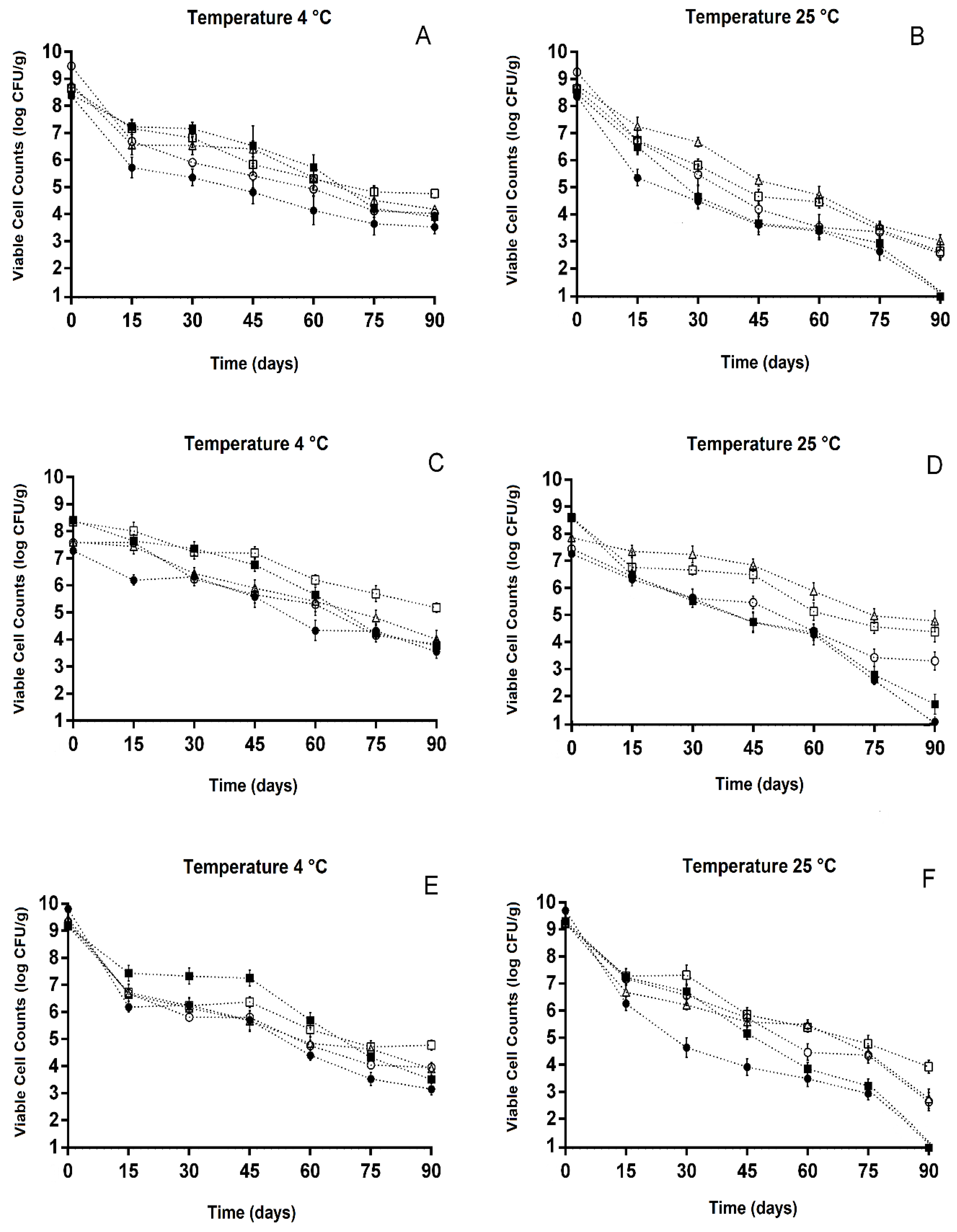
3.3. Viable Counts of Freeze-Dried Lactobacillus during Storage
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gonzalez-Aguilar, G.; Villa-Rodriguez, J.; Ayala-Zavala, J.; Yahia, E. Improvement of the antioxidant status of tropical fruits as a secondary response to some postharvest treatments. Trends Food Sci. Technol. 2010, 21, 475–482. [Google Scholar] [CrossRef]
- Silva, L.M.R.; Figueiredo, E.A.T.; Ricardo, N.M.P.S.; Vieira, I.G.P.; Figueiredo, R.W.; Brasil, I.M.; Gomes, C.L. Quantification of bioactive compounds in pulps and by-products of tropical fruits from Brazil. Food Chem. 2014, 143, 398–404. [Google Scholar] [CrossRef]
- Infante, J.; Selani, M.M.; Toledo, N.M.V.; Silveira-Diniz, M.F.; Alencar, S.M.; Spoto, M.H.F. Antioxidant activity of agroindustrial residues from tropical fruits. Braz. J. Food Nutr. 2013, 24, 87–91. [Google Scholar]
- Nóbrega, E.M.; Oliveira, E.L.; Genovese, M.I.; Correia, R.T.P. The Impact of Hot Air Drying on the Physical-Chemical Characteristics, Bioactive Compounds and Antioxidant Activity of Acerola (Malphigia emarginata) Residue. J. Food Process. Preserv. 2015, 39, 131–141. [Google Scholar] [CrossRef]
- Duarte, F.N.D.; Rodrigues, J.B.; Lima, M.C.; Lima, M.S.; Pacheco, M.T.B.; Pintado, M.M.E.; Aquino, J.S.; Souza, E.L. Potential prebiotic properties of cashew apple (Anacardium occidentale L.) agro-industrial byproduct on Lactobacillus sp. J. Sci. Food Agric. 2017, 97, 3712–3719. [Google Scholar] [CrossRef]
- Batista, K.S.; Alves, A.F.; Lima, M.D.S.; Silva, L.A.; Lins, P.P.; Gomes, J.A.S.; Silva, A.S.; Toscano, L.T.; Meireles, B.R.L.A.; Cordeiro, A.M.T.M.; et al. Beneficial effects of consumption of acerola, cashew or guava processing by-products on intestinal health and lipid metabolism in dyslipidaemic female Wistar rats. Br. J. Nutr. 2018, 119, 30–41. [Google Scholar] [CrossRef]
- Sun, Z.; Harris, H.M.B.; McCann, A.; Guo, C.; Argimón, S.; Zhang, W.; Yang, X.; Jeffery, I.B.; Cooney, J.C.; Kagawa, T.F.; et al. Expanding the biotechnology potential of lactobacilli through comparative genomics of 213 strains and associated genera. Nat. Commun. 2015, 6, 8322. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morrellj, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Garcia, E.F.; Araújo, A.O.; Luciano, W.A.; Albuquerque, T.M.R.; Arcanjo, N.M.O.; Madruga, M.S.; Lima, M.S.; Saarela, M.; Souza, E.L. The performance of five fruit-derived and freeze-dried potentially probiotic Lactobacillus strains in apple, orange, and grape juices. J. Sci. Food Agric. 2018, 98, 5000–5010. [Google Scholar] [CrossRef]
- Tymczyszyn, E.E.; Díaz, M.R.; Pataro, A.; Sandonato, N.; Gómez-Zavaglia, A.; Disalvo, E.A. Critical water activity for the preservation of Lactobacillus bulgaricus by vacuum drying. Int. J. Food Microbiol. 2008, 128, 342–347. [Google Scholar] [CrossRef]
- Velly, H.; Bouix, M.; Passot, S.; Penicaud, C.; Beinsteiner, H.; Ghorbal, S.; Lieben, P.; Fonseca, F. Cyclopropanation of unsaturated fatty acids and membrane rigidification improve the freeze-drying resistance of Lactococcus lactis subsp. Lactis TOMSC161. Appl. Microbiol. Biotechnol. 2015, 99, 907–918. [Google Scholar] [CrossRef]
- Nunes, G.L.; Etchepare, M.A.; Cichoski, A.J.; Zepka, L.Q.; Lopes, E.J.; Barin, J.S.; Flores, E.M.M.; Silva, C.B.; Menezes, C.R. Inulin, hi-maize, and trehalose as thermal protectants for increasing viability of Lactobacillus acidophilus encapsulated by spray drying. LWT-Food Sci. Technol. 2018, 89, 128–133. [Google Scholar] [CrossRef]
- Romano, N.; Schebor, C.; Mobili, P.; Gómez-Zavaglia, A. Role of mono- and oligosaccharides from FOS as stabilizing agents during freeze-drying and storage of Lactobacillus delbrueckii subsp. bulgaricus. Food Res. Int. 2016, 90, 251–258. [Google Scholar] [CrossRef]
- Tymczyszyn, E.E.; Gerbino, E.; Illanes, A.; Gomez-Zavaglia, A. Galacto-oligosaccharides as protective molecules in the preservation of Lactobacillus delbrueckii subsp. bulgaricus. Cryobiology 2011, 62, 123–129. [Google Scholar] [CrossRef]
- Ayala-Zavala, J.F.; Vega-Vega, V.; Rosas-Dominguez, C.; Palafox-Carlos, H.; Villa-Rodriguez, J.A.; Siddiqui, M. Agro-industrial potential of exotic fruit byproducts as a source of food additives. Food Res. Int. 2011, 44, 1866–1874. [Google Scholar] [CrossRef]
- Leslie, S.B.; Israeli, E.; Lighthart, B.; Crowe, J.H.; Crowe, L.M. Trehalose and sucrose protect both membranes and proteins in intact bacteria during drying. Appl. Environ. Microbiol. 1995, 61, 3592–3597. [Google Scholar] [CrossRef]
- Teixeira, P.; Castro, H.; Kirby, R. Evidence of membrane lipid oxidation of spray-dried Lactobacillus bulgaricus during storage. Lett. Appl. Microbiol. 1996, 22, 34–38. [Google Scholar] [CrossRef]
- Liu, X.; Yan, X.; Bi, J.; Liu, J.; Zhou, M.; Wu, X.; Chen, Q. Determination of phenolic compounds and antioxidant activities from peel, flesh and seed of guava (Psidium guajava L.). Electrophoresis 2018, 39, 1654–1662. [Google Scholar] [CrossRef]
- Quintana, G.; Gerbino, E.; Gómez-Zavaglia, A. Okara: A nutritionally valuable by-product able to stabilize Lactobacillus plantarum during freeze-drying, spray-drying, and storage. Front. Microbiol. 2017, 8, 641. [Google Scholar] [CrossRef]
- Ball, S.; Bullock, S.; Lloyd, L.; Mapp, K.P.; Ewen, A. Analysis of carbohydrates, alcohols, and organic acids by ion-exchange chromatography. In Agilent Hi-Plex Columns Applications Compendium; Agilent Technologies Inc.: Santa Clara, CA, USA, 2011; pp. 1–98. [Google Scholar]
- Padilha, C.V.S.; Miskinis, G.A.; Souza, M.E.A.O.; Pereira, G.E.; Oliveira, D.; Bordignon-Luiz, M.T.; Lima, M.S. Rapid determination of flavonoids and phenolic acids in grape juices and wines by RP-HPLC/DAD: Method validation and characterization of commercial products of the new Brazilian varieties of grape. Food Chem. 2017, 228, 106–115. [Google Scholar] [CrossRef]
- Horwitz, W.; Chichilo, P.; Reynolds, H. (Eds.) Official Methods of Analysis of the Association of Official Analytical Chemists, 18th ed.; 2005. Current Through Revision 3, 2010, met. 985.29; AOAC: Gaithersburg, MD, USA, 2010; Chapter 45; pp. 101–102. [Google Scholar]
- Liu, M.; Li, X.Q.; Weber, C.; Lee, C.Y.; Brown, J.; Liu, R.H. Antioxidant and antiproliferative activities of raspberries. J. Agric. Food Chem. 2002, 50, 2926–2930. [Google Scholar] [CrossRef]
- Sousa, M.S.B.; Vieira, L.M. Total phenolics and in vitro antioxidant capacity of tropical fruit pulp wastes. Braz. J. Food Technol. 2011, 14, 202–210. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Rockenbach, I.I.; Rodrigues, E.; Gonzaga, L.V.; Caliari, V.; Genovese, M.I.; Gonçalves, A.E.S.S.; Fett, R. Phenolic compounds content and antioxidant activity in pomace from selected red grapes (Vitis vinifera L. and Vitis labrusca L.) widely produced in Brazil. Food Chem. 2011, 127, 174–179. [Google Scholar] [CrossRef]
- Sariburun, E.; Sahin, S.; Demir, C.; Turkben, C.; Uylaser, V. Phenolic content and antioxidant activity of raspberry cultivars. J. Food Sci. 2010, 75, 328–335. [Google Scholar] [CrossRef]
- Sousa, S.; Pinto, J.; Pereira, C.; Malcata, F.X.; Pacheco, M.T.B.; Gomes, A.M.; Pintado, M. In vitro evaluation of yacon (Smallanthus sonchifolius) tuber flour prebiotic potential. Food Bioprod. Process. 2015, 95, 96–105. [Google Scholar] [CrossRef]
- Herigstad, B.; Hamilton, M.; Heersink, J. How to optimize the drop plate method for enumerating bacteria. J. Microbiol. Methods 2001, 44, 121–129. [Google Scholar] [CrossRef]
- Carrillo, M.G.; Ferrario, M.; Guerrero, S. Effectiveness of UV-C light assisted by mild heat on Saccharomyces cerevisiae KE 162 inactivation in carrot-orange juice blend studied by flow cytometry and transmission electron microscopy. Food Microbiol. 2018, 73, 1–10. [Google Scholar] [CrossRef]
- Kim, D.K.; Kim, S.J.; Kang, D.H. Bactericidal effect of 266 to 279 nm wavelength UVC-LEDs for inactivation of Gram positive and Gram negative foodborne pathogenic bacteria and yeasts. Food Res. Int. 2017, 97, 280–287. [Google Scholar] [CrossRef]
- Silva, F.; Ferreira, S.; Queiroz, J.A.; Domingues, F.C. Coriander (Coriandrum sativum L.) essential oil: Its antibacterial activity and mode of action evaluated by flow cytometry. J. Med. Microbiol. 2011, 60, 1479–1486. [Google Scholar] [CrossRef]
- Crowe, J.H.; Carpenter, J.F.; Crowe, L.M. The role of vitrification in anhydrobiosis. Annu. Rev. Physiol. 1998, 60, 73–103. [Google Scholar] [CrossRef]
- Gonzalez-Aguilar, G.; Robles-Sanchez, R.; Martinez-Tellez, M.; Olivas, G.; Alvarez-Parrilla, E.; de la Rosa, L. Bioactive compounds in fruits: Health benefits and effect of storage conditions. Postharvest Stewart Rev. 2008, 4, 1–10. [Google Scholar]
- Champagne, C.P.; Møllgaard, H. Production of Probiotic Cultures and Their Addition in Fermented Foods. In Handbook of Fermented Functional Foods, 2nd ed.; Farnworth, E.R., Ed.; CRC Press: Boca Raton, FL, USA, 2008; pp. 513–532. [Google Scholar]
- Champagne, C.P.; Mondou, F.; Raymond, Y.; Roy, D. Effect of polymers and storage temperature on the stability of freeze-dried lactic acid bacteria. Food Res. Int. 1996, 29, 555–562. [Google Scholar] [CrossRef]

| Parameters | Fruit Processing Coproducts | ||
|---|---|---|---|
| Acerola (ACE) | Cashew (CAS) | Guava (GUA) | |
| Simple sugars (g/100 g) | |||
| Fructose | 8.48 ± 0.01 a | 4.80 ± 0.01 b | 3.92 ± 0.01 c |
| Glucose | 5.31 ± 0.01 a | 4.88 ± 0.01 b | 3.17 ± 0.01 c |
| Maltose | 1.52 ± 0.01 b | 1.97 ± 0.01 a | 1.53 ± 0.01 b |
| Dietary fiber (g/100 g) | |||
| Insoluble dietary fiber | 61.16 ± 1.75 a | 47.49 ± 2.26 b | 49.12 ± 1.58 b |
| Soluble dietary fiber | 8.09 ± 0.69 b | 1.74 ± 0.53 c | 33.44 ± 3.63 a |
| Total dietary fiber | 69.25 ± 1.06 b | 49.22 ± 1.73 c | 82.55 ± 2.05 a |
| Phenolic compounds (mg/100 g) | |||
| Flavanols | |||
| Catechin | 3.12 ± 0.00 | ND | 1.95 ± 0.02 |
| Flavanones | |||
| Hesperetin | 1.43 ± 0.01 b | 1.25 ± 0.01 c | 1.61 ± 0.01 a |
| Naringenin | 1.37 ± 0.01 a | 0.42 ± 0.01 b | 0.31 ± 0.01 c |
| Flavonols | |||
| Kaempferol | 1.18 ± 0.01 a | 0.50 ± 0.02 c | 0.81 ± 0.02 b |
| Myricetin | 0.49 ± 0.01 c | 2.71 ± 0.06 a | 0.84 ± 0.00 b |
| Quercitin | 4.16 ± 0.01 a | 0.91 ± 0.02 b | 0.89 ± 0.03 b |
| Rutin | 1.19 ± 0.01 | 0.97 ± 0.02 | ND |
| Hydroxybenzoic acids | |||
| Syringic acid | ND | 0.91 ± 0.07 | 0.52 ± 0.03 |
| Hydroxycinnamic acids | |||
| Caffeic acid | 0.56 ± 0.01 b | 0.55 ± 0.01 b | 1.21 ± 0.01 a |
| p-Coumaric acid | 0.39 ± 0.01 | ND | ND |
| Caftaric acid | 0.92 ± 0.01 b | 1.32 ± 0.01 a | 0.64 ± 0.01 c |
| Chlorogenic acid | 0.35 ± 0.01 b | 0.31 ± 0.01 b | 0.62 ± 0.03 a |
| Polyphenols | |||
| Trans-resveratrol | 1.12 ± 0.02 a | 0.45 ± 0.01 b | 0.32 ± 0.01 c |
| Cis-resveratrol | 1.51 ± 0.07 a | 0.27 ± 0.01 c | 0.91 ± 0.05 b |
| Epicatechin gallate | 0.37 ± 0.01 c | 0.71 ± 0.01 b | 1.22 ± 0.02 a |
| Epicatechin | ND | 1.04 ± 0.05 | 1.25 ± 0.05 |
| Anthocyanins | |||
| Petunidin 3-glucoside | 0.49 ± 0.01 | 1.25 ± 0.05 | ND |
| Pelargonidin 3-glucoside | ND | 1.11 ± 0.01 | ND |
| Procyanidin B1 | ND | 0.62 ± 0.04 | 0.51 ± 0.01 |
| Procyanidin B2 | ND | 1.69 ± 0.09 | 0.43 ± 0.01 |
| Procyanidin A2 | ND | 1.05 ± 0.01 | 1.13 ± 0.01 |
| Total flavonoids (mg EC/100 g) 1 | 79.83 ± 0.23 a | 44.49 ± 0.61 b | 44.09 ± 1.01 b |
| Total phenolics (mg EAG/100 g) 2 | 492.107 ± 0.54 a | 368.520 ± 1.09 b | 304.057 ± 0.94 c |
| FRAP (µmol TEAC/g) 3 | 0.92 ± 0.01 a | 0.88 ± 0.01 b | 0.74 ± 0.01 c |
| ABTS (µmol TEAC/g) 3 | 16.14 ± 0.01 a | 15.29 ± 0.01 b | 14.54 ± 0.01 c |
| Treatments | Strains | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Lactobacillus paracasei L-10 | Lactobacillus casei L-26 | Lactobacillus acidophilus LA-05 | |||||||
| Before Freeze-Drying | After Freeze-Drying | Average log Reduction * | Before Freeze-Drying | After Freeze-Drying | Average log Reduction * | Before Freeze-Drying | After Freeze-Drying | Average log Reduction * | |
| NEC | 9.3 ± 0.1 ** | 8.7 ± 0.2 | 0.5 ± 0.1 aC | 10.2 ± 0.1 ** | 7.3 ± 0.4 | 2.9 ± 0.3 aA | 10.3 ± 0.2 ** | 9.4 ± 0.1 | 0.9 ± 0.1 aB |
| FOS | 9.1 ± 0.2 | 8.9 ± 0.1 | 0.2 ± 0.0 bC | 10.2 ± 0.1 ** | 8.9 ± 0.3 | 1.3 ± 0.1 bA | 10.3 ± 0.1 ** | 9.4 ± 0.2 | 0.8 ± 0.0 aB |
| ACE | 9.1 ± 0.2 | 9.0 ± 0.2 | 0.2 ± 0.1 bC | 10.3 ± 0.1 ** | 8.9 ± 0.2 | 1.4 ± 0.3 bA | 10.2 ± 0.2 ** | 9.3 ± 0.1 | 0.9 ± 0.1 aB |
| CAS | 9.5 ± 0.2 ** | 8.9 ± 0.1 | 0.6 ± 0.1 aC | 9.8 ± 0.1 ** | 7.9 ± 0.4 | 2.0 ± 0.3 bA | 10.2 ± 0.2 ** | 9.2 ± 0.1 | 1.0 ± 0.1 aB |
| GUA | 9.3 ± 0.1 ** | 9.1 ± 0.1 | 0.3 ± 0.0 bC | 9.4 ± 0.1 ** | 7.6 ± 0.4 | 1.8 ± 0.3 bA | 10.2 ± 0.1 ** | 9.3 ± 0.2 | 1.0 ± 0.1 aB |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Araújo, C.M.; Sampaio, K.B.; Menezes, F.N.D.D.; Almeida, E.T.d.C.; Lima, M.d.S.; Viera, V.B.; Garcia, E.F.; Gómez-Zavaglia, A.; de Souza, E.L.; de Oliveira, M.E.G. Protective Effects of Tropical Fruit Processing Coproducts on Probiotic Lactobacillus Strains during Freeze-Drying and Storage. Microorganisms 2020, 8, 96. https://doi.org/10.3390/microorganisms8010096
Araújo CM, Sampaio KB, Menezes FNDD, Almeida ETdC, Lima MdS, Viera VB, Garcia EF, Gómez-Zavaglia A, de Souza EL, de Oliveira MEG. Protective Effects of Tropical Fruit Processing Coproducts on Probiotic Lactobacillus Strains during Freeze-Drying and Storage. Microorganisms. 2020; 8(1):96. https://doi.org/10.3390/microorganisms8010096
Chicago/Turabian StyleAraújo, Caroliny Mesquita, Karoliny Brito Sampaio, Francisca Nayara Dantas Duarte Menezes, Erika Tayse da Cruz Almeida, Marcos dos Santos Lima, Vanessa Bordin Viera, Estefânia Fernandes Garcia, Andrea Gómez-Zavaglia, Evandro Leite de Souza, and Maria Elieidy Gomes de Oliveira. 2020. "Protective Effects of Tropical Fruit Processing Coproducts on Probiotic Lactobacillus Strains during Freeze-Drying and Storage" Microorganisms 8, no. 1: 96. https://doi.org/10.3390/microorganisms8010096
APA StyleAraújo, C. M., Sampaio, K. B., Menezes, F. N. D. D., Almeida, E. T. d. C., Lima, M. d. S., Viera, V. B., Garcia, E. F., Gómez-Zavaglia, A., de Souza, E. L., & de Oliveira, M. E. G. (2020). Protective Effects of Tropical Fruit Processing Coproducts on Probiotic Lactobacillus Strains during Freeze-Drying and Storage. Microorganisms, 8(1), 96. https://doi.org/10.3390/microorganisms8010096








