Adjuvant Strategies for More Effective Tuberculosis Vaccine Immunity
Abstract
1. Introduction
1.1. Subunit TB Vaccine Candidates
1.2. TB Vaccine Adjuvants
2. Adjuvants in Clinical-Stage TB Vaccines
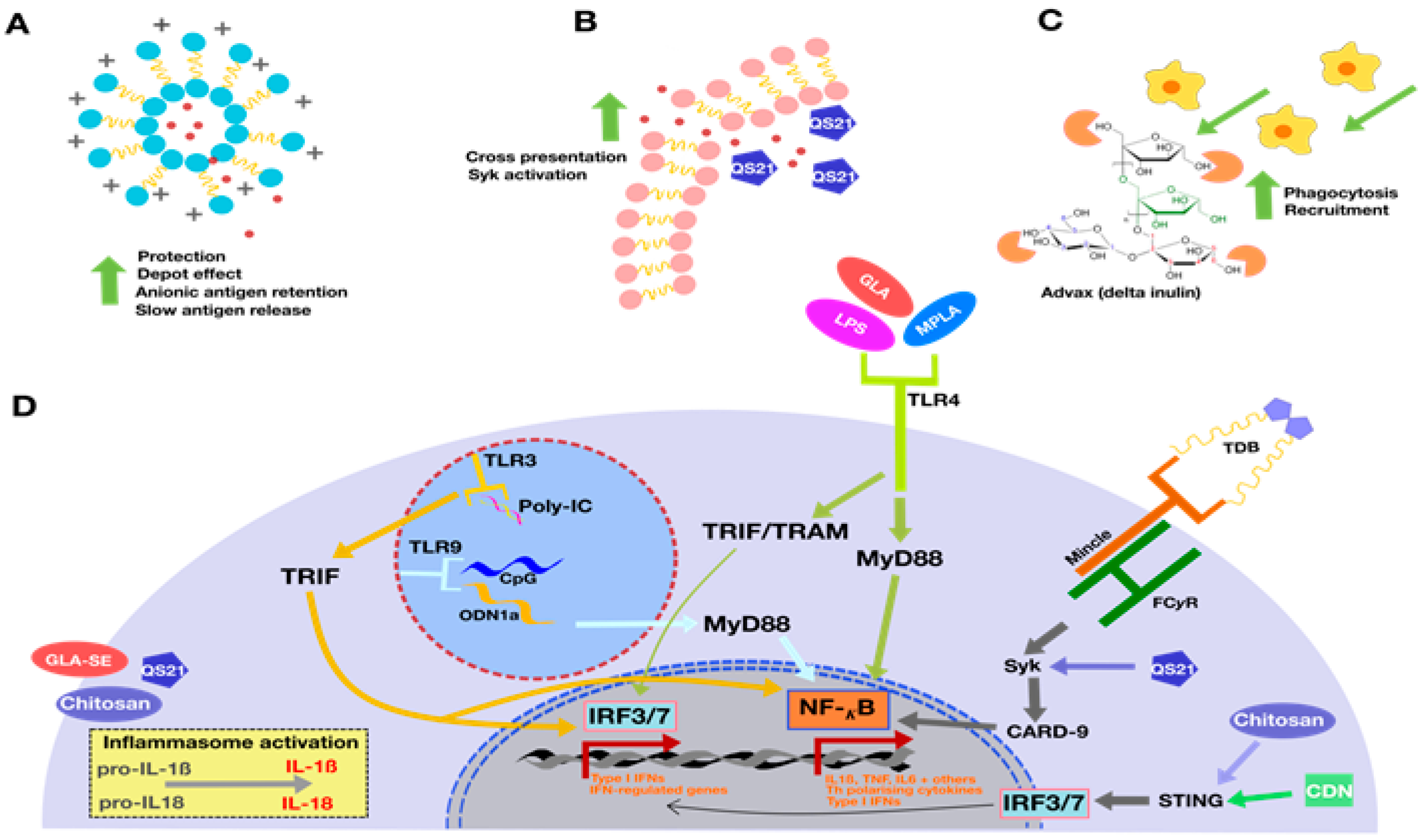
2.1. Liposomal Formulations and Emulsions—AS01, CAF01, and GLA-SE
2.2. Other Adjuvants in Clinical Trials: IC31 and GamTBVac
3. Novel TB Vaccine Adjuvants
3.1. Nanoparticles and Microparticles: Travelling Different Immune Pathways to Reach the Lymph Node
3.2. Adjuvants Derived from Nature: Plant and Microbial
4. Future Strategies and Developments
Funding
Conflicts of Interest
References
- World Health Organization. Global Tuberculosis Report 2018; Licence: CC BY-NC-SA 3.0 IGO; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Glaziou, P.; Floyd, K.; Raviglione, M.C. Global epidemiology of tuberculosis. Semin. Respir. Crit. Care Med. 2018, 39, 271–285. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Dockrell, H.M.; Ottenhoff, T.H.M.; Evans, T.G.; Zhang, Y. Tuberculosis vaccines: Opportunities and challenges. Respirology 2018, 23, 359–368. [Google Scholar] [CrossRef]
- Treatment Action Group. TB Prevention Pipeline Report 2018; Treatment Action Group: New York, NY, USA, 2018. [Google Scholar]
- Uthayakumar, D.; Paris, S.; Chapat, L.; Freyburger, L.; Poulet, H.; De Luca, K. Non-specific Effects of Vaccines Illustrated Through the BCG Example: From Observations to Demonstrations. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Aaby, P.; Roth, A.; Ravn, H.; Napirna, B.M.; Rodrigues, A.; Lisse, I.M.; Stensballe, L.; Diness, B.R.; Lausch, K.R.; Lund, N.; et al. Randomized trial of BCG vaccination at birth to low-birth-weight children: Beneficial nonspecific effects in the neonatal period? J. Infect. Dis. 2011, 204, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Samperio, P. Development of tuberculosis vaccines in clinical trials: Current status. Scand. J. Immunol. 2018, 88, e12710. [Google Scholar] [CrossRef] [PubMed]
- Billeskov, R.; Lindenstrøm, T.; Woodworth, J.; Vilaplana, C.; Cardona, P.-J.; Cassidy, J.P.; Mortensen, R.; Agger, E.M.; Andersen, P. High Antigen Dose is Detrimental to Post-Exposure Vaccine Protection against Tuberculosis. Front. Immunol. 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Tameris, M.; Hokey, D.A.; Nduba, V.; Sacarlal, J.; Laher, F.; Kiringa, G.; Gondo, K.; Lazarus, E.M.; Gray, G.E.; Nachman, S.; et al. A double-blind, randomised, placebo-controlled, dose-finding trial of the novel tuberculosis vaccine AERAS-402, an adenovirus-vectored fusion protein, in healthy, BCG-vaccinated infants. Vaccine 2015, 33, 2944–2954. [Google Scholar] [CrossRef] [PubMed]
- Tameris, M.D.D.; Hatherill, M.F.C.P.; Landry, B.S.M.P.H.; Scriba, T.J.P.; Snowden, M.A.M.P.H.; Lockhart, S.D.M.; Shea, J.E.P.; McClain, J.B.M.D.; Hussey, G.D.P.; Hanekom, W.A.P.; et al. Safety and efficacy of MVA85A, a new tuberculosis vaccine, in infants previously vaccinated with BCG: A randomised, placebo-controlled phase 2b trial. Lancet 2013, 381, 1021–1028. [Google Scholar] [CrossRef]
- Andersen, P. TB Vaccines: Progress and Problems; Elsevier Ltd: Oxford, UK, 2001; Volume 22, pp. 160–168. [Google Scholar]
- Counoupas, C.; Pinto, R.; Nagalingam, G.; Hill-Cawthorne, G.A.; Feng, C.G.; Britton, W.J.; Triccas, J.A. Mycobacterium tuberculosis components expressed during chronic infection of the lung contribute to long-term control of pulmonary tuberculosis in mice. NPJ Vaccines 2016, 1. [Google Scholar] [CrossRef]
- Jeyanathan, M.; Yao, Y.; Afkhami, S.; Smaill, F.; Xing, Z. New Tuberculosis Vaccine Strategies: Taking Aim at Un-Natural Immunity. Trends Immunol. 2018. [Google Scholar] [CrossRef]
- Counoupas, C.; Pinto, R.; Nagalingam, G.; Britton, W.J.; Petrovsky, N.; Triccas, J.A. Delta inulin-based adjuvants promote the generation of polyfunctional CD4(+) T cell responses and protection against Mycobacterium tuberculosis infection. Sci. Rep. 2017, 7, 8582. [Google Scholar] [CrossRef]
- Reed, S.G.; Orr, M.T.; Fox, C.B. Key roles of adjuvants in modern vaccines. Nat. Med. 2013, 19, 1597–1608. [Google Scholar] [CrossRef]
- De Gregorio, E.; Tritto, E.; Rappuoli, R. Alum adjuvanticity: Unraveling a century old mystery. Eur. J. Immunol. 2008, 38, 2068–2071. [Google Scholar] [CrossRef]
- Hutchison, S.; Benson, R.A.; Gibson, V.B.; Pollock, A.H.; Garside, P.; Brewer, J.M. Antigen depot is not required for alum adjuvanticity. FASEB J. 2012, 26, 1272–1279. [Google Scholar] [CrossRef]
- Moreno-Mendieta, S.A.; Rocha-Zavaleta, L.; Rodriguez-Sanoja, R. Adjuvants in tuberculosis vaccine development. FEMS Immunol. Med. Microbiol. 2010, 58, 75–84. [Google Scholar] [CrossRef]
- Agger, E.M. Novel adjuvant formulations for delivery of anti-tuberculosis vaccine candidates. Adv. Drug Deliv. Rev. 2016, 102, 73–82. [Google Scholar] [CrossRef]
- Khademi, F.; Taheri, R.A.; Momtazi-Borojeni, A.A.; Farnoosh, G.; Johnston, T.P.; Sahebkar, A. Potential of Cationic Liposomes as Adjuvants/Delivery Systems for Tuberculosis Subunit Vaccines. Rev. Physiol. Biochem. Pharmacol. 2018, 175, 47–69. [Google Scholar]
- Lyadova, I.V.; Panteleev, A.V. Th1 and Th17 Cells in Tuberculosis: Protection, Pathology, and Biomarkers. Mediat. Inflamm. 2015, 2015, 1–13. [Google Scholar] [CrossRef]
- Zygmunt, B.M.; Rharbaoui, F.; Groebe, L.; Guzman, C.A. Intranasal Immunization Promotes Th17 Immune Responses. J. Immunol. 2009, 183, 6933–6938. [Google Scholar] [CrossRef]
- Aguilo, N.; Alvarez-Arguedas, S.; Uranga, S.; Marinova, D.; Monzón, M.; Badiola, J.; Martin, C. Pulmonary but Not Subcutaneous Delivery of BCG Vaccine Confers Protection to Tuberculosis-Susceptible Mice by an Interleukin 17–Dependent Mechanism. J. Infect. Dis. 2015, 213, 831–839. [Google Scholar] [CrossRef]
- Lin, Y.; Slight, S.R.; Khader, S.A. Th17 cytokines and vaccine-induced immunity. Semin. Immunopathol. 2010, 32, 79–90. [Google Scholar] [CrossRef]
- Schoenen, H.; Bodendorfer, B.; Hitchens, K.; Manzanero, S.; Werninghaus, K.; Nimmerjahn, F.; Agger, E.M.; Stenger, S.; Andersen, P.; Ruland, J.; et al. Cutting edge: Mincle is essential for recognition and adjuvanticity of the mycobacterial cord factor and its synthetic analog trehalose-dibehenate. J. Immunol. 2010, 184, 2756–2760. [Google Scholar] [CrossRef]
- Van Dis, E.; Sogi, K.M.; Rae, C.S.; Sivick, K.E.; Surh, N.H.; Leong, M.L.; Kanne, D.B.; Metchette, K.; Leong, J.J.; Bruml, J.R.; et al. STING-Activating Adjuvants Elicit a Th17 Immune Response and Protect against Mycobacterium tuberculosis Infection. Cell Rep. 2018, 23, 1435–1447. [Google Scholar] [CrossRef]
- Kim, S.-H.; Jang, Y.-S. The development of mucosal vaccines for both mucosal and systemic immune induction and the roles played by adjuvants. Clin. Exp. Vaccine Res. 2017, 6, 15–21. [Google Scholar] [CrossRef]
- Lo, D.D. Vigilance or Subversion? Constitutive and Inducible M Cells in Mucosal Tissues. Trends Immunol. 2018, 39, 185–195. [Google Scholar] [CrossRef]
- Kim, S.-H.; Jang, Y.-S. Antigen targeting to M cells for enhancing the efficacy of mucosal vaccines. Exp. Mol. Med. 2014, 46, e85. [Google Scholar] [CrossRef]
- Zeng, L. Mucosal adjuvants: Opportunities and challenges. Hum. Vaccin Immunother. 2016, 12, 2456–2458. [Google Scholar] [CrossRef]
- Brewer, J.M.; Pollock, K.G.; Tetley, L.; Russell, D.G. Vesicle size influences the trafficking, processing, and presentation of antigens in lipid vesicles. J. Immunol. 2004, 173, 6143–6150. [Google Scholar] [CrossRef]
- Henriksen-Lacey, M.; Bramwell, V.W.; Christensen, D.; Agger, E.M.; Andersen, P.; Perrie, Y. Liposomes based on dimethyldioctadecylammonium promote a depot effect and enhance immunogenicity of soluble antigen. J. Control. Release 2010, 142, 180–186. [Google Scholar] [CrossRef]
- Rahnfeld, L.; Thamm, J.; Steiniger, F.; van Hoogevest, P.; Luciani, P. Study on the in situ aggregation of liposomes with negatively charged phospholipids for use as injectable depot formulation. Colloids Surf. B Biointerfaces 2018, 168, 10–17. [Google Scholar] [CrossRef]
- Wang, N.; Chen, M.; Wang, T. Liposomes used as a vaccine adjuvant-delivery system: From basics to clinical immunization. J. Control. Release 2019, 303, 130–150. [Google Scholar] [CrossRef]
- Gao, J.; Ochyl, L.J.; Yang, E.; Moon, J.J. Cationic liposomes promote antigen cross-presentation in dendritic cells by alkalizing the lysosomal pH and limiting the degradation of antigens. Int. J. Nanomed. 2017, 12, 1251–1264. [Google Scholar] [CrossRef]
- Maji, M.; Mazumder, S.; Bhattacharya, S.; Choudhury, S.T.; Sabur, A.; Shadab, M.; Bhattacharya, P.; Ali, N. A Lipid Based Antigen Delivery System Efficiently Facilitates MHC Class-I Antigen Presentation in Dendritic Cells to Stimulate CD8+ T Cells. Sci. Rep. 2016, 6, 27206. [Google Scholar] [CrossRef]
- Taneichi, M.; Ishida, H.; Kajino, K.; Ogasawara, K.; Tanaka, Y.; Kasai, M.; Mori, M.; Nishida, M.; Yamamura, H.; Mizuguchi, J.; et al. Antigen Chemically Coupled to the Surface of Liposomes Are Cross-Presented to CD8+ T Cells and Induce Potent Antitumor Immunity. J. Immunol. 2006, 177, 2324. [Google Scholar] [CrossRef]
- Fox, B.C. Squalene Emulsions for Parenteral Vaccine and Drug Delivery. Molecules 2009, 14, 3286. [Google Scholar] [CrossRef]
- Stassijns, J.; Bollaerts, K.; Baay, M.; Verstraeten, T. A systematic review and meta-analysis on the safety of newly adjuvanted vaccines among children. Vaccine 2016, 34, 714–722. [Google Scholar] [CrossRef]
- Van Der Meeren, O.; Hatherill, M.; Nduba, V.; Wilkinson, R.J.; Muyoyeta, M.; Van Brakel, E.; Ayles, H.M.; Henostroza, G.; Thienemann, F.; Scriba, T.J.; et al. Phase 2b Controlled Trial of M72/AS01E Vaccine to Prevent Tuberculosis. N. Engl. J. Med. 2018. [Google Scholar] [CrossRef]
- Didierlaurent, A.M.; Laupèze, B.; Di Pasquale, A.; Hergli, N.; Collignon, C.; Garçon, N. Adjuvant system AS01: Helping to overcome the challenges of modern vaccines. Exp. Rev. Vaccines 2017, 16, 55–63. [Google Scholar] [CrossRef]
- MacLeod, M.K.L.; McKee, A.S.; David, A.; Wang, J.; Mason, R.; Kappler, J.W.; Marrack, P. Vaccine adjuvants aluminum and monophosphoryl lipid A provide distinct signals to generate protective cytotoxic memory CD8 T cells. Proc. Natl Acad. Sci. USA 2011, 108, 7914. [Google Scholar] [CrossRef]
- Del Giudice, G.; Rappuoli, R.; Didierlaurent, A.M. Correlates of adjuvanticity: A review on adjuvants in licensed vaccines. Semin. Immunol. 2018, 39, 14–21. [Google Scholar] [CrossRef]
- Marciani, D.J. Elucidating the Mechanisms of Action of Saponin-Derived Adjuvants. Trends Pharmacol. Sci. 2018, 39, 573–585. [Google Scholar] [CrossRef]
- Sun, H.-X.; Xie, Y.; Ye, Y.-P. Advances in saponin-based adjuvants. Vaccine 2009, 27, 1787–1796. [Google Scholar] [CrossRef]
- Coccia, M.; Collignon, C.; Hervé, C.; Chalon, A.; Welsby, I.; Detienne, S.; van Helden, M.J.; Dutta, S.; Genito, C.J.; Waters, N.C.; et al. Cellular and molecular synergy in AS01-adjuvanted vaccines results in an early IFNγ response promoting vaccine immunogenicity. NPJ Vaccines 2017, 2, 25. [Google Scholar] [CrossRef]
- Montoya, J.; Solon, J.A.; Cunanan, S.R.C.; Acosta, L.; Bollaerts, A.; Moris, P.; Janssens, M.; Jongert, E.; Demoitié, M.-A.; Mettens, P.; et al. A Randomized, Controlled Dose-Finding Phase II Study of the M72/AS01 Candidate Tuberculosis Vaccine in Healthy PPD-Positive Adults. J. Clin. Immunol. 2013, 33, 1360–1375. [Google Scholar] [CrossRef]
- Kumarasamy, N.; Poongulali, S.; Bollaerts, A.; Moris, P.; Beulah, F.E.; Ayuk, L.N.; Demoitié, M.-A.; Jongert, E.; Ofori-Anyinam, O. A Randomized, Controlled Safety, and Immunogenicity Trial of the M72/AS01 Candidate Tuberculosis Vaccine in HIV-Positive Indian Adults. Medicine 2016, 95, e2459. [Google Scholar] [CrossRef]
- Van Dissel, J.T.; Joosten, S.A.; Hoff, S.T.; Soonawala, D.; Prins, C.; Hokey, D.A.; O’Dee, D.M.; Graves, A.; Thierry-Carstensen, B.; Andreasen, L.V.; et al. A novel liposomal adjuvant system, CAF01, promotes long-lived Mycobacterium tuberculosis-specific T-cell responses in human. Vaccine 2014, 32, 7098–7107. [Google Scholar] [CrossRef]
- Davidsen, J.; Rosenkrands, I.; Christensen, D.; Vangala, A.; Kirby, D.; Perrie, Y.; Agger, E.M.; Andersen, P. Characterization of cationic liposomes based on dimethyldioctadecylammonium and synthetic cord factor from M. tuberculosis (trehalose 6,6′-dibehenate)—A novel adjuvant inducing both strong CMI and antibody responses. Biochim. Biophy. Acta 2005, 1718, 22–31. [Google Scholar] [CrossRef]
- Ishikawa, E.; Ishikawa, T.; Morita, Y.S.; Toyonaga, K.; Yamada, H.; Takeuchi, O.; Kinoshita, T.; Akira, S.; Yoshikai, Y.; Yamasaki, S. Direct recognition of the mycobacterial glycolipid, trehalose dimycolate, by C-type lectin Mincle. J. Exp. Med. 2009, 206, 2879–2888. [Google Scholar] [CrossRef]
- Desel, C.; Werninghaus, K.; Ritter, M.; Jozefowski, K.; Wenzel, J.; Russkamp, N.; Schleicher, U.; Christensen, D.; Wirtz, S.; Kirschning, C.; et al. The Mincle-Activating Adjuvant TDB Induces MyD88-Dependent Th1 and Th17 Responses through IL-1R Signaling. PLoS ONE 2013, 8, e53531. [Google Scholar] [CrossRef]
- Christensen, D.; Mortensen, R.; Rosenkrands, I.; Dietrich, J.; Andersen, P. Vaccine-induced Th17 cells are established as resident memory cells in the lung and promote local IgA responses. Mucosal Immunol. 2017, 10, 260–270. [Google Scholar] [CrossRef]
- Aagaard, C.; Hoang, T.; Dietrich, J.; Cardona, P.J.; Izzo, A.; Dolganov, G.; Schoolnik, G.K.; Cassidy, J.P.; Billeskov, R.; Andersen, P. A multistage tuberculosis vaccine that confers efficient protection before and after exposure. Nat. Med. 2011, 17, 189–194. [Google Scholar] [CrossRef]
- Woodworth, J.S.; Cohen, S.B.; Moguche, A.O.; Plumlee, C.R.; Agger, E.M.; Urdahl, K.B.; Andersen, P. Subunit vaccine H56/CAF01 induces a population of circulating CD4 T cells that traffic into the Mycobacterium tuberculosis-infected lung. Mucosal Immunol. 2017, 10, 555–564. [Google Scholar] [CrossRef]
- Penn-Nicholson, A.; Tameris, M.; Smit, E.; Day, T.A.; Musvosvi, M.; Jayashankar, L.; Vergara, J.; Mabwe, S.; Bilek, N.; Geldenhuys, H.; et al. Safety and immunogenicity of the novel tuberculosis vaccine ID93 + GLA-SE in BCG-vaccinated healthy adults in South Africa: A randomised, double-blind, placebo-controlled phase 1 trial. Lancet Respir. Med. 2018, 6, 287–298. [Google Scholar] [CrossRef]
- Orr, M.T.; Duthie, M.S.; Windish, H.P.; Lucas, E.A.; Guderian, J.A.; Hudson, T.E.; Shaverdian, N.; O’Donnell, J.; Desbien, A.L.; Reed, S.G.; et al. MyD88 and TRIF synergistic interaction is required for TH1-cell polarization with a synthetic TLR4 agonist adjuvant. Eur. J. Immunol. 2013, 43, 2398–2408. [Google Scholar] [CrossRef]
- Desbien, A.L.; Reed, S.J.; Bailor, H.R.; Cauwelaert, N.D.; Laurance, J.D.; Orr, M.T.; Fox, C.B.; Carter, D.; Reed, S.G.; Duthie, M.S. Squalene emulsion potentiates the adjuvant activity of the TLR4 agonist, GLA, via inflammatory caspases, IL-18, and IFN-γ. Eur. J. Immunol. 2015, 45, 407–417. [Google Scholar] [CrossRef]
- Nemes, E.; Geldenhuys, H.; Rozot, V.; Rutkowski, K.T.; Ratangee, F.; Bilek, N.; Mabwe, S.; Makhethe, L.; Erasmus, M.; Toefy, A.; et al. Prevention of M-tuberculosis Infection with H4:IC31 Vaccine or BCG Revaccination. N. Engl. J. Med. 2018, 379, 138–149. [Google Scholar] [CrossRef]
- Schellack, C.; Prinz, K.; Egyed, A.; Fritz, J.H.; Wittmann, B.; Ginzler, M.; Swatosch, G.; Zauner, W.; Kast, C.; Akira, S.; et al. IC31, a novel adjuvant signaling via TLR9, induces potent cellular and humoral immune responses. Vaccine 2006, 24, 5461–5472. [Google Scholar] [CrossRef]
- Aichinger, M.C.; Ginzler, M.; Weghuber, J.; Zimmermann, L.; Riedl, K.; Schütz, G.; Nagy, E.; von Gabain, A.; Schweyen, R.; Henics, T.; et al. Adjuvating the adjuvant: Facilitated delivery of an immunomodulatory oligonucleotide to TLR9 by a cationic antimicrobial peptide in dendritic cells. Vaccine 2011, 29, 426–436. [Google Scholar] [CrossRef]
- Jantaruk, P.; Roytrakul, S.; Sitthisak, S.; Kunthalert, D. Potential role of an antimicrobial peptide, KLK in inhibiting lipopolysaccharide-induced macrophage inflammation. PLoS ONE 2017, 12, e0183852. [Google Scholar] [CrossRef]
- Fritz, J.H.; Brunner, S.; Birnstiel, M.L.; Buschle, M.; Gabain, A.; Mattner, F.; Zauner, W. The artificial antimicrobial peptide KLKLLLLLKLK induces predominantly a TH2-type immune response to co-injected antigens. Vaccine 2004, 22, 3274–3284. [Google Scholar] [CrossRef]
- Tkachuk, A.P.; Gushchin, V.A.; Potapov, V.D.; Demidenko, A.V.; Lunin, V.G.; Gintsburg, A.L. Multi-subunit BCG booster vaccine GamTBvac: Assessment of immunogenicity and protective efficacy in murine and guinea pig TB models. PLoS ONE 2017, 12, e0176784. [Google Scholar] [CrossRef]
- Pustylnikov, S.; Sagar, D.; Jain, P.; Khan, Z.K. Targeting the C-type lectins-mediated host-pathogen interactions with dextran. J. Pharm. Pharm. Sci. 2014, 17, 371–392. [Google Scholar] [CrossRef]
- Perdomo, C.; Zedler, U.; Kuhl, A.A.; Lozza, L.; Saikali, P.; Sander, L.E.; Vogelzang, A.; Kaufmann, S.H.E.; Kupz, A. Mucosal BCG Vaccination Induces Protective Lung-Resident Memory T Cell Populations against Tuberculosis. MBio 2016, 7, e01686-16. [Google Scholar] [CrossRef]
- Lai, R.; Afkhami, S.; Haddadi, S.; Jeyanathan, M.; Xing, Z. Mucosal immunity and novel tuberculosis vaccine strategies: Route of immunisation-determined T-cell homing to restricted lung mucosal compartments. Eur. Respir. Rev. 2015, 24, 356–360. [Google Scholar] [CrossRef]
- Caccamo, N.; Guggino, G.; Joosten, S.A.; Gelsomino, G.; Di Carlo, P.; Titone, L.; Galati, D.; Bocchino, M.; Matarese, A.; Salerno, A.; et al. Multifunctional CD4+ T cells correlate with active Mycobacterium tuberculosis infection. Eur. J. Immunol. 2010, 40, 2211–2220. [Google Scholar] [CrossRef]
- Qiu, Z.; Zhang, M.; Zhu, Y.; Zheng, F.; Lu, P.; Liu, H.; Graner, M.W.; Zhou, B.; Chen, X. Multifunctional CD4 T cell responses in patients with active tuberculosis. Sci. Rep. 2012, 2, 216. [Google Scholar] [CrossRef]
- Rodo, M.J.; Rozot, V.; Nemes, E.; Dintwe, O.; Hatherill, M.; Little, F.; Scriba, T.J. A comparison of antigen-specific T cell responses induced by six novel tuberculosis vaccine candidates. PLoS Pathog. 2019, 15, e1007643. [Google Scholar] [CrossRef]
- Penn-Nicholson, A.; Geldenhuys, H.; Burny, W.; van der Most, R.; Day, C.L.; Jongert, E.; Moris, P.; Hatherill, M.; Ofori-Anyinam, O.; Hanekom, W.; et al. Safety and immunogenicity of candidate vaccine M72/AS01E in adolescents in a TB endemic setting. Vaccine 2015, 33, 4025–4034. [Google Scholar] [CrossRef]
- Marty-Roix, R.; Vladimer, G.I.; Pouliot, K.; Weng, D.; Buglione-Corbett, R.; West, K.; MacMicking, J.D.; Chee, J.D.; Wang, S.; Lu, S.; et al. Identification of QS-21 as an Inflammasome-activating Molecular Component of Saponin Adjuvants. J. Biol. Chem. 2016, 291, 1123–1136. [Google Scholar] [CrossRef]
- Reljic, R.; Sibley, L.; Huang, J.-M.; Pepponi, I.; Hoppe, A.; Hong, H.A.; Cutting, S.M. Mucosal Vaccination against Tuberculosis Using Inert Bioparticles. Infect. Immun. 2013, 81, 4071. [Google Scholar] [CrossRef]
- Comparative analysis of Bacillus subtilis spores and monophosphoryl lipid A as adjuvants of protein-based mycobacterium tuberculosis-based vaccines: Partial requirement for interleukin-17a for induction of protective immunity. Clin. Vaccine Immunol. 2014, 21, 501. [CrossRef]
- Ostrop, J.; Jozefowski, K.; Zimmermann, S.; Hofmann, K.; Strasser, E.; Lepenies, B.; Lang, R. Contribution of MINCLE—SYK Signaling to Activation of Primary Human APCs by Mycobacterial Cord Factor and the Novel Adjuvant TDB. J. Immunol. 2015, 195, 2417–2428. [Google Scholar] [CrossRef]
- Lindenstrøm, T.; Woodworth, J.; Dietrich, J.; Aagaard, C.; Andersen, P.; Agger, E.M. Vaccine-Induced Th17 Cells Are Maintained Long-Term Postvaccination as a Distinct and Phenotypically Stable Memory Subset. Infect. Immun. 2012, 80, 3533–3544. [Google Scholar] [CrossRef]
- Carroll, E.C.; Jin, L.; Mori, A.; Muñoz-Wolf, N.; Oleszycka, E.; Moran, H.B.T.; Mansouri, S.; McEntee, C.P.; Lambe, E.; Agger, E.M.; et al. The Vaccine Adjuvant Chitosan Promotes Cellular Immunity via DNA Sensor cGAS-STING-Dependent Induction of Type I Interferons. Immunity 2016, 44, 597–608. [Google Scholar] [CrossRef]
- Khademi, F.; Taheri, R.A.; Yousefi Avarvand, A.; Vaez, H.; Momtazi-Borojeni, A.A.; Soleimanpour, S. Are chitosan natural polymers suitable as adjuvant/delivery system for anti-tuberculosis vaccines? Microb. Pathog. 2018, 121, 218–223. [Google Scholar] [CrossRef]
- Seydoux, E.; Liang, H.; Dubois Cauwelaert, N.; Archer, M.; Rintala, N.D.; Kramer, R.; Carter, D.; Fox, C.B.; Orr, M.T. Effective Combination Adjuvants Engage Both TLR and Inflammasome Pathways To Promote Potent Adaptive Immune Responses. J. Immunol. 2018, 201, 98–112. [Google Scholar] [CrossRef]
- Luabeya, A.K.K.; Kagina, B.M.N.; Tameris, M.D.; Geldenhuys, H.; Hoff, S.T.; Shi, Z.; Kromann, I.; Hatherill, M.; Mahomed, H.; Hanekom, W.A.; et al. First-in-human trial of the post-exposure tuberculosis vaccine H56:IC31 in Mycobacterium tuberculosis infected and non-infected healthy adults. Vaccine 2015, 33, 4130–4140. [Google Scholar] [CrossRef]
- Hussein, J.; Zewdie, M.; Yamuah, L.; Bedru, A.; Abebe, M.; Dagnew, A.F.; Chanyalew, M.; Yohannes, A.G.; Ahmed, J.; Engers, H.; et al. A phase I, open-label trial on the safety and immunogenicity of the adjuvanted tuberculosis subunit vaccine H1/IC31® in people living in a TB-endemic area. Trials 2018, 19, 24. [Google Scholar] [CrossRef]
- Morelli, A.B.; Becher, D.; Koernig, S.; Silva, A.; Drane, D.; Maraskovsky, E. ISCOMATRIX: A novel adjuvant for use in prophylactic and therapeutic vaccines against infectious diseases. J. Med. Microbiol. 2012, 61, 935–943. [Google Scholar] [CrossRef]
- Andersen, C.S.; Dietrich, J.; Agger, E.M.; Lycke, N.Y.; Lövgren, K.; Andersen, P. The combined CTA1-DD/ISCOMs vector is an effective intranasal adjuvant for boosting prior Mycobacterium bovis BCG immunity to Mycobacterium tuberculosis. Infect. Immun. 2007, 75, 408–416. [Google Scholar] [CrossRef]
- Pabreja, S.; Garg, T.; Rath, G.; Goyal, A.K. Mucosal vaccination against tuberculosis using Ag85A-loaded immunostimulating complexes. Artif. Cells Nanomed. Biotechnol. 2016, 44, 532–539. [Google Scholar] [CrossRef]
- Tyne, A.S.; Chan, J.G.; Shanahan, E.R.; Atmosukarto, I.; Chan, H.K.; Britton, W.J.; West, N.P. TLR2-targeted secreted proteins from Mycobacterium tuberculosis are protective as powdered pulmonary vaccines. Vaccine 2013, 31, 4322–4329. [Google Scholar] [CrossRef]
- Ahmed, M.; Smith, D.M.; Hamouda, T.; Rangel-Moreno, J.; Fattom, A.; Khader, S.A. A novel nanoemulsion vaccine induces mucosal Interleukin-17 responses and confers protection upon Mycobacterium tuberculosis challenge in mice. Vaccine 2017, 35, 4983–4989. [Google Scholar] [CrossRef]
- Bielinska, A.U.; Gerber, M.; Blanco, L.P.; Makidon, P.E.; Janczak, K.W.; Beer, M.; Swanson, B.; Baker, J.R., Jr. Induction of Th17 cellular immunity with a novel nanoemulsion adjuvant. Crit. Rev. Immunol. 2010, 30, 189–199. [Google Scholar] [CrossRef]
- Ashhurst, A.S.; Parumasivam, T.; Chan, J.G.Y.; Lin, L.C.W.; Flórido, M.; West, N.P.; Chan, H.-K.; Britton, W.J. PLGA particulate subunit tuberculosis vaccines promote humoral and Th17 responses but do not enhance control of Mycobacterium tuberculosis infection. PLoS ONE 2018, 13, e0194620. [Google Scholar] [CrossRef]
- Kirby, D.J.; Rosenkrands, I.; Agger, E.M.; Andersen, P.; Coombes, A.G.A.; Perrie, Y. PLGA microspheres for the delivery of a novel subunit TB vaccine. J. Drug Target. 2008, 16, 282–293. [Google Scholar] [CrossRef]
- Speth, M.T.; Repnik, U.; Muller, E.; Spanier, J.; Kalinke, U.; Corthay, A.; Griffiths, G. Poly(I:C)-Encapsulating Nanoparticles Enhance Innate Immune Responses to the Tuberculosis Vaccine Bacille Calmette-Guerin (BCG) via Synergistic Activation of Innate Immune Receptors. Mol. Pharm. 2017, 14, 4098–4112. [Google Scholar] [CrossRef]
- Huang, Q.; Yu, W.; Hu, T. Potent Antigen-Adjuvant Delivery System by Conjugation of Mycobacterium tuberculosis Ag85B-HspX Fusion Protein with Arabinogalactan-Poly(I:C) Conjugate. Bioconjugate Chem. 2016, 27, 1165–1174. [Google Scholar] [CrossRef]
- Stylianou, E.; Diogo, G.R.; Pepponi, I.; van Dolleweerd, C.; Arias, M.A.; Locht, C.; Rider, C.C.; Sibley, L.; Cutting, S.M.; Loxley, A.; et al. Mucosal delivery of antigen-coated nanoparticles to lungs confers protective immunity against tuberculosis infection in mice. Eur. J. Immunol. 2014, 44, 440–449. [Google Scholar] [CrossRef]
- Pati, R.; Shevtsov, M.; Sonawane, A. Nanoparticle Vaccines Against Infectious Diseases. Front. Immunol. 2018, 9, 2224. [Google Scholar] [CrossRef]
- Anderson, J.M.; Shive, M.S. Biodegradation and biocompatibility of PLA and PLGA microspheres. Adv. Drug Deliv. Rev. 1997, 28, 5–24. [Google Scholar] [CrossRef]
- Silva, A.L.; Soema, P.C.; Slütter, B.; Ossendorp, F.; Jiskoot, W. PLGA particulate delivery systems for subunit vaccines: Linking particle properties to immunogenicity. Hum. Vaccines Immunother. 2016, 12, 1056–1069. [Google Scholar] [CrossRef]
- Parumasivam, T.; Leung, S.S.Y.; Quan, D.H.; Triccas, J.A.; Britton, W.J.; Chan, H.-K. Rifapentine-loaded PLGA microparticles for tuberculosis inhaled therapy: Preparation and in vitro aerosol characterization. Eur. J. Pharm. Sci. 2016, 88, 1–11. [Google Scholar] [CrossRef]
- dos Santos, S.A.; Zárate-Bladés, C.R.; de Sá Galetti, F.C.; Brandão, I.T.; Masson, A.P.; Soares, E.G.; Araújo, A.P.U.; Silva, C.L. A subunit vaccine based on biodegradable microspheres carrying rHsp65 protein and KLK protects BALB/c mice against tuberculosis infection. Hum. Vaccines 2010, 6, 1047–1053. [Google Scholar] [CrossRef]
- Shi, S.; Hickey, A.J. PLGA Microparticles in Respirable Sizes Enhance an In Vitro T Cell Response to Recombinant Mycobacterium Tuberculosis Antigen TB10.4-Ag85B. Pharm. Res. 2010, 27, 350–360. [Google Scholar] [CrossRef]
- Karimi, S.M.; Sankian, M.; Khademi, F.; Tafaghodi, M. Chitosan (CHT) and trimethylchitosan (TMC) nanoparticles as adjuvant/delivery system for parenteral and nasal immunization against Mycobacterium tuberculosis (MTb) ESAT-6 antigen. Nanomed. J. 2016, 3, 223–229. [Google Scholar] [CrossRef]
- Song, M.; Hong, H.A.; Huang, J.M.; Colenutt, C.; Khang, D.D.; Nguyen, T.V.; Park, S.M.; Shim, B.S.; Song, H.H.; Cheon, I.S.; et al. Killed Bacillus subtilis spores as a mucosal adjuvant for an H5N1 vaccine. Vaccine 2012, 30, 3266–3277. [Google Scholar] [CrossRef]
- Sun, B.; Yu, S.; Zhao, D.; Guo, S.; Wang, X.; Zhao, K. Polysaccharides as vaccine adjuvants. Vaccine 2018, 36, 5226–5234. [Google Scholar] [CrossRef]
- Oscarson, S.; Sehgelmeble, F.W. Chemical Syntheses of Inulin and Levan Structures. J. Organ. Chem. 2002, 67, 8457–8462. [Google Scholar] [CrossRef]
- Cooper, P.D.; Petrovsky, N. Delta inulin: A novel, immunologically active, stable packing structure comprising β-D-[2- > 1] poly(fructo-furanosyl) α-D-glucose polymers. Glycobiology 2011, 21, 595–606. [Google Scholar] [CrossRef]
- Gotze, O.; Muller-Eberhard, H.J. The c3-activator system: An alternate pathway of complement activation. J. Exp. Med. 1971, 134, 90–108. [Google Scholar]
- Hayashi, M.; Aoshi, T.; Haseda, Y.; Kobiyama, K.; Wijaya, E.; Nakatsu, N.; Igarashi, Y.; Standley, D.M.; Yamada, H.; Honda-Okubo, Y.; et al. Advax, a Delta Inulin Microparticle, Potentiates In-built Adjuvant Property of Co-administered Vaccines. EBioMedicine 2017, 15, 127–136. [Google Scholar] [CrossRef]
- Honda-Okubo, Y.; Saade, F.; Petrovsky, N. Advax™, a polysaccharide adjuvant derived from delta inulin, provides improved influenza vaccine protection through broad-based enhancement of adaptive immune responses. Vaccine 2012, 30, 5373–5381. [Google Scholar] [CrossRef]
- Gordon, D.L.; Sajkov, D.; Honda-Okubo, Y.; Wilks, S.H.; Aban, M.; Barr, I.G.; Petrovsky, N. Human Phase 1 trial of low-dose inactivated seasonal influenza vaccine formulated with Advax delta inulin adjuvant. Vaccine 2016, 34, 3780–3786. [Google Scholar] [CrossRef]
- Gordon, D.; Kelley, P.; Heinzel, S.; Cooper, P.; Petrovsky, N. Immunogenicity and safety of Advax, a novel polysaccharide adjuvant based on delta inulin, when formulated with hepatitis B surface antigen: A randomized controlled Phase 1 study. Vaccine 2014, 32, 6469–6477. [Google Scholar] [CrossRef]
- Gordon, D.L.; Sajkov, D.; Woodman, R.J.; Honda-Okubo, Y.; Cox, M.M.; Heinzel, S.; Petrovsky, N. Randomized clinical trial of immunogenicity and safety of a recombinant H1N1/2009 pandemic influenza vaccine containing Advax polysaccharide adjuvant. Vaccine 2012, 30, 5407–5416. [Google Scholar] [CrossRef]
- Petrovsky, N.; Cooper, P.D. Advax™, a novel microcrystalline polysaccharide particle engineered from delta inulin, provides robust adjuvant potency together with tolerability and safety. Vaccine 2015, 33, 5920–5926. [Google Scholar] [CrossRef]
- Murugappan, S.; Frijlink, H.W.; Petrovsky, N.; Hinrichs, W.L. Enhanced pulmonary immunization with aerosolized inactivated influenza vaccine containing delta inulin adjuvant. Eur. J. Pharm. Sci. 2015, 66, 118–122. [Google Scholar] [CrossRef]
- Wilson, N.S.; Yang, B.; Morelli, A.B.; Koernig, S.; Yang, A.; Loeser, S.; Airey, D.; Provan, L.; Hass, P.; Braley, H.; et al. ISCOMATRIX vaccines mediate CD8+ T-cell cross-priming by a MyD88-dependent signaling pathway. Immunol. Cell Biol. 2012, 90, 540–552. [Google Scholar] [CrossRef]
- Agren, L.C.; Ekman, L.; Lowenadler, B.; Lycke, N.Y. Genetically engineered nontoxic vaccine adjuvant that combines B cell targeting with immunomodulation by cholera toxin A1 subunit. J. Immunol. 1997, 158, 3936–3946. [Google Scholar]
- Eriksson, A.M.; Schon, K.M.; Lycke, N.Y. The cholera toxin-derived CTA1-DD vaccine adjuvant administered intranasally does not cause inflammation or accumulate in the nervous tissues. J. Immunol. 2004, 173, 3310–3319. [Google Scholar] [CrossRef]
- Kumar, K.; Kon, O.M. Diagnosis and treatment of tuberculosis: Latest developments and future priorities. Ann. Res. Hosp. 2017, 1, 1. [Google Scholar] [CrossRef]
- Bhatt, K.; Verma, S.; Ellner, J.J.; Salgame, P. Quest for correlates of protection against tuberculosis. Clin. Vaccine Immunol. 2015, 22, 258–266. [Google Scholar] [CrossRef]
- Sallin, M.A.; Sakai, S.; Kauffman, K.D.; Young, H.A.; Zhu, J.F.; Barber, D.L. Th1 Differentiation Drives the Accumulation of Intravascular, Non-protective CD4 T Cells during Tuberculosis. Cell Rep. 2017, 18, 3091–3104. [Google Scholar] [CrossRef]
- Aagaard, C.; Hoang, T.T.K.T.; Izzo, A.; Billeskov, R.; Troudt, J.; Arnett, K.; Keyser, A.; Elvang, T.; Andersen, P.; Dietrich, J. Protection and Polyfunctional T Cells Induced by Ag85B-TB10.4/IC31® against Mycobacterium tuberculosis Is Highly Dependent on the Antigen Dose. PLoS ONE 2009, 4, e5930. [Google Scholar] [CrossRef]
- Sakai, S.; Mayer-Barber, K.D.; Barber, D.L. Defining features of protective CD4 T cell responses to Mycobacterium tuberculosis. Curr. Opin. Immunol. 2014, 29, 137–142. [Google Scholar] [CrossRef]
- Sakai, S.; Kauffman, K.D.; Schenkel, J.M.; McBerry, C.C.; Mayer-Barber, K.D.; Masopust, D.; Barber, D.L. Cutting Edge: Control of Mycobacterium tuberculosis Infection by a Subset of Lung Parenchyma-Homing CD4 T Cells. J. Immunol. 2014, 192, 2965–2969. [Google Scholar] [CrossRef]
| Adjuvant/Delivery System | Components | Antigen | Proposed Mechanism of Action | Immune Readout | Testing Status | References |
|---|---|---|---|---|---|---|
| Advax | Delta inulin particles | Ag85B, CysD (CysVac2) | Enhanced phagocytosis, immune cell recruitment, low reactogenicity | Th1, Th17 | Preclinical | [12,14] |
| AS01 | MPLA and QS21 | Mtb32, Mtb 39 (M72) | TLR4 activation (MPLA), liposomal disruption and Syk activation, CD2 activation on T-cells, NLRP3 inflammasome (QS21) | Th1 | Phase IIb (54% efficacy) | [40,71,72] |
| B. subtilis spores | MPT64; Acr-Ag85B | Mucoadhesive, resistant to enzymatic degradation, suitable for mucosal administration | Th1, IgA, low Th17 | Preclinical | [73,74] | |
| CAF01 | DDA and TDB | Ag85B, ESAT-6 (H1) | TDB activates Mincle, MyD88-dependent Th1/Th17 polarising cytokines. DDA forms cationic liposomes that are stabilised by TDB. | Th1, Th17 | Phase I | [49,52,75,76] |
| Chitosan and derivatives | Ag85B, ESAT-6 (H1) | Activates cGAS-STING pathway, mucoadhesive and mucosal epithelial penetration properties, suitable for mucosal administration | Th1, low Th17 | Preclinical | [77,78] | |
| Cyclic dinucleotides | Synthetic dinucleotide analogue of cyclic diguanylate | Ag85B, ESAT-6, Rv1733c, Rv2626c, RpfD (5Ag) | STING activation (IRF-3 type I IFN production, NFkB, STAT-6 chemokine expression) | Th17, low Th1 | Preclinical | [26] |
| Dextran | Ag85A, ESAT-6-CFP10 | Activates DC-SIGN receptor family, mannose receptor, langerin | Th1/Th2 | Phase I | [64,65] | |
| GLA-SE | GLA in squalene emulsion | Rv2608, Rv3619, Rv3620, Rv18183 (ID93) | GLA is a synthetic TLR4 agonist, in squalene in water emulsion activates NLRP3 inflammasome | Th1 | Phase IIa | [56,57,58,79] |
| IC31 | KLK and ODN1a | Ag85V, ESAT-6 (H1); Ag85B, ESAT-6 and Rv2660c (H56) and Ag 85B, TB10.4 (H4) | ODN1a binds TLR9, KLK forms aggregates with ODN1a and enhances translocation into cells | Th1 | Phase IIa (H56:IC31; 30.5% efficacy) | [46,59,80,81] |
| ISCOMs | Immune stimulatory complexes (saponin, cholesterol and phospholipid) | Ag85B, ESAT-6 (H1); Ag85A | TLR independent, may be inflammasome mediated (under investigation) | Th1/Th2 | Preclinical | [82,83,84] |
| Lipokel | PamCys2 and 3NTA | Culp 1, Culp 6 | PamCys2 is a TLR2 ligand and 3NTA is a chelating entity that allows antigen binding | Th1 | Phase I | [85] |
| Nanoemulsion | Soybean oil phase mixed into aqueous phase | ESAT-6, Ag85B | Mucoadhesive, highly tolerated, suitable for mucosal administration | Th17, Th1 | Preclinical | [86,87] |
| PLGA (poly(lactic-co-glycolic acid)) | Microsphere delivery system | Ag85B, ESAT-6 (H1); MPT83 | Antigen protection, depot formation, controlled release, enhanced phagocytosis, biodegradable, suitable for mucosal administration | Th1, Th17 | Preclinical | [88,89] |
| PolyI:C | dsRNA | BCG; Ag85B, HspX | TLR3 agonist | Th1, Th2 | Preclinical | [90,91] |
| Yellow carnauba wax nanoparticles | Incorporated with heparin-binding hemagglutinin adhesion (HBHA) protein | Ag85B | Enhanced adherence to alveolar epithelium (HBHA), highly tolerated (particles), suitable for mucosal administration | Th1 | Preclinical | [92] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stewart, E.; Triccas, J.A.; Petrovsky, N. Adjuvant Strategies for More Effective Tuberculosis Vaccine Immunity. Microorganisms 2019, 7, 255. https://doi.org/10.3390/microorganisms7080255
Stewart E, Triccas JA, Petrovsky N. Adjuvant Strategies for More Effective Tuberculosis Vaccine Immunity. Microorganisms. 2019; 7(8):255. https://doi.org/10.3390/microorganisms7080255
Chicago/Turabian StyleStewart, Erica, James A Triccas, and Nikolai Petrovsky. 2019. "Adjuvant Strategies for More Effective Tuberculosis Vaccine Immunity" Microorganisms 7, no. 8: 255. https://doi.org/10.3390/microorganisms7080255
APA StyleStewart, E., Triccas, J. A., & Petrovsky, N. (2019). Adjuvant Strategies for More Effective Tuberculosis Vaccine Immunity. Microorganisms, 7(8), 255. https://doi.org/10.3390/microorganisms7080255





