Tuberculosis Progression Does Not Necessarily Equate with a Failure of Immune Control
Abstract
:1. Introduction.
2. To Progress or Not to Progress
3. What Do We Know of Immune Protection?
4. Immune Control Is Filtered Through the Host Phagocyte
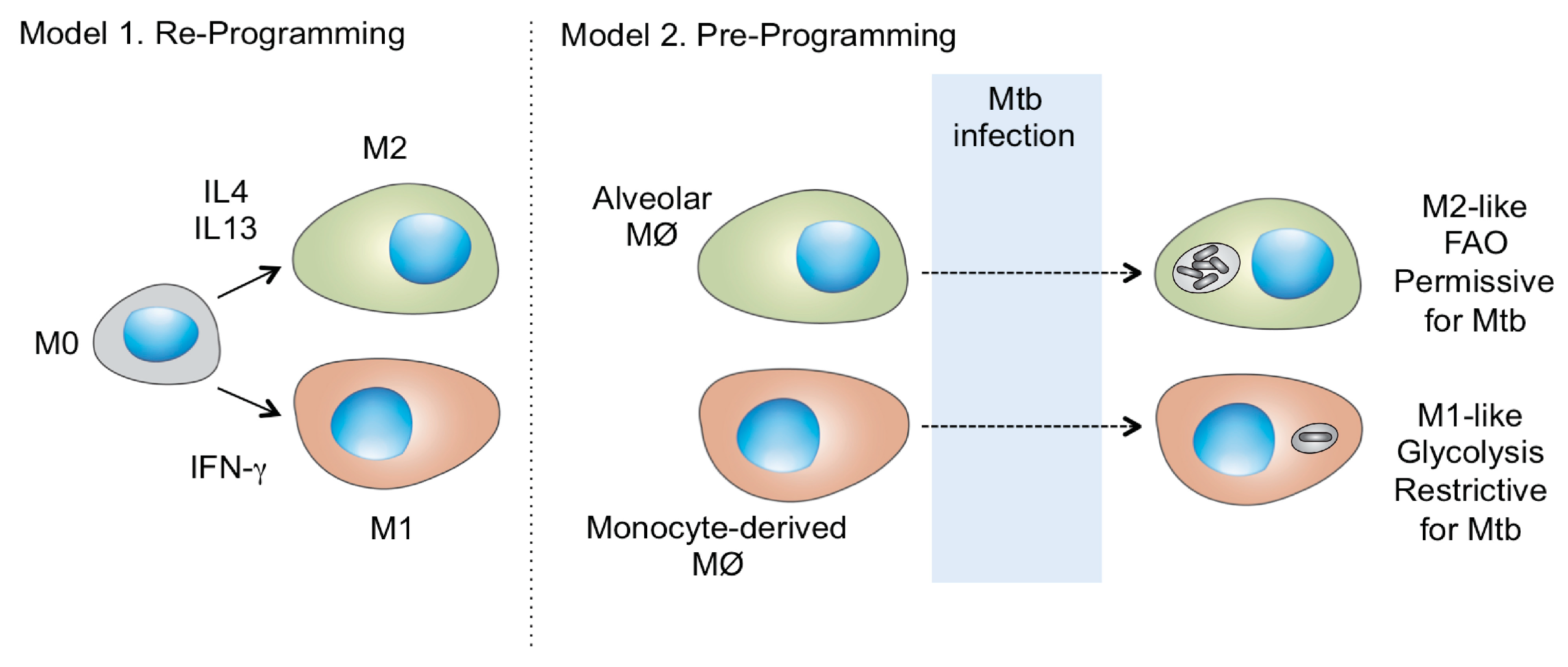
5. Is Host Macrophage Ontogeny a Missing Link in Tuberculosis Progression?
6. Final Comments
Funding
Conflicts of Interest
References
- Hershberg, R.; Lipatov, M.; Small, P.M.; Sheffer, H.; Niemann, S.; Homolka, S.; Roach, J.C.; Kremer, K.; Petrov, D.A.; Feldman, M.W.; et al. High functional diversity in Mycobacterium tuberculosis driven by genetic drift and human demography. PLoS Biol. 2008, 6, e311. [Google Scholar] [CrossRef] [PubMed]
- Wirth, T.; Hildebrand, F.; Allix-Beguec, C.; Wolbeling, F.; Kubica, T.; Kremer, K.; van Soolingen, D.; Rusch-Gerdes, S.; Locht, C.; Brisse, S.; et al. Origin, spread and demography of the Mycobacterium tuberculosis complex. PLoS Pathog. 2008, 4, e1000160. [Google Scholar] [CrossRef] [PubMed]
- Russell, D.G.; Barry, C.E., II.; Flynn, J.L. Tuberculosis: what we don’t know can and does, hurt us. Science 2010, 328, 852–856. [Google Scholar] [CrossRef] [PubMed]
- Houben, R.M.; Dodd, P.J. The Global Burden of Latent Tuberculosis Infection: A Re-estimation Using Mathematical Modelling. PLoS Med. 2016, 13, e1002152. [Google Scholar] [CrossRef] [PubMed]
- Behr, M.A.; PEdelstein, H.; Ramakrishnan, L. Revisiting the timetable of tuberculosis. BMJ 2018, 362, k2738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Houben, R.M.; Crampin, A.C.; Ndhlovu, R.; Sonnenberg, P.; Godfrey-Faussett, P.; Haas, W.H.; Engelmann, G.; Lombard, C.J.; Wilkinson, D.; Bruchfeld, J.; et al. Human immunodeficiency virus associated tuberculosis more often due to recent infection than reactivation of latent infection. Int. J. Tuberc. Lung Dis. 2011, 15, 24–31. [Google Scholar] [PubMed]
- Berry, M.P.; Graham, C.M.; McNab, F.W.; Xu, Z.; Bloch, S.A.; Oni, T.; Wilkinson, K.A.; Banchereau, R.; Skinner, J.; Wilkinson, R.J.; et al. An interferon-inducible neutrophil-driven blood transcriptional signature in human tuberculosis. Nature 2010, 466, 973–977. [Google Scholar] [CrossRef] [Green Version]
- Fiore-Gartland, A.; Carpp, L.N.; Naidoo, K.; Thompson, E.; Zak, D.E.; Self, S.; Churchyard, G.; Walzl, G.; Penn-Nicholson, A.; Scriba, T.J.; et al. Considerations for biomarker-targeted intervention strategies for tuberculosis disease prevention. Tuberculosis 2018, 109, 61–68. [Google Scholar] [CrossRef]
- Musvosvi, M.; Duffy, D.; Filander, E.; Africa, H.; Mabwe, S.; Jaxa, L.; Bilek, N.; Llibre, A.; Rouilly, V.; Hatherill, M.; et al. T-cell biomarkers for diagnosis of tuberculosis: candidate evaluation by a simple whole blood assay for clinical translation. Eur. Respir. J. 2018, 51. [Google Scholar] [CrossRef]
- Suliman, S.; Thompson, E.; Sutherland, J.; Weiner Rd, J.; Ota, M.O.C.; Shankar, S.; Penn-Nicholson, A.; Thiel, B.; Erasmus, M.; Maertzdorf, J.; et al. GC6-74 and ACS Cohort Study groups. Four-gene Pan-African Blood Signature Predicts Progression to Tuberculosis. Am. J. Respir. Crit. Care Med. 2018. [Google Scholar] [CrossRef]
- North, R.J.; Jung, Y.J. Immunity to tuberculosis. Annu. Rev. Immunol. 2004, 22, 599–623. [Google Scholar] [CrossRef] [PubMed]
- Ginhoux, F.; Guilliams, M. Tissue-Resident Macrophage Ontogeny and Homeostasis. Immunity 2016, 44, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Ginhoux, F.; Jung, S. Monocytes and macrophages: developmental pathways and tissue homeostasis. Nat. Rev. Immunol. 2014, 14, 392–404. [Google Scholar] [CrossRef] [PubMed]
- Eguiluz-Gracia, I.; Schultz, H.H.; Sikkeland, L.I.; Danilova, E.; Holm, A.M.; Pronk, C.J.; Agace, W.W.; Iversen, M.; Andersen, C.; Jahnsen, F.L.; et al. Long-term persistence of human donor alveolar macrophages in lung transplant recipients. Thorax 2016, 71, 1006–1011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nayak, D.K.; Zhou, F.; Xu, M.; Huang, J.; Tsuji, M.; Hachem, R.; Mohanakumar, T. Long-Term Persistence of Donor Alveolar Macrophages in Human Lung Transplant Recipients That Influences Donor-Specific Immune Responses. Am. J. Transpl. 2016, 16, 2300–2311. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Nazarova, E.V.; Tan, S.; Liu, Y.; Russell, D.G. Growth of Mycobacterium tuberculosis in vivo segregates with host macrophage metabolism and ontogeny. J. Exp. Med. 2018, 215, 1135–1152. [Google Scholar] [CrossRef]
- Sukumar, N.; Tan, S.; Aldridge, B.B.; Russell, D.G. Exploitation of Mycobacterium tuberculosis reporter strains to probe the impact of vaccination at sites of infection. PLoS Pathog. 2014, 10, e1004394. [Google Scholar] [CrossRef]
- Tan, S.; Sukumar, N.; Abramovitch, R.B.; Parish, T.; Russell, D.G. Mycobacterium tuberculosis responds to chloride and pH as synergistic cues to the immune status of its host cell. PLoS Pathog 2013, 9, e1003282. [Google Scholar] [CrossRef]
- Russell, D.G.; Huang, L.; VanderVen, B.C. Immunometabolism at the interface between macrophages and pathogens. Nat. Rev. Immunol. 2019, 19, 291–304. [Google Scholar] [CrossRef]
- Lee, S.H.; Charmoy, M.; Romano, A.; Paun, A.; Chaves, M.M.; Cope, F.O.; Ralph, D.A.; Sacks, D.L. Mannose receptor high, M2 dermal macrophages mediate nonhealing Leishmania major infection in a Th1 immune environment. J. Exp. Med. 2018, 215, 357–375. [Google Scholar] [CrossRef]
- Mould, K.J.; Barthel, L.; Mohning, M.P.; Thomas, S.M.; McCubbrey, A.L.; Danhorn, T.; Leach, S.M.; Fingerlin, T.E.; O’Connor, B.P.; Reisz, J.A.; et al. Cell Origin Dictates Programming of Resident versus Recruited Macrophages during Acute Lung Injury. Am. J. Respir. Cell. Mol. Biol. 2017, 57, 294–306. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Russell, D.G. Tuberculosis Progression Does Not Necessarily Equate with a Failure of Immune Control. Microorganisms 2019, 7, 185. https://doi.org/10.3390/microorganisms7070185
Russell DG. Tuberculosis Progression Does Not Necessarily Equate with a Failure of Immune Control. Microorganisms. 2019; 7(7):185. https://doi.org/10.3390/microorganisms7070185
Chicago/Turabian StyleRussell, David G. 2019. "Tuberculosis Progression Does Not Necessarily Equate with a Failure of Immune Control" Microorganisms 7, no. 7: 185. https://doi.org/10.3390/microorganisms7070185
APA StyleRussell, D. G. (2019). Tuberculosis Progression Does Not Necessarily Equate with a Failure of Immune Control. Microorganisms, 7(7), 185. https://doi.org/10.3390/microorganisms7070185




