Host Richness Increases Tuberculosis Disease Risk in Game-Managed Areas
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Surveys and Disease
2.2. Host Abundances
2.3. Species and Community Competence
2.4. Statistics
2.5. Ethics Statement
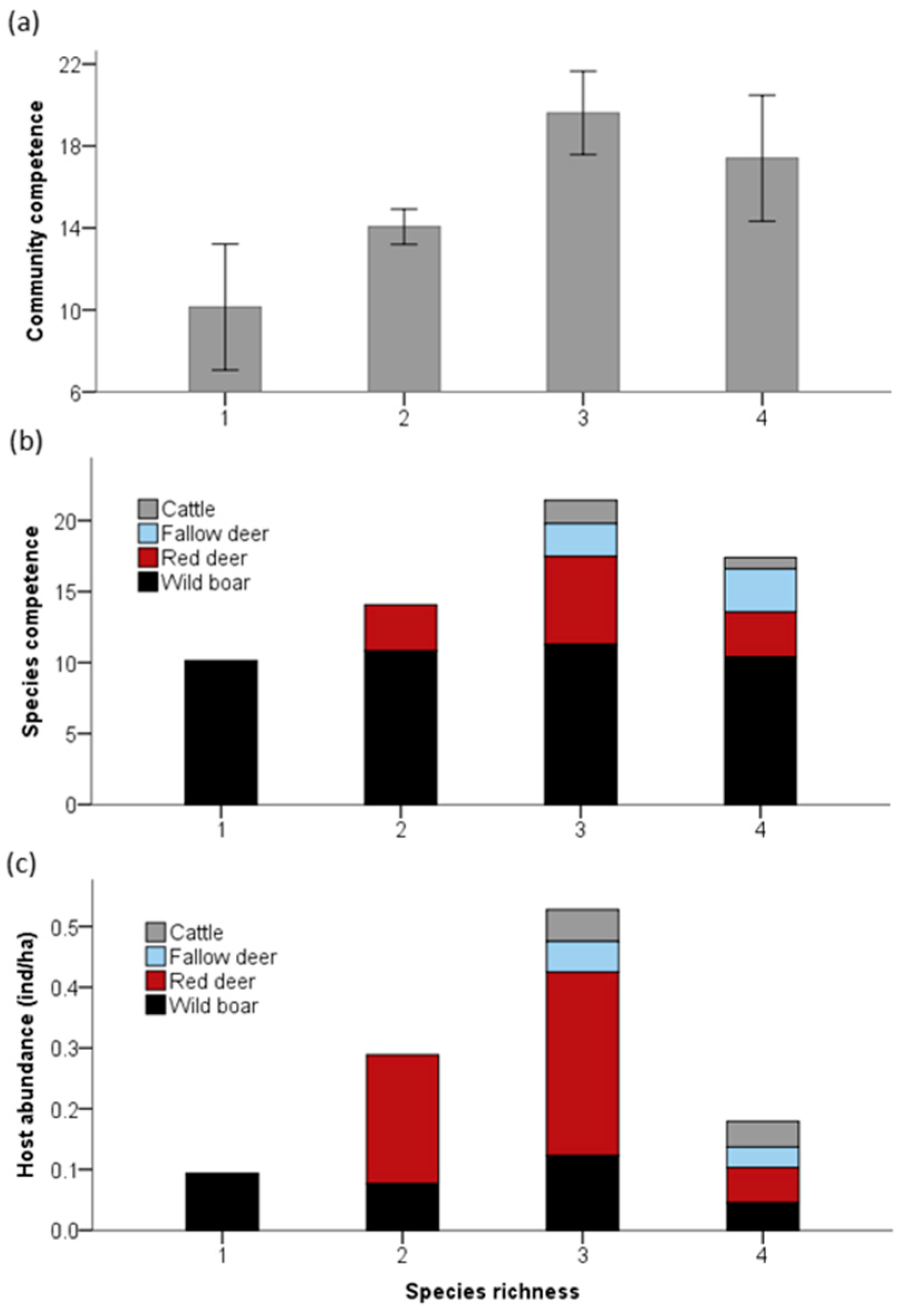
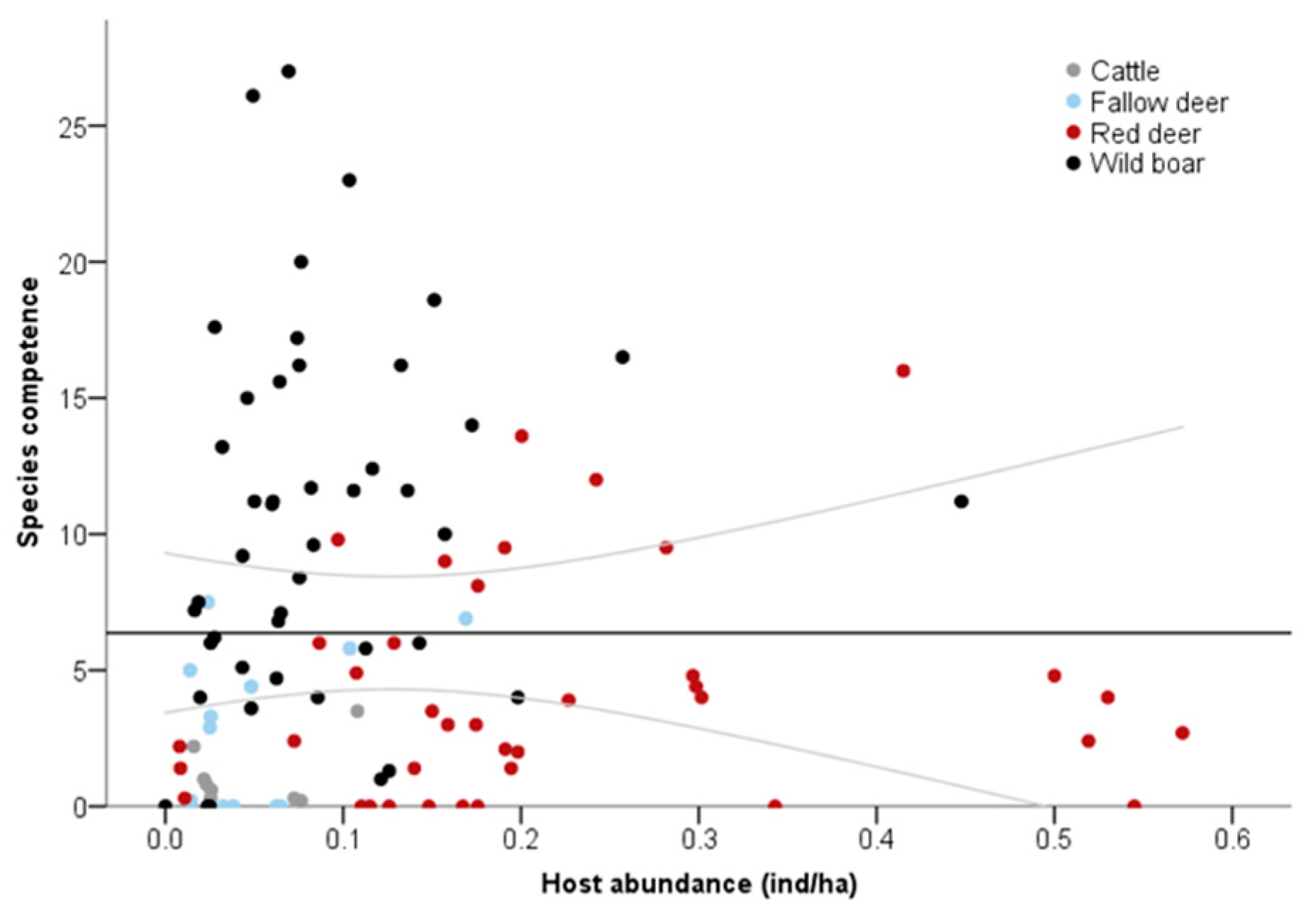
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Johnson, P.T.J.; Preston, D.L.; Hoverman, J.T.; Richgels, K.L.D. Biodiversity decreases disease through predictable changes in host community competence. Nature 2013, 494, 230–234. [Google Scholar] [CrossRef]
- Salkeld, D.J.; Padgett, K.A.; Jones, J.H. Meta-analysis suggesting that the relationship between biodiversity and risk of zoonotic pathogen transmission isidiosyncratic. Ecol. Lett. 2013, 16, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Hudson, P.J.; Dobson, A.P.; Lafferty, K.D. Is a healthy ecosystem one that is rich in parasites? Trends Ecol. Evol. 2006, 21, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.E.; Patel, N.G.; Levy, M.A.; Storeygard, A.; Balk, D.; Gittleman, J.L.; Daszak, P. Global trends in emerging infectious diseases. Nature 2008, 451, 990–994. [Google Scholar] [CrossRef] [PubMed]
- Wood, C.L.; Lafferty, K.D.; Micheli, F. Fishing out marine parasites? Impacts of fishing on rates of parasitism in the ocean. Ecol. Lett. 2010, 13, 761–775. [Google Scholar] [CrossRef] [PubMed]
- Keesing, F.; Holt, R.D.; Ostfeld, R.S. Effects of species diversity on disease risk. Ecol. Lett. 2006, 9, 485–498. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.Y.; de Boer, W.F.; van Langevelde, F.; Xu, C.; Jebara, K.B.; Berlingieri, F.; Prins, H.H. Dilution effect in bovine tuberculosis: Risk factors for regional disease occurrence in Africa. Proc. R. Soc. Lond. B Biol. Sci. 2013, 280, 20130624. [Google Scholar] [CrossRef] [PubMed]
- LoGiudice, K.; Duerr, S.T.; Newhouse, M.J.; Schmidt, K.A.; Killilea, M.E.; Ostfeld, R.S. Impact of host community composition on Lyme disease risk. Ecology 2008, 89, 2841–2849. [Google Scholar] [CrossRef] [PubMed]
- Salkeld, D.J.; Salathe, M.; Stapp, P.; Jones, J.H. Plague outbreaks in prairie dog populations explained by percolation thresholds of alternate host abundance. Proc. Natl. Acad. Sci. USA 2010, 107, 14247–14250. [Google Scholar] [CrossRef] [PubMed]
- Hardstaff, J.L.; Marion, G.; Hutchings, M.R.; White, P.C. Evaluating the tuberculosis hazard posed to cattle from wildlife across Europe. Res. Vet. Sci. 2014, 97, S86–S93. [Google Scholar] [CrossRef]
- Humblet, M.F.; Boschiroli, M.L.; Saegerman, C. Classification of worldwide bovine tuberculosis risk factors in cattle: A stratified approach. Vet. Res. 2009, 40, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Ward, A.I.; Smith, G.C.; Etherington, T.R.; Delahay, R.J. Estimating the risk of cattle exposure to tuberculosis posed by wild deer relative to badgers in England and Wales. J. Wildl. Dis. 2009, 45, 1104–1120. [Google Scholar] [CrossRef] [PubMed]
- Roche, B.; Dobson, A.P.; Guégan, J.F.; Rohani, P. Linking community and disease ecology: The impact of biodiversity on pathogen transmission. Philos Trans. R. Soc. Lond. B Biol. Sci. 2012, 367, 2807–2813. [Google Scholar] [CrossRef] [PubMed]
- Sintayehu, D.W.; Heitkönig, I.M.; Prins, H.H.; Tessema, Z.K.; De Boer, W.F. Effect of host diversity and species assemblage composition on bovine tuberculosis (bTB) risk in Ethiopian cattle. Parasitology 2017, 144, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Vicente, J.; Barasona, J.A.; Acevedo, P.; Ruiz-Fons, J.F.; Boadella, M.; Diez-Delgado, I.; Gortazar, C. Temporal trend of tuberculosis in wild ungulates from Mediterranean Spain. Transbound. Emerg. Dis. 2013, 60, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Barasona, J.A.; Latham, M.C.; Acevedo, P.; Armenteros, J.A.; Latham, A.D.M.; Gortazar, C.; Carro, F.; Soriguer, R.C.; Vicente, J. Spatiotemporal interactions between wild boar and cattle: Implications for cross-species disease transmission. Vet. Res. 2014, 45, 122. [Google Scholar] [CrossRef] [PubMed]
- Vicente, J.; Höfle, U.; Fernández de Mera, I.G.; Gortázar, C. The importance of parasite life-history and host density in predicting the impact of infections in red deer. Oecologia 2007, 152, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Barasona, J.A.; Acevedo, P.; Diez-Delgado, I.; Queiros, J.; Carrasco-García, R.; Gortazar, C.; Vicente, J. Tuberculosis-associated death among adult wild boars, Spain, 2009–2014. Emerg. Infect. Dis. 2016, 22, 2178. [Google Scholar] [CrossRef]
- Gortazar, C.; Torres, M.J.; Acevedo, P.; Aznar, J.; Negro, J.J.; de la Fuente, J.; Vicente, J. Fine-tuning the space, time, and host distribution of mycobacteria in wildlife. BMC Microbiol. 2011, 11, 27. [Google Scholar] [CrossRef]
- Acevedo, P.; Vicente, J.; Höfle, U.; Cassinello, J.; Ruiz-Fons, F.; Gortazar, C. Estimation of European wild boar relative abundance and aggregation: A novel method in epidemiological risk assessment. Epid. Inf. 2007, 135, 519–527. [Google Scholar] [CrossRef]
- Acevedo, P.; Quirós-Fernández, F.; Casal, J.; Vicente, J. Spatial distribution of wild boar population abundance: Basic information for spatial epidemiology and wildlife management. Ecol. Indic. 2014, 36, 594–600. [Google Scholar] [CrossRef]
- Association for the Study of Animal Behaviour (ASAB). Guidelines for the treatment of animals in behavioural research and teaching. Anim. Behav. 2006, 71, 245–253. [Google Scholar] [CrossRef]
- Dobson, A.P. Comparisons of some characteristics of the life histories of microparasites, macroparasites, and predators. In Population Biology of Infectious Diseases; Anderson, R.M., May, R.M., Eds.; Springer-Verlag: New York, NY, USA, 1982. [Google Scholar]
- Pfennig, K.S. Evolution of pathogen virulence: The role of variation in host phenotype. Proc. R. Soc. Lond. B 2011, 268, 755–760. [Google Scholar] [CrossRef] [PubMed]
- Barasona, J.A.; Vicente, J.; Díez-Delgado, I.; Aznar, J.; Gortázar, C.; Torres, M.J. Environmental presence of Mycobacterium tuberculosis complex in aggregation points at the wildlife/livestock interface. Transbound. Emerg. Dis. 2017, 64, 1148–1158. [Google Scholar] [CrossRef] [PubMed]
- Fine, A.E.; Bolin, C.A.; Gardiner, J.C.; Kaneene, J.B. A study of the persistence of Mycobacterium bovis in the environment under natural weather conditions in Michigan, USA. Vet. Med. Int. 2011, 2011. [Google Scholar] [CrossRef]
- Civitello, D.J.; Cohen, J.; Fatima, H.; Halstead, N.T.; Liriano, J.; McMahon, T.A.; Rohr, J.R. Biodiversity inhibits parasites: Broad evidence for the dilution effect. Proc. Natl. Acad. Sci. USA 2015, 112, 8667–8671. [Google Scholar] [CrossRef] [PubMed]
- Luis, A.D.; Kuenzi, A.J.; Mills, J.N. Species diversity concurrently dilutes and amplifies transmission in a zoonotic host–pathogen system through competing mechanisms. Proc. Natl. Acad. Sci. USA 2018, 115, 7979–7984. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barasona, J.A.; Gortázar, C.; de la Fuente, J.; Vicente, J. Host Richness Increases Tuberculosis Disease Risk in Game-Managed Areas. Microorganisms 2019, 7, 182. https://doi.org/10.3390/microorganisms7060182
Barasona JA, Gortázar C, de la Fuente J, Vicente J. Host Richness Increases Tuberculosis Disease Risk in Game-Managed Areas. Microorganisms. 2019; 7(6):182. https://doi.org/10.3390/microorganisms7060182
Chicago/Turabian StyleBarasona, Jose Angel, Christian Gortázar, José de la Fuente, and Joaquín Vicente. 2019. "Host Richness Increases Tuberculosis Disease Risk in Game-Managed Areas" Microorganisms 7, no. 6: 182. https://doi.org/10.3390/microorganisms7060182
APA StyleBarasona, J. A., Gortázar, C., de la Fuente, J., & Vicente, J. (2019). Host Richness Increases Tuberculosis Disease Risk in Game-Managed Areas. Microorganisms, 7(6), 182. https://doi.org/10.3390/microorganisms7060182





