Multifaceted Applications of Microbial Pigments: Current Knowledge, Challenges and Future Directions for Public Health Implications
Abstract
:1. Introduction
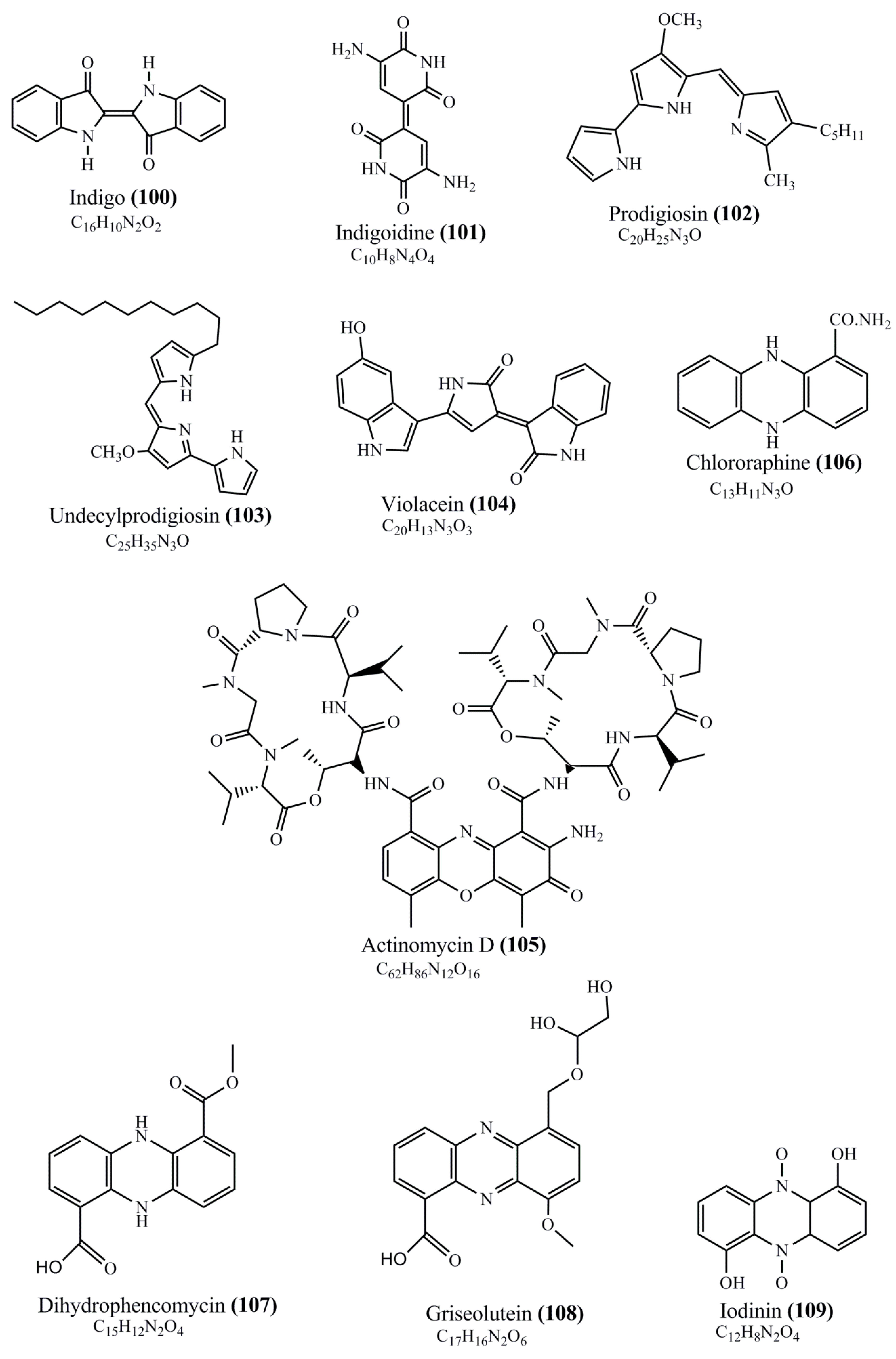
2. Microbial Pigments and Chemical Structures
3. Brief Historical Note on Microbial Pigments
4. Host Pigmented Compounds Said to Be of Microbial Origin
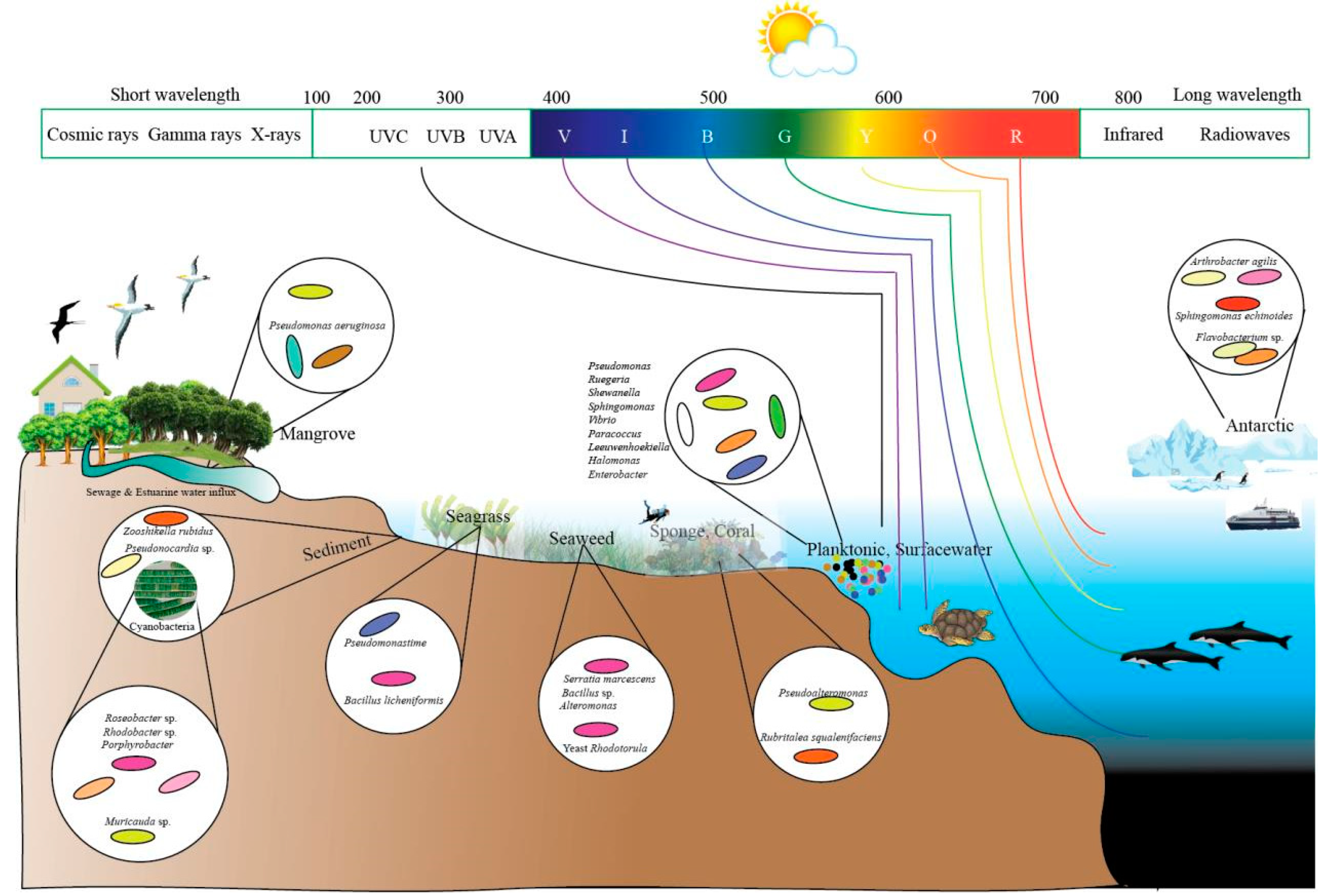
5. Ecology and Habitats of Pigmented Microorganisms
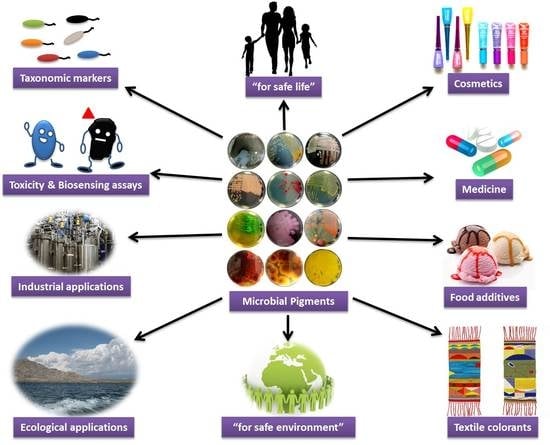
6. Uses of Microbial Pigments
6.1. Biological Significance
6.2. Industrial Significance
6.2.1. Antibacterial Activity
6.2.2. Antifungal Activity
6.2.3. Antiviral Activity
6.2.4. Antimetastatic Activity
6.2.5. Immunosuppressive Activity
6.2.6. Antitumor Activity
6.2.7. Anti-Alzhelmeric Activity
6.2.8. Antiatherosclerosis Activity
6.2.9. Antihypertensive Activity
6.2.10. Anticancer Activity or Antineoplastic Activity
6.2.11. Anti-Tuberculosis Activity
6.2.12. Antifouling Activity
6.2.13. Anti-Algicidal Activity
6.2.14. Anti-Insecticidal Activity
6.2.15. Anti-Herbicidal Activity
6.2.16. Antiparasitic Activity
6.2.17. Antiprotozoal Activity
6.2.18. Antileishmanial Activity
6.2.19. Antiulcerogenic Activity
6.2.20. Antilipoperoxidant Activity
6.2.21. Anti-HIV Activity
6.2.22. Anti-Malarial Activity
6.2.23. Antitrypanosomal Activity
6.2.24. Antinematodal Activity
6.2.25. Anti-Inflammatory Activity
6.2.26. Antihypertriglyceridemia Activity
6.2.27. Anti-Atherosclerotic Activity
6.2.28. Antioxidant Activity
6.2.29. Anti-Proliferation Activity
6.2.30. Anti-Aging Activity
6.2.31. Anti-Obesity Activity
6.2.32. Anti-Diabetic Activity
6.2.33. Antiadipogenic Activity
6.2.34. Ichthyodeterrent Activity
6.2.35. Conjugated Antibodies
6.2.36. Cytotoxic Activity
6.2.37. Inducing Activity as Larval Metamorphosis
6.2.38. Miscellaneous Activities
7. Factors Affecting Pigment Production
8. Challenges in Pigment Compound Development
9. Pathogenicity of Pigmented Microbes
10. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aylward, F.O.; Eppley, J.M.; Smith, J.M.; Chavez, F.P.; Scholin, C.A.; DeLong, E.F. Microbial community transcriptional networks are conserved in three domains at ocean basin scales. Proc. Natl. Acad. Sci. USA 2015, 112, 5443–5448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soliev, A.B.; Hosokawa, K.; Enomoto, K. Bioactive pigments from marine bacteria: Applications and physiological roles. Evid.-Based Complement. Altern. Med. 2011, 2011, 670349. [Google Scholar] [CrossRef] [PubMed]
- Venil, C.K.; Zakaria, Z.A.; Ahmad, W.A. Bacterial pigments and their applications. Process Biochem. 2013, 48, 1065–1079. [Google Scholar] [CrossRef]
- Narsing Rao, M.P.; Xiao, M.; Li, W.-J. Fungal and Bacterial Pigments: Secondary Metabolites with WideApplications. Front. Microbiol. 2017, 8, 1113. [Google Scholar] [CrossRef] [PubMed]
- Numan, M.; Bashir, S.; Mumtaz, R.; Tayyab, S.; Rehman, N.U.; Khan, A.L.; Shinwari, Z.K.; Al-Harrasi, A. Therapeutic applications of bacterial pigments: A review of current status and future opportunities. 3 Biotech 2018, 8, 207. [Google Scholar] [CrossRef] [PubMed]
- Mumtaz, R.; Bashir, S.; Numan, M.; ShinwarI, Z.K.; Ali, M. Pigments from Soil Bacteria and Their Therapeutic Properties: A Mini Review. Curr. Microbiol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, C.H.; Vinithkumar, N.V.; Kirubagaran, R. Marine pigmented bacteria: A prospective source of antibacterial compounds. J. Nat. Sci. Biol. Med. 2019, in press. [Google Scholar]
- Kim, S. Marine Biomaterials: Characterization, Isolation and Applications; CRC Press: New York, NY, USA, 2013; pp. 1–787. [Google Scholar]
- Amaro, H.M.; Sousa-Pinto, I.; Malcata, F.X.; Guedes, A.C. Microalgae as a source of pigments extraction and purification methods. In Marine Microorganisms Extraction and Analysis of Bioactive Compounds; Leo, M.L.N., Ed.; CRC Press: Boca Raton, FL, USA, 2016; pp. 99–128. [Google Scholar]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2014, 31, 160–258. [Google Scholar] [CrossRef] [Green Version]
- Kirti, K.; Amita, S.; Priti, S.; Kumar, A.M.; Jyoti, S. Colorful World of Microbes: Carotenoids and Their Applications. Adv. Biol. 2014, 2014, 837891. [Google Scholar] [CrossRef]
- Britton, G.; Liaaen-Jensen, S.; Pfander, H. Carotenoids—Handbook; Birkhäuser Verlag: Basel, Switzerland, 2004; pp. 1–625. [Google Scholar]
- Soliev, A.B.; Enomoto, K. Antitumor Pigments from Marine Bacteria. In Marine Biomaterials: Characterization, Isolation and Applications; Kim, S., Ed.; CRC Press: London, UK, 2013; pp. 149–171. [Google Scholar]
- Williamson, N.R.; Fineran, P.C.; Gristwood, T.; Chawrai, S.R.; Leeper, F.J.; Salmond, G.P.C. Anticancer and immunosuppressive properties of bacterial prodiginines. Future Microbiol. 2007, 2, 605–618. [Google Scholar] [CrossRef]
- Gürses, A.; Açıkyıldız, M.; Güneş, K.; Gürses, M.S. Dyes and Pigments; Springer: Cham, Switzerland, 2016; pp. 1–83. [Google Scholar]
- Delgado-Vargas, F.; Paredes-López, O. Natural Colorants for Food and Nutraceutical Uses; CRC Press LLC: Boca Raton, FL, USA, 2003; pp. 1–344. [Google Scholar]
- Babitha, S. Microbial Pigments. In Biotechnology for Agro-Industrial Residues Utilisation; Nigam, P.S., Pandey, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 147–162. [Google Scholar]
- Caro, Y.; Anamale, L.; Fouillaud, M.; Laurent, P.; Petit, T.; Dufosse, L. Natural hydroxyanthraquinoid pigmentsas potent food grade colorants: An overview. Nat. Prod. Bioprospect. 2012, 2, 174–193. [Google Scholar] [CrossRef]
- Misawa, N. Carotenoid β-ring hydroxylase and ketolase from marine bacteria-promiscuous enzymes for synthesizing functional xanthophylls. Mar. Drugs 2011, 9, 757–771. [Google Scholar] [CrossRef] [PubMed]
- Heider, S.A.; Peters-Wendisch, P.; Wendisch, V.F.; Beekwilder, J.; Brautaset, T. Metabolic engineering for the microbial production of carotenoids and related products with a focus on the rare C50 carotenoids. Appl. Microbiol. Biotechnol. 2014, 98, 4355–4368. [Google Scholar] [CrossRef] [PubMed]
- Dufossé, L. Current and Potential Natural Pigments from Microorganisms (Bacteria, Yeasts, Fungi, Microalgae). In Handbook on Natural Pigments in Food and Beverages; Carle, R., Schweiggert, R.M., Eds.; Elsevier Ltd.: Cambridge, UK, 2016; pp. 337–354. [Google Scholar]
- Revuelta, J.L.; Ledesma-Amaro, R.; Jiménez, A. Industrial Production of Vitamin B2 by Microbial Fermentation. In Industrial Biotechnology of Vitamins, Biopigments, and Antioxidants, 1st ed.; Vandamme, E.J., Revuelta, J.L., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2016; pp. 17–40. [Google Scholar]
- Baxter, B.K.; Gunde-Cimerman, N.; Oren, A. Salty sisters: The women of halophiles. Front. Microbiol. 2014, 5, 192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelis, H.J.; de Leenheer, A.P. Microbial production of carotenoids other than β-carotene. In Biotechnology of Vitamins, Pigments and Growth Factors; Vandamme, J., Ed.; Elsevier: Essex, UK, 1989; pp. 43–80. [Google Scholar]
- Joshi, V.K.; Attri, D.; Bala, A.; Bhushan, S. Microbial pigments. Indian J. Biotechnol. 2003, 2, 362–369. [Google Scholar]
- Feng, Y.; Shao, Y.; Zhou, Y.; Chen, W.; Chen, F. Monascus Pigments. In Industrial Biotechnology of Vitamins, Biopigments, and Antioxidants, 1st ed.; Vandamme, E.J., Revuelta, J.L., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2016; pp. 497–535. [Google Scholar]
- Hodgkiss, W.; Liston, M.; Godwin, T.W.; Jamikorn, M. The isolation and description of two marine micro-organisms with special reference to their pigment production. J. Gen. Microbiol. 1954, 11, 488–4150. [Google Scholar] [CrossRef] [PubMed]
- Giddings, L.; Newman, D.J. Bioactive Compounds from Terrestrial Extremophiles; Springer: Cham, Switzerland, 2015; pp. 1–75. [Google Scholar]
- Okazaki, T.; Kitahara, T.; Okami, Y. Studies on marine microorganisms. IV. A new antibiotic SS-228 Y produced by Chainia isolated from shallow sea mud. J. Antibiot. 1975, 28, 176–184. [Google Scholar] [CrossRef]
- Franks, A.; Haywood, P.; Holmstöm, C.; Egan, S.; Kjelleberg, S.; Kumar, N. Isolation and structure elucidation of a novel yellow pigment from the marine bacterium Pseudoalteromonas tunicata. Molecules 2005, 10, 1286–1291. [Google Scholar] [CrossRef]
- Blackman, A.; Li, C. New tambjamine alkaloids from the marine bryozoan Bugula dentata. Aust. J. Chem. 1994, 47, 1625–1629. [Google Scholar] [CrossRef]
- Pinkerton, D.M.; Banwell, M.G.; Garson, M.J.; Kumar, N.; de Moraes, M.O.; Cavalcanti, B.C.; Barros, F.W.; Pessoa, C. Antimicrobial and cytotoxic activities of synthetically derived tambjamines C and E–J, BE-18591, and a related alkaloid from the marine bacterium Pseudoalteromonas tunicata. Chem. Biodivers. 2010, 7, 1311–1324. [Google Scholar] [CrossRef]
- Hermansson, M.; Jones, G.W.; Kjelleberg, S. Frequency of antibiotic and heavy metal resis-tance, pigmentation, and plasmids in bacteria of the marine airwater interface. Appl. Environ. Microbiol. 1987, 53, 2338–2342. [Google Scholar] [PubMed]
- Miteva, V.I.; Sheridan, P.P.; Brenchley, J.E. Phylogenetic and physiological diversity of micro-organisms isolated from a deep greenland glacier ice core. Appl. Environ. Microbiol. 2004, 70, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.F.; Yao, T.D.; Tian, L.D.; Xu, S.J.; An, L.Z. Phylogenetic and physiological diversity of bacteria isolated from puruogangri ice core. Microb. Ecol. 2008, 55, 476–488. [Google Scholar] [CrossRef]
- Agogué, H.; Joux, F.; Obernosterer, I.; Lebaron, P. Resistance of marine bacterioneuston to solar radiation. Appl. Environ. Microbiol. 2005, 71, 5282–5289. [Google Scholar] [CrossRef] [PubMed]
- Khanafari, A.; Khavarinejad, D.; Mashinchian, A. Solar salt lake as natural environmental source for extraction halophilic pigments. Iran. J. Microbiol. 2009, 2, 103–109. [Google Scholar]
- Yurkov, V.V.; Krieger, S.; Stackebrandt, E.; Beatty, J.T. Citromicrobium bathyomarinum, a novel aerobic bacterium isolated from deep-sea hydrothermal vent plume waters that contains photosynthetic pigment-protein complexes. J. Bacteriol. 1999, 181, 4517–4525. [Google Scholar] [PubMed]
- Hathaway, J.J.M.; Garcia, M.G.; Balasch, M.M.; Spilde, M.N.; Stone, F.D.; Dapkevicius, M.D.L.N.; Amorim, I.R.; Gabriel, R.; Borges, P.A.; Northup, D.E. Comparison of bacterial diversity in Azorean and Hawaiian lava cave microbial mats. Geomicrobiol. J. 2014, 31, 205–220. [Google Scholar] [CrossRef]
- Ramesh, C.H.; Mohanraju, R.; Narayana, S.; Murthy, K.N.; Karthick, P. Molecular characterization of marine pigmented bacteria showing antibacterial activity. Indian J. Mar. Sci. 2017, 46, 2081–2087. [Google Scholar]
- Nedashkovskaya, O.I.; Ludwig, W. Family II. Cyclobacteriaceae fam. nov. In Bergey’s Manual of Systematic Bacteriology, 2nd ed.; Krieg, N.R., Ludwig, W., Whitman, W., Hedlund, B.P., Paster, B.J., Staley, J.T., Ward, N., Brown, D., Parte, A., Eds.; Springer: New York, NY, USA, 2011; Volume 4, pp. 423–444. [Google Scholar]
- Méndez-Zavala, A.; Contreras-Esquivel, J.C.; Lara-Victoriano, F.; Rodríguez-Herrera, R.; Aguilar, C.N. Fungal production of the red pigment using a xerophilic strain Penicillium purpurogenum GH-2. Revista Mexicana de Ingeniería Química 2007, 6, 267–273. [Google Scholar]
- Van den Hoek, C.; Mann, D.G.; Jahns, H.M. Algae: An Introduction to Phycology; Cambridge University Press: Cambridge, UK, 1995; 637p. [Google Scholar]
- Lee, R.E. Phycology; Cambridge University Press: Cambridge, UK, 1999; pp. 1–624. [Google Scholar]
- Graham, L.; Wilcox, L. Algae; Prentice-Hall: Englewood Cliffs, NJ, USA, 2000; 700p. [Google Scholar]
- Likens, G.E. Plankton of Inland Waters: A Derivative of Encyclopedia of Inland Waters, 1st ed.; Academic Press/Elsevier: New York, NY, USA, 2010; 412p. [Google Scholar]
- Takaichi, S. Carotenoids in Algae: Distributions, Biosyntheses and Functions. Mar. Drugs 2011, 9, 1101–1118. [Google Scholar] [CrossRef]
- Roy, S.; Llewellyn, C.; Egeland, E.S.; Johnsen, G. Phytoplankton Pigments: Characterization, Chemotaxonomy and Applications in Oceanography; Cambridge University Press: Cambridge, UK, 2011; 845p. [Google Scholar]
- Stafsnes, M.H.; Bruheim, P. Pigmented Marine Heterotrophic Bacteria. In Marine Biomaterials: Characterization, Isolation and Applications; Kim, S., Ed.; CRC Press, Taylor & Francis Group: London, UK, 2013; pp. 117–148. [Google Scholar]
- Dieser, M.; Greenwood, M.; Foreman, C.M. Carotenoid pigmentation in Antarctic hetero-trophic bacteria as a strategy to withstand environmental stresses. Arct. Antarct. Alp. Res. 2010, 42, 396–405. [Google Scholar] [CrossRef]
- Egan, S.; James, S.; Holmström, C.; Kjelleberg, S. Correlation between pigmentation and antifouling compounds produced by Pseudoalteromonas tunicata. Environ. Microbiol. 2002, 4, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Mandelli, F.; Miranda, V.; Rodrigues, E.; Mercadante, A. Identification of carotenoids with high antioxidant capacity produced by extremophile microorganisms. World J. Microbiol. Biotechnol. 2012, 28, 1781–1790. [Google Scholar] [CrossRef] [PubMed]
- Chew, B.P.; Park, J.S. Carotenoid action on the immune response. J. Nutr. 2004, 134, 257S–261S. [Google Scholar] [CrossRef] [PubMed]
- Konzen, M.; Marco, D.D.; Cordova, C.A.S.; Vieira, T.O.; Antônio, R.V.; Creczynski-Pasa, T.B. Antioxidant properties of violacein: Possible relation on its biological function. Bioorg. Med. Chem. 2006, 14, 8307–8313. [Google Scholar] [CrossRef] [PubMed]
- Pierson, L.; Pierson, E. Metabolism and function of phenazines in bacteria: Impacts on the behavior of bacteria in the environment and biotechnological processes. Appl. Microbiol. Biotechnol. 2010, 86, 1659–1670. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, L.; Costa, M.S. The Family Thermaceae. In The Prokaryotes—Other Major Lineages of Bacteria and the Archaea; Rosenberg, E., DeLong, E.F., Lory, S., Stackebrandt, E., Thompson, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 955–987. [Google Scholar]
- Boric, M.; Danevcic, T.; Stopar, D. Prodigiosin from Vibrio sp. DSM 14379: A new UV-protective pigment. Microb. Ecol. 2011, 62, 528–536. [Google Scholar] [CrossRef]
- Plonka, P.M.; Grabacka, M. Melanin synthesis in microorganisms—Biotechnological and medical aspects. Acta Biochim. Pol. 2006, 53, 429–443. [Google Scholar]
- Coyne, V.E.; Al-Harthi, L. Induction of melanin biosynthesis in Vibrio cholerae. Appl. Environ. Microbiol. 1992, 58, 2861–2865. [Google Scholar]
- Matz, C.; Deines, P.; Boenigt, J.; Arndt, H.; Eberl, L.; Kjelleberg, S.; Jürgens, K. Impact of violacein-producing bacteria on survival and feeding of bacterivorous nanoflagellates. Appl. Environ. Microbiol. 2004, 70, 1593–1599. [Google Scholar] [CrossRef]
- Visca, P.; Imperi, F.; Lamont, I.L. Pyoverdine siderophores: From biogenesis to biosignificance. Trends Microbiol. 2006, 15, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.; Chandramohan, D.; Bharathi, P.A.L. Differential sensitivity of pigmented and non-pigmented marine bacteria to metals and antibiotics. Water Res. 1992, 26, 431–434. [Google Scholar] [CrossRef]
- Margalith, P.Z. Pigment Microbiology; Chapman & Hall: London, UK, 1992; pp. 1–156. [Google Scholar]
- Gessler, N.N.; Egorova, A.S.; Belozerskaya, T.A. Fungal anthraquinones. Appl. Biochem. Microbiol. 2013, 49, 85–99. [Google Scholar] [CrossRef]
- Soni, S.K. Microbes: A Source of Energy for the 21st Century; New India Publishing Agency: New Delhi, India, 2007; pp. 1–590. [Google Scholar]
- Tong, Y.Y.; Lighthart, B. Solar radiation is shown to select for pigmented bacteria in the ambient outdoor atmosphere. Photochem. Photobiol. 1997, 65, 103–106. [Google Scholar] [CrossRef]
- Stafsnes, M.; Josefsen, K.; Kildahl-Andersen, G.; Valla, S.; Ellingsen, T.; Bruheim, P. Isolation and characterization of marine pigmented bacteria from Norwegian coastal waters and screening for carotenoids with UVA-blue light absorbing properties. J. Microbiol. 2010, 48, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Asada, C.; Sawada, T. Production of antibacterial violet pigment by psychrotropic bacterium RT102 strain. Biotechnol. Bioprocess Eng. 2003, 8, 37–40. [Google Scholar] [CrossRef]
- Liu, G.Y.; Nizet, V. Color me bad: Microbial pigments as virulence factors. Trends Microbiol. 2009, 17, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Oren, A. A short history of the symposia on halophilic microorganisms: From Rehovot 1978 to Beijing 2010. In Halophiles and Hypersaline Environments: Current Research and Future Trends; Ventosa, A., Oren, A., Ma, Y., Eds.; Springer: Berlin, Germany, 2011; pp. 373–382. [Google Scholar]
- Lev-Yadun, S.; Halpern, M. Ergot (Claviceps purpurea)—An aposematic fungus. Symbiosis 2007, 43, 105–108. [Google Scholar]
- Venil, C.K.; Aruldass, C.A.; Dufossé, L.; Zakaria, Z.A.; Ahmad, W.A. Current perspective on bacterial pigments: Emergingsustainable compounds with coloring and biological properties for the industry—An incisive evaluation. RSC Adv. 2014, 4, 39523–39529. [Google Scholar] [CrossRef]
- Nigam, P.S.; Luke, J.S. Food additives: Production of microbial pigments and their antioxidant properties. Curr. Opin. Food Sci. 2016, 7, 93–100. [Google Scholar] [CrossRef]
- Capelli, G.C.; Cysewski, G. The Worlds’ Best Kept Health Secret Natural Astaxanthin; Cyanotech Corporation: Kailua-Kona, HI, USA, 2013; pp. 1–202. [Google Scholar]
- Ananya, A.K.; Ahmad, I.Z. Cyanobacteria “the blue green algae” and its novel applications: A brief review. Int. J. Innov. Appl. Stud. 2014, 7, 251–261. [Google Scholar]
- Rao, M. Microbes and Non-Flowering Plants: Impact and Applications; Ane Books Pvt Ltd.: New Delhi, India, 2009; 565p. [Google Scholar]
- Sonani, R.R.; Rastogi, R.P.; Patel, R.; Madamwar, D. Recent advances in production, purification and applications of phycobiliproteins. World J. Biol. Chem. 2016, 26, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Konuray, G.; Erginkaya, Z. Antimicrobial and antioxidant properties of pigments synthesized from microorganisms. In The Battle against Microbial Pathogens: Basic Science, Technological Advances and Educational Programs; Méndez-Vilas, A., Ed.; Formatex Research Center: Badajoz, Spain, 2015; pp. 27–33. [Google Scholar]
- Begum, H.; Yusoff, F.M.; Banerjee, S.; Khatoon, H.; Shariff, M. Availability and utilization of pigments from microalgae. Crit. Rev. Food Sci. Nutr. 2015, 56, 2209–2222. [Google Scholar] [CrossRef] [PubMed]
- Stankovic, N.; Senerovic, L.; Ilic-Tomic, T.; Vasiljevic, B.; Nikodinovic-Runic, J. Properties and applications of undecylprodigiosin and other bacterial prodigiosins. Appl. Microbiol. Biotechnol. 2014, 98, 3841–3858. [Google Scholar] [CrossRef] [PubMed]
- Durán, N.; Justo, G.Z.; Ferreira, C.V.; Melo, P.S.; Cordi, L.; Martins, D. Violacein: Properties and biological activities. Biotechnol. Appl. Biochem. 2007, 48, 127–133. [Google Scholar] [PubMed]
- Andersen, R.J.; Wolfe, M.S.; Faulkner, D.J. Autotoxic antibiotic production by a marine Chromobacterium. Mar. Biol. 1974, 27, 281–285. [Google Scholar] [CrossRef]
- Schneemann, I.; Wiese, J.; Kunz, A.L.; Imhoff, J.F. Genetic approach for the fast discovery of phenazine producing bacteria. Mar. Drugs 2011, 9, 772–789. [Google Scholar] [CrossRef]
- Shirata, A.; Tsukamoto, T.; Yasui, H.; Kato, H.; Hayasaka, S.; Kojima, A. Production of bluish-purple pigments by Janthinobacterium lividum isolated from the raw silk and dyeing with them. Nippon Sanshigaku Zasshi 1997, 66, 377–385. [Google Scholar]
- Koyama, J. Anti-infective quinone derivatives of recent patents. Recent Pat. Anti-Infect. Drug Discov. 2006, 1, 113–125. [Google Scholar] [CrossRef]
- Zhang, J.; Shen, Y.; Liu, J.; Wei, D. Antimetastatic effect of prodigiosin through inhibition of tumor invasion. Biochem. Pharmacol. 2005, 69, 407–414. [Google Scholar] [CrossRef]
- Kawauchi, K.; Shibutani, K.; Yagisawa, H.; Kamata, H.; Nakatsuji, S.I.; Anzai, H.; Yokoyama, Y.; Ikegami, Y.; Moriyama, Y.; Hirata, H. A possible immunosuppressant, cycloprodigiosin hydrochloride, obtained from Pseudoalteromonas denitrificans. Biochem. Biophys. Res. Commun. 1997, 237, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Han, S.B.; Kim, H.M.; Kim, Y.H.; Lee, C.W.; Jang, E.S.; Son, K.H.; Sung, U.K.; Kim, Y.K. T-cell specific immunosuppression by prodigiosin isolated from Serratia marcescens. Int. J. Immunopharmacol. 1998, 20, 1–13. [Google Scholar] [CrossRef]
- Songia, S.; Mortellaro, A.; Taverna, S.; Fornasiero, C.; Scheiber, E.A.; Erba, E.; Colotta, F.; Mantovani, A.; Isetta, A.M.; Golay, J. Characterization of the new immunosuppressive drug undecylprodigiosin in human lymphocytes: Retinoblastoma protein, cyclin-dependent kinase-2, and cyclin-dependent kinase-4 as molecular targets. J. Immunol. 1997, 158, 3987–3995. [Google Scholar] [PubMed]
- Fouillaud, M.; Venkatachalam, M.; Girard-Valenciennes, E.; Caro, Y.; Dufossé, L. Anthraquinones and derivatives from marine-derived fungi: Structural diversity and selected biological activities. Mar. Drugs 2016, 14, 64. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.L.; Hung, H.K.; Wang, J.J.; Pan, T.M. Red mold dioscorea has greater hypolipidemic and antiatherosclerotic effect than tradi-tional redmold rice andunfermented dioscorea in hamsters. J. Agric. Food Chem. 2007, 55, 7162–7169. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.L.; Lee, C.L.; Pan, T.M. Red mold dioscorea has a greater antihypertensive effect than traditional red mold rice in spontaneously hypertensive rats. J. Agric. Food Chem. 2009, 57, 5035–5041. [Google Scholar] [CrossRef]
- Fox, R.D. Spirulina, the alga that can end malnutrition. Futurist 1985, 19, 30–35. [Google Scholar]
- Montaner, B.; Pérez-Tomás, R. Prodigiosin-induced apoptosis in human colon cancer cells. Life Sci. 2001, 68, 2025–2036. [Google Scholar] [CrossRef]
- Zheng, L.; Yan, X.; Han, X.; Chen, H.; Lin, W.; Lee, F.S.; Wang, X. Identification of norharman as the cytotoxic compound pro-duced by the sponge (Hymeniacidon perleve) associated marine bacterium Pseudoalteromonas piscicida and its apoptotic effect on cancer cells. Biotechnol. Appl. Biochem. 2006, 44, 135–142. [Google Scholar]
- Chincholkar, S.; Thomashow, L. Microbial Phenazines: Biosynthesis, Agriculture and Health; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1–243. [Google Scholar]
- Mojib, N.; Philpott, R.; Huang, J.P.; Niederweis, M.; Bej, A.K. Antimycobacterial activity of in vitro of pigments isolated from Antartic bacteria. Antonie van Leeuwenhoek 2010, 98, 531–540. [Google Scholar] [CrossRef]
- Holmström, C.; Egan, S.; Franks, A.; McCloy, S.; Kjelleberg, S. Antifouling activities expressed by marine surface associated Pseudoalteromonas species. FEMS Microbiol. Ecol. 2002, 41, 47–58. [Google Scholar] [CrossRef]
- Priya, K.A.; Satheesh, S.; Ashokkumar, B.; Varalakshmi, P.; Selvakumar, G.; Sivakumar, N. Antifouling activity of prodigiosin from estuarine isolate of Serratia marcescens CMST 07. In Microbiological Research in Agroecosystem Management; Velu, R.K., Ed.; Springer: New Delhi, India, 2013; Volume XVI, pp. 11–21. [Google Scholar]
- Jeong, H.; Yim, J.H.; Lee, C.; Choi, S.H.; Park, Y.K.; Yoon, S.H.; Hur, C.G.; Kang, H.Y.; Kim, D.; Lee, H.H.; et al. Genomic blueprint of Hahella chejuensis, a marine microbe producing an algicidal agent. Nucleic Acids Res. 2005, 33, 7066–7073. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Kim, J.; Yim, J.H.; Kwon, S.K.; Lee, C.H.; Lee, H.K. Red to red—The marine bacterium Hahella chejuensis and its product prodigiosin for mitigation of harmful algal blooms. J. Microbiol. Biotechnol. 2008, 18, 1621–1629. [Google Scholar] [PubMed]
- Sakaki, T.; Shibata, M.; Mukai, K.; Sakai, M.; Wakamatsu, K.; Miyauchi, S. Chlorociboria aeruginosa pigment as algicide. Japanese Kokai Tokkyo Koho 2002, 2002, 2002291493. [Google Scholar]
- Rai, M. Advances in Fungal Biotechnology; I. K. International Pvt Ltd.: New Delhi, India, 2009; p. 545. [Google Scholar]
- Quereshi, S.; Khan, A.A.; Pandey; Khim, A.K. Anthraquinone pigment with herbicidal potential from Phoma herbarum FGCC#54. Chem. Nat. Compd. 2011, 47, 521. [Google Scholar]
- Haun, M.; Pereira, M.F.; Hoffman, M.E.; Joyas, A.; Campos, V.; Filardi, L.D.; De Castro, S.L.; Duran, N. Bacterial chemistry. VI. Biological activities and cytotoxicity of 1, 3-dihydro-2H-indol-2-one derivatives. Biol. Res. 1992, 25, 21–25. [Google Scholar] [PubMed]
- Lopes, S.C.P.; Blanco, Y.C.; Justo, G.Z.; Nogueira, P.A.; Rodrigues, F.L.S.; Goelnitz, U.; Wunderlich, G.; Facchini, G.; Brocchi, M.; Duran, N.; et al. Violacein extracted from Chromobacterium violaceum inhibits Plasmodium growth in vitro and in vivo. Antimicrob. Agents Chemother. 2009, 53, 2149–2152. [Google Scholar] [CrossRef]
- Williams, R.P.; Quadri, S.M. The pigments of Serratia. In The genus Serratia; Graevenitz, A.V., Rubin, S.J., Eds.; CRC Press Inc.: Boca Raton, FL, USA, 1980; pp. 31–75. [Google Scholar]
- Leon, L.L.; Miranda, C.C.; De Souza, A.O.; Duran, N. Antileishmanial activity of the violacein extracted from Chromobacterium violaceum. J. Antimicrob. Chemother. 2001, 3, 449–450. [Google Scholar] [CrossRef]
- Moraes, C.S.; Seabra, S.H.; Castro, D.P.; Brazil, R.P.; de Souza, W.; Garcia, E.S.; Azambuja, P. Leishmania chagasi interactions with Serratia marcescens: Ultrastructural studies, lysis and carbohydrate effects. Exp. Parasitol. 2008, 118, 561–568. [Google Scholar] [CrossRef]
- Durản, N.; Justo, G.Z.; Melo, P.S.; DeAzevedo, M.B.M.; Brito, A.R.M.S.; Almeida, A.B.; Haun, M. Evaluation of the antiulcerogenic activity of violacein and its modulation by the inclusion complexation with beta-cyclodextrin. Can. J. Physiol. Pharmacol. 2003, 81, 387–396. [Google Scholar] [CrossRef]
- Duran, N.; Melo, P.S.; Haun, M. In Vitro evaluation of violacein on AIDS-related lumphoma and human tumor cell lines. In Proceedings of the 25th Annual Meetings of the Brazilian Society of Biochemistry and Molecular Biology, Sociedade Brasi-leira de Bioquimica e Biologia Molecular (SBBq), Caxambu, Brazil, 1996; Available online: https://scholar.google.co.in/scholar?hl=en&as_sdt=0%2C5&q=In+Vitro+evaluation+of+violacein+on+AIDS-related+lumphoma+and+human+tumor+cell+lines&btnG= (accessed on 31 December 2016).
- Kim, H.S.; Hayashi, M.; Shibata, Y.; Wataya, Y.; Mitamura, T.; Horii, T.; Kawauchi, K.; Hirata, H.; Tsuboi, S.; Moriyama, Y. Cycloprodigiosin hydrochloride obtained from Pseudoalteromonas denitrificansis a potent antimalarial agent. Biol. Pharm. Bull. 1999, 22, 532–534. [Google Scholar] [CrossRef] [PubMed]
- Lazaro, J.E.; Nitcheu, J.; Predicala, R.Z.; Mangalindan, G.C.; Nesslany, F.; Marzin, D.; Concepcion, G.P.; Diquet, B. Heptyl prodigiosin, a bacterial metabolite, is anti-malarial in vivo and nonmutagenic in vitro. J. Nat. Toxins 2002, 11, 367–377. [Google Scholar] [PubMed]
- Liu, Y.T.; Sui, M.J.; Ji, D.D.; Wu, I.H.; Chou, C.; Chen, C.C. Protection from UV irradiation by melanin of mosquitocidal activity of Bt.var. israeliensis. J. Invertebr. Pathol. 1993, 62, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Genes, C.; Baquero, E.; Echeverri, F.; Maya, J.D.; Triana, O. Mitochondrial dysfunction in Trypanosoma cruzi: The role of Serratia marcescens prodigiosin in the alternative treatment of Chagas disease. Parasites Vectors 2011, 4, 66. [Google Scholar] [CrossRef] [PubMed]
- Azambuja, P.; Feder, D.; Garcia, E.S. Isolation of Serratia marcescens in the midgut of Rhodnius prolixus: Impact on the establishment of the parasite Trypanosoma cruzi in the vector. Exp. Parasitol. 2004, 107, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Sankari, M.K.; Jonathan, E.I.; Ardhanareeswaran, N. Isolation of phenazine and its activity against root-knot nematode, Meloidogyne incognita. Indian J. Biotehnol. 2014, 43, 180–183. [Google Scholar]
- Shi, Y.C.; Liao, J.W.; Pan, T.M. Antihypertriglyceridemia and anti-inflammatory activities of Monascus-fermented dioscorea in streptozotocin-induced diabetic rats. Exp. Diabetes Res. 2011, 2011, 710635. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.H.; Hsu, W.H.; Liao, T.H.; Pan, T.M. The Monascus metabolite monascin against TNF-α-induced insulin resistance via suppressing PPAR-γphos-phorylation in C2C12 myotubes. Food Chem. Toxicol. 2011, 49, 2609–2617. [Google Scholar] [CrossRef]
- Lee, C.; Hung, Y.; Hsu, Y.; Pan, T. Monascin and Ankaflavin have more anti-atherosclerosis effect and less side effect involving increasing creatinine phosphokinase activity than Monacolin K under the same dosages. J. Agric. Food Chem. 2013, 61, 143–150. [Google Scholar] [CrossRef]
- Kishimoto, Y.; Yoshida, H.; Kondo, K. Potential Anti-Atherosclerotic Properties of Astaxanthin. Mar. Drugs 2016, 14, 35. [Google Scholar] [CrossRef]
- Osawa, A.; Ishii, Y.; Sasamura, N.; Morita, M.; Kasai, H.; Maoka, T.; Shindo, K. Characterization and antioxidative activities of rare C50 carotenoids-sarcinaxanthin, sarcinaxanthin monoglucoside, and sarcinaxanthin diglucoside obtained from Micrococcus yunnanensis. J. Oleo Sci. 2010, 59, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Martin, H.; Kock, S.; Scherrers, R.; Lutter, K.; Wagener, T.; Hundsdörfer, C.; Frixel, S.; Schaper, K.; Ernst, H.; Schrader, W.; et al. 3,3′-Dihydroxyisorenieratene, a Natural Carotenoid with Superior Antioxidant and Photoprotective Properties. Angew. Chem. 2009, 48, 400–403. [Google Scholar] [CrossRef] [PubMed]
- Teo, I.T.; Chui, C.H.; Tang, J.C.O.; Lau, F.Y.; Cheng, G.Y.M.; Wong, R. Antiproliferation and induction of cell death of Phaffia rhodozyma (Xanthophyllomyces dendrorhous) extract fermented by brewer malt waste on breast cancer cells. Int. J. Mol. Med. 2005, 16, 931–936. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kim, Y.; Kim, Y. The effect of beta-carotene on neuroblastoma stemness. FASEB J. 2012, 26. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, Y.O.; Jeun, J.; Choi, D.Y.; Shin, C.S. L-Trp and L-Leu-OEt derivatives of the Monascus pigment exert high anti-obesity effects on mice. Biosci. Biotechnol. Biochem. 2010, 74, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Jou, P.C.; Ho, B.Y.; Hsu, Y.W.; Pan, T.M. The effect of Monascus secondary polyketide metabolites, monascin and ankaflavin, on adipogenesis and lipolysis activity in 3T3-L1. J. Agric. Food Chem. 2010, 58, 12703–12709. [Google Scholar] [CrossRef]
- Choe, D.; Lee, J.; Woo, S.; Shin, C.S. Evaluation of the amine derivatives of Monascus pigment with anti-obesity activities. Food Chem. 2012, 134, 315–323. [Google Scholar] [CrossRef]
- Wang, Z.; Lin, B.; Mostaghim, A.; Rubin, R.A.; Glaser, E.R.; Mittraparp-arthorn, P.; Thompson, J.R.; Vuddhakul, V.; Vora, G.J. Vibrio campbellii hmgA-mediated pyomelanization impairs quorum sensing, virulence, and cellular fitness. Front. Microbiol. 2013, 4, 379. [Google Scholar] [CrossRef]
- Srianta, I.; Kusumawati, N.; Nugerahani, I.; Artanti, N.; Xu, G.R. In vitroα-glucosidase inhibitory activity of Monascus-fermented durian seed extracts. Int. Food Res. J. 2013, 20, 533–536. [Google Scholar]
- Lung, T.; Liao, L.; Wang, J.; Wei, B.; Huang, P.; Lee, C. Metals of deep ocean water increase the anti-adipogenesis effect of monascus-fermented product via modulating the monascin and ankaflavin production. Mar. Drugs 2016, 14, 106. [Google Scholar] [CrossRef]
- Lindquist, N.; Fenical, W. New tambjamine class alkaloids from the marine ascidian Atapozoa sp. and its nudibranch predators—Origins of the tambjamines in atapozoa. Experientia 1991, 47, 504–506. [Google Scholar] [CrossRef]
- Chakdar, H.; Pabbi, S. Extraction and purification of Phycoerythrin from Anabaena variabilis (CCC421). Phykos 2012, 42, 25–31. [Google Scholar]
- Speitling, M.; Smetanina, O.F.; Kuznetsova, O.F.; Laatsch, H. Bromoalterochromides A and A′, unprecedented chromopeptides from a marine Pseudoalteromonas maricaloris strain KMM 636T. J. Antibiot. 2007, 60, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Grossart, H.; Thorwest, M.; Plitzko, I.; Brinkhoff, T.; Simon, M.; Zeeck, A. Production of a blue pigment (Glaukothalin) by marine Rheinheimera spp. Int. J. Microbiol. 2009, 2009, 701735. [Google Scholar] [CrossRef] [PubMed]
- Tebben, J.; Tapiolas, D.M.; Motti, C.A.; Abrego, D.; Negri, A.P.; Blackall, L.L.; Steinberg, P.D.; Harder, T. Induction of larval metamorphosis of the coral Acropora millepora by tetrabromopyrrole isolated from a Pseudoalteromonas bacterium. PLoS ONE 2011, 6, e19082. [Google Scholar] [CrossRef]
- George, S.B.; Lawrence, J.M.; Lawrence, A.L.; Smiley, J.; Plank, L. Carotenoids in the adult diet enhance egg and juvenile production in the sea urchin Lytechinus variegatus. Aquaculture 2001, 199, 353–369. [Google Scholar] [CrossRef]
- Meena, K.K.; Kumar, M.; Kalyuzhnaya, M.G.; Yandigen, M.S.; Singh, D.P.; Saxena, A.K.; Arora, D. Epiphytic pink-pigmentedmethylotrophic bacteria enhance germination and seedling growth of wheat (Triticum aestivum) by producing phytohormone. Antonie Van Leeuwenhoek 2012, 101, 777–786. [Google Scholar] [CrossRef]
- Martínkovả, L. Biological activities of oligoketide pigments of Monascus purpureus. Food Addit. Contam. 1999, 16, 15–24. [Google Scholar] [CrossRef]
- Buck, J.D. Effects of medium composition on the recovery of bacteria from sea water. J. Exp. Mar. Biol. Ecol. 1974, 15, 25–34. [Google Scholar] [CrossRef]
- Reichenbach, H.; Kleinig, H.; Achenbach, H. The pigments of Flexibacter elegans: Novel and chemosystematically useful compounds. Arch. Microbiol. 1974, 101, 131–144. [Google Scholar] [CrossRef]
- Hajjaj, H.; Blanc, P.; Groussac, E.; Uribelarrea, J.L.; Goma, G.; Loubiere, P. Kinetic analysis of red pigment and citrinin by Monascus rubber as a function of organic acid accumulation. Enzym. Microb. Technol. 2000, 27, 619–625. [Google Scholar] [CrossRef]
- De Carvalho, J.C. Microbial Pigments. In Biotransformation of Waste Biomass into High Value Biochemicals; Brar, S.K., Dhillon, G.S., Soccol, C.R., Eds.; Springer: New York, NY, USA, 2014; pp. 73–98. [Google Scholar]
- Ghosh, A.; Goyal, A.; Jain, R.K. Study of methanol-induced phenotypic changes in a novel strain of Acinetobacter lwoffii. Arch. Microbiol. 2007, 188, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Saviola, B. Pigments and Pathogenesis. J. Mycobact. Dis. 2014, 4. [Google Scholar] [CrossRef] [Green Version]
- Robledo, J.A.; Murillo, A.M.; Rouzaud, F. Physiological Role and Potential Clinical Interest of Mycobacterial Pigments. IUBMB Life 2011, 63, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Mulders, K.J.M. Phototrophic pigment production with microalgae: Biological constraints and opportunities. J. Phycol. 2014, 50, 229–242. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, M.; Nakagawa, Y.; Li, H.; Matsuyama, T. Silica gel-dependent production of prodigiosin and serrawettins by Serratia marcescens in a liquid culture. Microbes Environ. 2001, 16, 250–254. [Google Scholar] [CrossRef]
- Chen, W.C.; Yu, W.J.; Chang, C.C.; Chang, J.S.; Huang, S.H.; Chang, C.H.; Chen, S.Y.; Chien, C.C.; Yao, C.L.; Chen, W.M.; et al. Enhancing production of prodigiosin from Serratia marcescens C3 by statistical experimental design and porous carrier addition strategy. Biochem. Eng. J. 2013, 78, 93–100. [Google Scholar] [CrossRef]
- Guedes, A.C.; Amaro, H.M.; Malcata, F.X. Microalgae as Sources of Carotenoids. Mar. Drugs 2011, 9, 625–644. [Google Scholar] [CrossRef]
- Velmurugan, P.; Kamala-Kannan, S.; Balachandar, V.; Lakshmanaperumalsamy, P.; Chae, J.; Oh, B. Natural pigment extraction from five filamentous fungi for industrial applications and dyeing of leather. Carbohydr. Polym. 2010, 79, 262–268. [Google Scholar] [CrossRef]
- Yurkova, A.M.; Vustin, M.M.; Tyaglov, B.V.; Maksimova, I.A.; Sineokiy, S.P. Pigmented basidiomycetous yeasts are a promising source of carotenoids and ubiquinone Q10. Microbiology 2008, 77, 1–6. [Google Scholar] [CrossRef]
- Ahmad, W.A.; Venil, C.K.; Aruldass, C.A. Production of Violacein by Chromobacterium violaceum Grown in Liquid Pineapple Waste: Current Scenario. In Beneficial Microorganisms in Agriculture, Aquaculture and Other Areas, Microbiology Monographs; Liong, M.T., Ed.; Springer: Berlin/Heidelberg, Germany, 2015; Volume 29, pp. 45–58. [Google Scholar]
- Carvalho, J.C.; Bicas, J.L.; Fernández, D.E.R.; Woiciechowski, A.L.; Medeiros, A.B.P.; Soccol, C.R. Natural Colorants from Microorganisms. In Biotechnological Production of Natural Ingredients for Food Industry, 1st ed.; Bicas, J.L., Maróstica Jr, M.R., Pastore, G.M., Eds.; Bentham Science Publishers: Sharjah, UAE, 2016; pp. 288–321. [Google Scholar] [Green Version]
- Taskin, M.; Sisman, T.; Erdal, S.; Kurbanoglu, E.B. Use of waste chicken feathers as peptone for production of carotenoids in submerged culture of Rhodotorula glutinis MT-5. Eur. Food Res. Technol. 2011, 233, 657–665. [Google Scholar] [CrossRef]
- Wang, Y.; Nakajima, A.; Hosokawa, K.; Soliev, A.B.; Osaka, I.; Arakawa, R.; Enomoto, K. Cytotoxic prodigiosin family pigments from Pseudoalteromonas sp.1020R isolated from the Pacific coast of Japan. Biosci. Biotechnol. Biochem. 2012, 76, 1229–1232. [Google Scholar] [CrossRef] [PubMed]
- Piersimoni, C.; Scarparo, C. Extrapulmonary infections associated with nontuberculous Mycobacteria in immunocompetent persons. Emerg. Infect. Dis. 2009, 15, 1351–1548. [Google Scholar] [CrossRef] [PubMed]
- Duran, N.; Menck, C.F. Chromobacterium violaceum: A review of pharmacological and industiral perspectives. Crit. Rev. Microbiol. 2001, 27, 201–222. [Google Scholar] [CrossRef] [PubMed]
- Ryan, R.P.; Monchy, S.; Cardinale, M.; Taghavi, S.; Crossman, L.; Avison, M.B.; Berg, G.; Van Der Lelie, D.; Dow, J.M. The versatility and adaptation of bacteria from the genus Stenotrophomonas. Nat. Rev. Microbiol. 2009, 7, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Jeffries, J.L.; Jia, J.; Choi, W.; Choe, S.; Miao, J.; Xu, Y.; Powell, R.; Lin, J.; Kuang, Z.; Gaskins, H.R.; et al. Pseudomonas aeruginosa pyocyanin modulates mucin glycosylation with sialyl-Lewisx to increase binding to airway epithelial cells. Mucosal Immunol. 2015, 9, 1039–1050. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Dwivedi, P.; Sharma, A.K.; Sankar, M.; Patil, R.D.; Singh, N.D. Apoptosis and lipid peroxidation in ochratoxin A- and citrinin-induced nephrotoxicity in rabbits. Toxicol. Ind. Health 2014, 30, 90–98. [Google Scholar] [CrossRef]
- Mapari, S.A.S.; Thrane, U.; Meyer, A.S. Fungal polyketide azaphilone pigments as future natural food colorants? Trends Biotechnol. 2014, 28, 300–307. [Google Scholar] [CrossRef]
- Frisvad, J.C.; Yilmaz, N.; Thrane, U.; Rasmussen, K.B.; Houbraken, J.; Samson, R.A. Talaromyces atroroseus, a new species efficiently producing industrially relevant red pigments. PLoS ONE 2013, 8, e84102. [Google Scholar] [CrossRef]
- Medentsev, A.G.; Akimenko, V.K. Naphthoquinone metabolites of the fungi. Phytochemistry 1998, 47, 935–959. [Google Scholar] [CrossRef]




















| Pigment | Media/Supplement | Incubation Temperature | Reference |
|---|---|---|---|
| Prodigiosin | Casein hydrolysate agar | 24–28 °C | [63] |
| Violacein | Lactose and tryptophan | 22 °C | |
| Indigo | Potato-glucose-peptone agar, Phosphate agar—incorporation of 2-hydroxypyridine and/or Tryptophan | ||
| Naphthoquinones | Glucose—mineral salt medium with ammonium sulphate, zinc, and magnesium ions—and Glucose—asparagine medium with small amounts of aspartic or glutamic acid and 5-fluorouracil | ||
| Monascus pigments | Suitable media with glucose, peptone or amino acids, and corn and potato starch | 25–28 °C | |
| Pyocyanine | Glycerol, leucine, glycine, alanine, and mineral salts | ||
| Phenazine | Shikimic acid, chorismic acid, glucose, glycerol, gluconate, and glutamine | ||
| Riboflavin | Cornsteep liquor, corn oil, and glycine | 26–28 °C | |
| Melanin | Tyrosine agar, Peptone-yeast extract iron agar, Tyrosine, Zn, Cu, Co, and 3-chlorobenzoate | ||
| Carotenoids | Mevalonic acid, trisporic acid, and Isopentenyl pyrophosphate | ||
| Anthraquinones | Sucrose, molasses, corn extract, yeast extract, zinc sulfate, and magnesium sulphate | 27–29 °C | 21 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramesh, C.; Vinithkumar, N.V.; Kirubagaran, R.; Venil, C.K.; Dufossé, L. Multifaceted Applications of Microbial Pigments: Current Knowledge, Challenges and Future Directions for Public Health Implications. Microorganisms 2019, 7, 186. https://doi.org/10.3390/microorganisms7070186
Ramesh C, Vinithkumar NV, Kirubagaran R, Venil CK, Dufossé L. Multifaceted Applications of Microbial Pigments: Current Knowledge, Challenges and Future Directions for Public Health Implications. Microorganisms. 2019; 7(7):186. https://doi.org/10.3390/microorganisms7070186
Chicago/Turabian StyleRamesh, Chatragadda, Nambali Valsalan Vinithkumar, Ramalingam Kirubagaran, Chidambaram Kulandaisamy Venil, and Laurent Dufossé. 2019. "Multifaceted Applications of Microbial Pigments: Current Knowledge, Challenges and Future Directions for Public Health Implications" Microorganisms 7, no. 7: 186. https://doi.org/10.3390/microorganisms7070186
APA StyleRamesh, C., Vinithkumar, N. V., Kirubagaran, R., Venil, C. K., & Dufossé, L. (2019). Multifaceted Applications of Microbial Pigments: Current Knowledge, Challenges and Future Directions for Public Health Implications. Microorganisms, 7(7), 186. https://doi.org/10.3390/microorganisms7070186