Comparing the Metabolic Capabilities of Bacteria in the Mycobacterium tuberculosis Complex
Abstract
1. Introduction
2. Pathogenic Processes of the Canonical Pathogen, Mycobacterium tuberculosis
3. The Metabolic Capabilities of M. tuberculosis
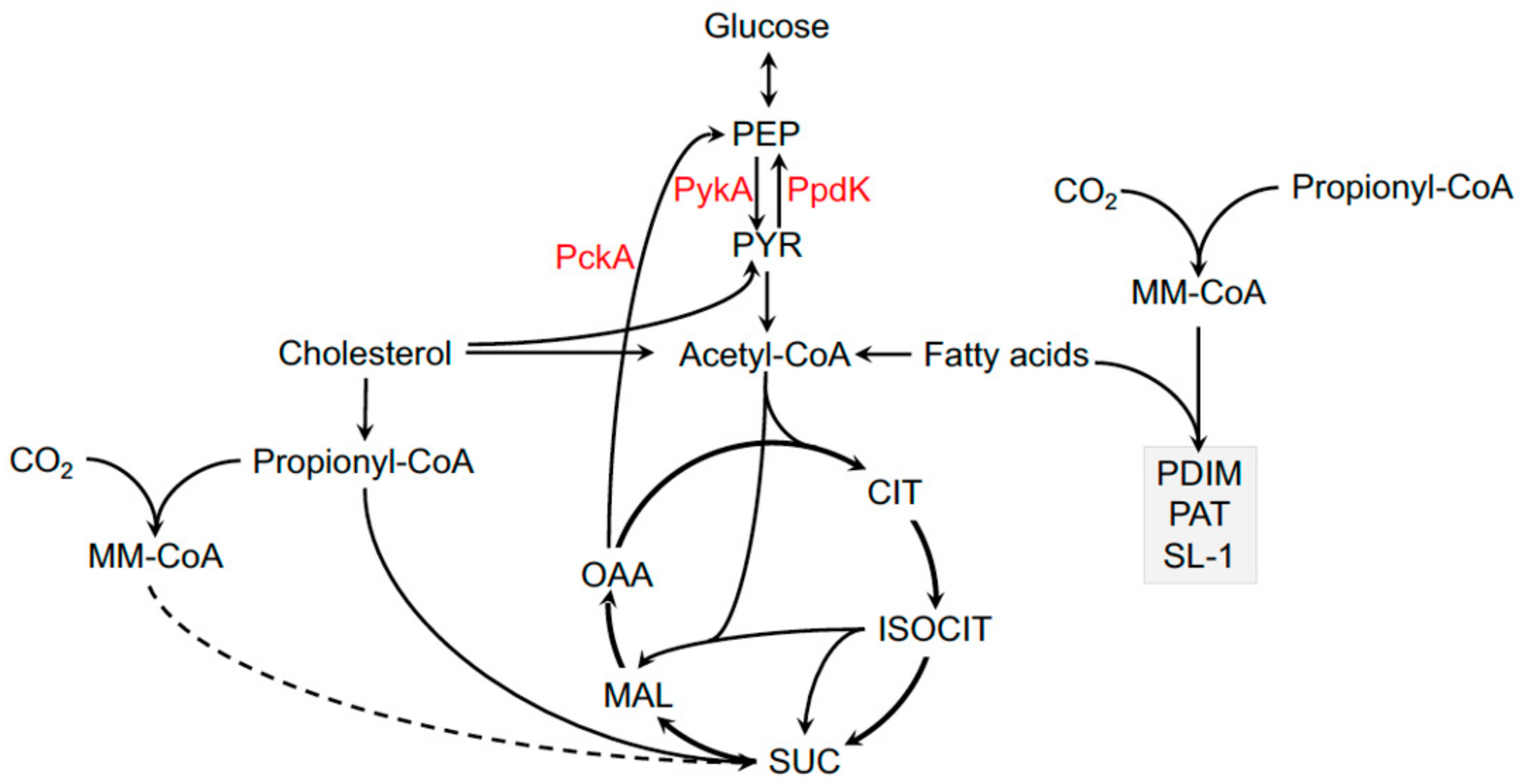
4. Carbon Metabolism
5. Lipid Import
6. Fatty Acid Metabolism in M. tuberculosis
7. Cholesterol Metabolism in M. tuberculosis
8. Coupled Metabolism of Fatty Acids and Cholesterol in the Macrophage
9. M. tuberculosis Metabolic Flexibility and Macrophage Heterogeneity
10. Nitrogen from Amino Acid Metabolism in M. tuberculosis
11. Metals and Metabolism in M. tuberculosis
12. Molybdenum Cofactor in M. tuberculosis
13. Immunological Pressures on M. tuberculosis Metabolism
14. Acidic pH and PhoPR
15. Concluding Remarks
Funding
Conflicts of Interest
References
- De Jong, B.C.; Antonio, M.; Gagneux, S. Mycobacterium africanum—Review of an important cause of human tuberculosis in West Africa. PLoS Negl. Trop. Dis. 2010, 4, e744. [Google Scholar] [CrossRef] [PubMed]
- Niemann, S.; Richter, E.; Dalügge-Tamm, H.; Schlesinger, H.; Graupner, D.; Königstein, B.; Gurath, G.; Greinert, U.; Rüsch-Gerdes, S. Two cases of Mycobacterium microti derived tuberculosis in HIV-negative immunocompetent patients. Emerg. Infect. Dis. 2000, 6, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Van Soolingen, D.; van der Zanden, A.G.; de Haas, P.E.; Noordhoek, G.T.; Kiers, A.; Foudraine, N.A.; Portaels, F.; Kolk, A.H.; Kremer, K.; van Embden, J.D. Diagnosis of Mycobacterium microti infections among humans by using novel genetic markers. J. Clin. Microbiol. 1998, 36, 1840–1845. [Google Scholar] [PubMed]
- Malone, K.M.; Gordon, S.V. Strain Variation in the Mycobacterium tuberculosis Complex.: Its Role in Biology, Epidemiology and Control; Gagneux, S., Ed.; Springer International Publishing AG: Basel, Switzerland, 2017. [Google Scholar]
- Blazquez, J.; Espinosa de Los Monteros, L.E.; Samper, S.; Martín, C.; Guerrero, A.; Cobo, J.; Van Embden, J.; Baquero, F.; Gómez-Mampaso, E. Genetic characterization of multidrug-resistant Mycobacterium bovis strains from a hospital outbreak involving human immunodeficiency virus-positive patients. J. Clin. Microbiol. 1997, 35, 1390–1393. [Google Scholar] [PubMed]
- Dippenaar, A.; Dippenaar, A.; Parsons, S.D.C.; Sampson, S.L.; van der Merwe, R.G.; Drewe, J.A.; Abdallah, A.M.; Siame, K.K.; Gey van Pittius, N.C.; van Helden, P.D.; et al. Whole genome sequence analysis of Mycobacterium suricattae. Tuberculosis 2015, 95, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Parsons, S.D.; Drewe, J.A.; Gey van Pittius, N.C.; Warren, R.M.; van Helden, P.D. Novel cause of tuberculosis in meerkats, South Africa. Emerg. Infect. Dis. 2013, 19, 2004–2007. [Google Scholar] [CrossRef] [PubMed]
- Alexander, K.A.; Laver, P.N.; Michel, A.L.; Williams, M.; van Helden, P.D.; Warren, R.M.; Gey van Pittius, N.C. Novel Mycobacterium tuberculosis complex pathogen, M. mungi. Emerg. Infect. Dis. 2010, 16, 1296–1299. [Google Scholar] [CrossRef] [PubMed]
- Marcos, L.A.; Spitzer, E.D.; Mahapatra, R.; Ma, Y.; Halse, T.A.; Shea, J.; Isabelle, M.; Lapierre, P.; Escuyer, V.E. Mycobacterium orygis Lymphadenitis in New York, USA. Emerg. Infect. Dis. 2017, 23, 1749–1751. [Google Scholar] [CrossRef]
- Rahim, Z.; Thapa, J.; Fukushima, Y.; van der Zanden, A.G.M.; Gordon, S.V.; Suzuki, Y.; Nakajima, C. Tuberculosis Caused by Mycobacterium orygis in Dairy Cattle and Captured Monkeys in Bangladesh: A New Scenario of Tuberculosis in South Asia. Transbound. Emerg. Dis. 2017, 64, 1965–1969. [Google Scholar] [CrossRef]
- Cousins, D.V.; Bastida, R.; Cataldi, A.; Quse, V.; Redrobe, S.; Dow, S.; Duignan, P.; Murray, A.; Dupont, C.; Ahmed, N.; et al. Tuberculosis in seals caused by a novel member of the Mycobacterium tuberculosis complex: Mycobacterium pinnipedii sp. nov. Int. J. Syst. Evol. Microbiol. 2003, 53, 1305–1314. [Google Scholar] [CrossRef]
- Forshaw, D.; Phelps, G.R. Tuberculosis in a captive colony of pinnipeds. J. Wildl. Dis. 1991, 27, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Moser, I.; Prodinger, W.M.; Hotzel, H.; Greenwald, R.; Lyashchenko, K.P.; Bakker, D.; Gomis, D.; Seidler, T.; Ellenberger, C.; Hetzel, U.; et al. Mycobacterium pinnipedii: Transmission from South American sea lion (Otaria byronia) to Bactrian camel (Camelus bactrianus bactrianus) and Malayan tapirs (Tapirus indicus). Vet. Microbiol. 2008, 127, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Thompson, P.J.; Cousins, D.V.; Gow, B.L.; Collins, D.M.; Williamson, B.H.; Dagnia, H.T. Seals, seal trainers, and mycobacterial infection. Am. Rev. Respir Dis. 1993, 147, 164–167. [Google Scholar] [CrossRef] [PubMed]
- Pfyffer, G.E.; Auckenthaler, R.; van Embden, J.D.; van Soolingen, D. Mycobacterium canettii, the smooth variant of M. tuberculosis, isolated from a swiss patient exposed in Africa. Emerg. Infect. Dis. 1998, 4, 631–634. [Google Scholar] [CrossRef] [PubMed]
- Van Soolingen, D.; Hoogenboezem, T.; de Haas, P.E.; Hermans, P.W.; Koedam, M.A.; Teppema, K.S.; Brennan, P.J.; Besra, G.S.; Portaels, F.; Top, J.; et al. A novel pathogenic taxon of the Mycobacterium tuberculosis complex, canetti: Characterization of an exceptional isolate from Africa. Int. J. Syst. Bacteriol. 1997, 47, 1236–1245. [Google Scholar] [CrossRef] [PubMed]
- Behr, M.A.; Wilson, M.A.; Gill, W.P.; Salamon, H.; Schoolnik, G.K.; Rane, S.; Small, P.M. Comparative genomics of BCG vaccines by whole-genome DNA microarray. Science 1999, 284, 1520–1523. [Google Scholar] [CrossRef]
- Brosch, R.; Gordon, S.V.; Billault, A.; Garnier, T.; Eiglmeier, K.; Soravito, C.; Barrell, B.G.; Cole, S.T. Use of a Mycobacterium tuberculosis H37Rv bacterial artificial chromosome library for genome mapping, sequencing, and comparative genomics. Infect. Immun. 1998, 66, 2221–2229. [Google Scholar]
- Brosch, R.; Gordon, S.V.; Marmiesse, M.; Brodin, P.; Buchrieser, C.; Eiglmeier, K.; Garnier, T.; Gutierrez, C.; Hewinson, G.; Kremer, K.; et al. A new evolutionary scenario for the Mycobacterium tuberculosis complex. PNAS 2002, 99, 3684–3689. [Google Scholar] [CrossRef]
- Gordon, S.V.; Brosch, R.; Billault, A.; Garnier, T.; Eiglmeier, K.; Cole, S.T. Identification of variable regions in the genomes of tubercle bacilli using bacterial artificial chromosome arrays. Mol. Microbiol. 1999, 32, 643–655. [Google Scholar] [CrossRef]
- Mahairas, G.G.; Sabo, P.J.; Hickey, M.J.; Singh, D.C.; Stover, C.K. Molecular analysis of genetic differences between Mycobacterium bovis BCG and virulent M. bovis. J. Bacteriol. 1996, 178, 1274–1282. [Google Scholar] [CrossRef]
- Mostowy, S.; Tsolaki, A.G.; Small, P.M.; Behr, M.A. The in vitro evolution of BCG vaccines. Vaccine 2003, 21, 4270–4274. [Google Scholar] [CrossRef]
- Brosch, R.; Philipp, W.J.; Stavropoulos, E.; Colston, M.J.; Cole, S.T.; Gordon, S.V. Genomic analysis reveals variation between Mycobacterium tuberculosis H37Rv and the attenuated M. tuberculosis H37Ra strain. Infect. Immun. 1999, 67, 5768–5774. [Google Scholar] [PubMed]
- Mostowy, S.; Onipede, A.; Gagneux, S.; Niemann, S.; Kremer, K.; Desmond, E.P.; Kato-Maeda, M.; Behr, M. Genomic analysis distinguishes Mycobacterium africanum. J. Clin. Microbiol. 2004, 42, 3594–3599. [Google Scholar] [CrossRef] [PubMed]
- Comas, I.; Chakravartti, J.; Small, P.M.; Galagan, J.; Niemann, S.; Kremer, K.; Ernst, J.D.; Gagneux, S. Human T cell epitopes of Mycobacterium tuberculosis are evolutionarily hyperconserved. Nat. Genet. 2010, 42, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Coscolla, M.; Copin, R.; Sutherland, J.; Gehre, F.; de Jong, B.; Owolabi, O.; Mbayo, G.; Giardina, F.; Ernst, J.D.; Gagneux, S. M. tuberculosis T Cell Epitope Analysis Reveals Paucity of Antigenic Variation and Identifies Rare Variable TB Antigens. Cell Host Microbe 2015, 18, 538–548. [Google Scholar] [CrossRef] [PubMed]
- Pepperell, C.S.; Casto, A.M.; Kitchen, A.; Granka, J.M.; Cornejo, O.E.; Holmes, E.C.; Birren, B.; Galagan, J.; Feldman, M.W. The role of selection in shaping diversity of natural M. tuberculosis populations. PLoS Pathog. 2013, 9, e1003543. [Google Scholar] [CrossRef]
- Copin, R.; Wang, X.; Louie, E.; Escuyer, V.; Coscolla, M.; Gagneux, S.; Palmer, G.H.; Ernst, J.D. Within Host Evolution Selects for a Dominant Genotype of Mycobacterium tuberculosis while T Cells Increase Pathogen Genetic Diversity. PLoS Pathog. 2016, 12, e1006111. [Google Scholar] [CrossRef]
- Alexander, K.A.; Laver, P.N.; Williams, M.C.; Sanderson, C.E.; Kanipe, C.; Palmer, M.V. Pathology of the Emerging Mycobacterium tuberculosis Complex Pathogen, Mycobacterium mungi, in the Banded Mongoose (Mungos mungo). Vet. Pathol. 2018, 55, 303–309. [Google Scholar] [CrossRef]
- Amato, B.; Capucchio, T.M.; Biasibetti, E.; Mangano, E.; Boniotti, B.M.; Pacciarini, L.M.; Migliore, S.; Vitale, M.; Fiasconaro, M.; Di Marco Lo Presti, V. Pathology and genetic findings in a rare case of mycobacterium caprae infection in a sow. Vet. Microbiol. 2017, 205, 71–74. [Google Scholar] [CrossRef]
- Bouzid, F.; Brégeon, F.; Lepidi, H.; Donoghue, H.D.; Minnikin, D.E.; Drancourt, M. Ready Experimental Translocation of Mycobacterium canettii Yields Pulmonary Tuberculosis. Infect. Immun. 2017, 85. [Google Scholar] [CrossRef]
- Coscolla, M.; Lewin, A.; Metzger, S.; Maetz-Rennsing, K.; Calvignac-Spencer, S.; Nitsche, A.; Dabrowski, P.W.; Radonic, A.; Niemann, S.; Parkhill, J.; et al. Novel Mycobacterium tuberculosis complex isolate from a wild chimpanzee. Emerg. Infect. Dis. 2013, 19, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Drewe, J.A.; Foote, A.K.; Sutcliffe, R.L.; Pearce, G.P. Pathology of Mycobacterium bovis infection in wild meerkats (Suricata suricatta). J. Comp. Pathol. 2009, 140, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Kipar, A.; Burthe, S.J.; Hetzel, U.; Rokia, M.A.; Telfer, S.; Lambin, X.; Birtles, R.J.; Begon, M.; Bennett, M. Mycobacterium microti tuberculosis in its maintenance host, the field vole (Microtus agrestis): Characterization of the disease and possible routes of transmission. Vet. Pathol. 2014, 51, 903–914. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pollock, J.M.; Neill, S.D. Mycobacterium bovis infection and tuberculosis in cattle. Vet. J. 2002, 163, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Roe, W.D.; Lenting, B.; Kokosinska, A.; Hunter, S.; Duignan, P.J.; Gartrell, B.; Rogers, L.; Collins, D.M.; de Lisle, G.W.; Gedye, K.; et al. Pathology and molecular epidemiology of Mycobacterium pinnipedii tuberculosis in native New Zealand marine mammals. PLoS ONE 2019, 14, e0212363. [Google Scholar] [CrossRef] [PubMed]
- Russell, D.G.; Huang, L.; VanderVen, B.C. Immunometabolism at the interface between macrophages and pathogens. Nat. Rev. Immunol. 2019, 19, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Yruela, I.; Contreras-Moreira, B.; Magalhaes, C.; Osorio, N.S.; Gonzalo-Asensio, J. Mycobacterium tuberculosis Complex Exhibits Lineage-Specific Variations Affecting Protein Ductility and Epitope Recognition. Genome Biol. Evol. 2016, 8, 3751–3764. [Google Scholar]
- WHO. Global Tuberculosis Report 2018. Available online: https://www.who.int/tb/publications/global_report/en/ (accessed on 1 May 2019).
- Pai, M.; Behr, M.A.; Dowdy, D.; Dheda, K.; Divangahi, M.; Boehme, C.C.; Ginsberg, A.; Swaminathan, S.; Spigelman, M.; Getahun, H.; et al. Tuberculosis. Nat. Rev. Dis. Primers 2016, 2, 16076. [Google Scholar] [CrossRef]
- Cohen, S.B.; Gern, B.H.; Delahaye, J.L.; Adams, K.N.; Plumlee, C.R.; Winkler, J.K.; Sherman, D.R.; Gerner, M.Y.; Urdahl, K.B. Alveolar Macrophages Provide an Early Mycobacterium tuberculosis Niche and Initiate Dissemination. Cell Host Microbe 2018, 24, 439–446.e434. [Google Scholar] [CrossRef] [PubMed]
- Russell, D.G. The evolutionary pressures that have molded Mycobacterium tuberculosis into an infectious adjuvant. Curr. Opin. Microbiol. 2013, 16, 78–84. [Google Scholar] [CrossRef]
- Domingo-Gonzalez, R.; Prince, O.; Cooper, A.; Khader, S.A. Cytokines and Chemokines in Mycobacterium tuberculosis Infection. Microbiol. Spectr. 2016, 4. [Google Scholar]
- Norris, B.A.; Ernst, J.D. Mononuclear cell dynamics in M. tuberculosis infection provide opportunities for therapeutic intervention. PLoS Pathog. 2018, 14, e1007154. [Google Scholar] [CrossRef] [PubMed]
- O’Garra, A.; Redford, P.S.; McNab, F.W.; Bloom, C.I.; Wilkinson, R.J.; Berry, M.P. The immune response in tuberculosis. Annu. Rev. Immunol. 2013, 31, 475–527. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.J.; Carey, A.F.; Fortune, S.M. A bug’s life in the granuloma. Semin. Immunopathol. 2016, 38, 213–220. [Google Scholar] [CrossRef] [PubMed]
- McClean, C.M.; Tobin, D.M. Macrophage form, function, and phenotype in mycobacterial infection: Lessons from tuberculosis and other diseases. Pathog. Dis. 2016, 74. [Google Scholar] [CrossRef] [PubMed]
- Russell, D.G.; VanderVen, B.C.; Lee, W.; Abramovitch, R.B.; Kim, M.J.; Homolka, S.; Niemann, S.; Rohde, K.H. Mycobacterium tuberculosis Wears What It Eats. Cell Host Microbe 2010, 8, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Stutz, M.D.; Clark, M.P.; Doerflinger, M.; Pellegrini, M. Mycobacterium tuberculosis: Rewiring host cell signaling to promote infection. J. Leukoc. Biol. 2018, 103, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Russell, D.G.; Dant, J.; Sturgill-Koszycki, S. Mycobacterium avium- and Mycobacterium tuberculosis-containing vacuoles are dynamic, fusion-competent vesicles that are accessible to glycosphingolipids from the host cell plasmalemma. J. Immunol. 1996, 156, 4764–4773. [Google Scholar]
- Sturgill-Koszycki, S.; Schaible, U.E.; Russell, D.G. Mycobacterium-containing phagosomes are accessible to early endosomes and reflect a transitional state in normal phagosome biogenesis. Embo J. 1996, 15, 6960–6968. [Google Scholar] [CrossRef]
- Pethe, K.; Swenson, D.L.; Alonso, S.; Anderson, J.; Wang, C.; Russell, D.G. Isolation of Mycobacterium tuberculosis mutants defective in the arrest of phagosome maturation. Proc. Natl. Acad. Sci. USA 2004, 101, 13642–13647. [Google Scholar] [CrossRef]
- Clemens, D.L.; Horwitz, M.A. The Mycobacterium tuberculosis phagosome interacts with early endosomes and is accessible to exogenously administered transferrin. J. Exp Med. 1996, 184, 1349–1355. [Google Scholar] [CrossRef] [PubMed]
- Fratti, R.A.; Chua, J.; Deretic, V. Cellubrevin alterations and Mycobacterium tuberculosis phagosome maturation arrest. J. Biol. Chem. 2002, 277, 17320–17326. [Google Scholar] [CrossRef] [PubMed]
- Gouzy, A.; Poquet, Y.; Neyrolles, O. Amino acid capture and utilization within the Mycobacterium tuberculosis phagosome. Future Microbiol. 2014, 9, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; VanderVen, B.C.; Fahey, R.J.; Russell, D.G. Intracellular Mycobacterium tuberculosis exploits host-derived fatty acids to limit metabolic stress. J. Biol. Chem. 2013, 288, 6788–6800. [Google Scholar] [CrossRef] [PubMed]
- Nazarova, E.V.; Montague, C.R.; La, T.; Wilburn, K.M.; Sukumar, N.; Lee, W.; Caldwell, S.; Russell, D.G.; VanderVen, B.C. Rv3723/LucA coordinates fatty acid and cholesterol uptake in Mycobacterium tuberculosis. eLife 2017, 6, e26969. [Google Scholar] [CrossRef] [PubMed]
- Nazarova, E.V.; Podinovskaia, M.; Russell, D.G.; VanderVen, B.C. Flow Cytometric Quantification of Fatty Acid Uptake by Mycobacterium tuberculosis in Macrophages. Bio-Protocol 2018, 8, e2734. [Google Scholar] [CrossRef]
- Neyrolles, O.; Wolschendorf, F.; Mitra, A.; Niederweis, M. Mycobacteria, metals, and the macrophage. Immunol. Rev. 2015, 264, 249–263. [Google Scholar] [CrossRef] [PubMed]
- Schaible, U.E.; Sturgill-Koszycki, S.; Schlesinger, P.H.; Russell, D.G. Cytokine activation leads to acidification and increases maturation of Mycobacterium avium-containing phagosomes in murine macrophages. J. Immunol. 1998, 160, 1290–1296. [Google Scholar] [PubMed]
- Via, L.E.; Fratti, R.A.; McFalone, M.; Pagan-Ramos, E.; Deretic, D.; Deretic, V. Effects of cytokines on mycobacterial phagosome maturation. J. Cell Sci. 1998, 111, 897–905. [Google Scholar] [PubMed]
- Alonso, S.; Pethe, K.; Russell, D.G.; Purdy, G.E. Lysosomal killing of mycobacterium mediated by ubiquitin-derived peptides is enhanced by autophagy. Proc. Natl. Acad. Sci. USA 2007, 104, 6031–6036. [Google Scholar] [CrossRef]
- MacMicking, J.D.; North, R.J.; LaCourse, R.; Mudgett, J.S.; Shah, S.K.; Nathan, C.F. Identification of nitric oxide synthase as a protective locus against tuberculosis. Proc. Natl. Acad. Sci. USA 1997, 94, 5243–5248. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.K.; Sassetti, C.M. Mycobacterial persistence requires the utilization of host cholesterol. Proc. Natl. Acad. Sci. USA 2008, 105, 4376–4380. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, S.; Mittal, E.; Philips, J.A. Tuberculosis and the art of macrophage manipulation. Pathog. Dis. 2018, 76. [Google Scholar] [CrossRef] [PubMed]
- McKinney, J.D.; Höner zu Bentrup, K.; Muñoz-Elías, E.J.; Miczak, A.; Chen, B.; Chan, W.T.; Swenson, D.; Sacchettini, J.C.; Jacobs, W.R.J.; Russell, D.G. Persistence of Mycobacterium tuberculosis in macrophages and mice requires the glyoxylate shunt enzyme isocitrate lyase. Nature 2000, 406, 735–738. [Google Scholar] [CrossRef] [PubMed]
- Alkhuder, K.; Meibom, K.L.; Dubail, I.; Dupuis, M.; Charbit, A. Glutathione provides a source of cysteine essential for intracellular multiplication of Francisella tularensis. PLoS Pathog. 2009, 5, e1000284. [Google Scholar] [CrossRef] [PubMed]
- George, J.R.; Pine, L.; Reeves, M.W.; Harrell, W.K. Amino acid requirements of Legionella pneumophila. J. Clin. Microbiol. 1980, 11, 286–291. [Google Scholar] [PubMed]
- Cole, S.T.; Brosch, R.; Parkhill, J.; Garnier, T.; Churcher, C.; Harris, D.; Gordon, S.V.; Eiglmeier, K.; Gas, S.; Barry, C.E., III; et al. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 1998, 393, 537–544. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, L.P.; Fischer, S.M.; Marrero, J.; Nathan, C.; Ehrt, S.; Rhee, K.Y. Metabolomics of Mycobacterium tuberculosis reveals compartmentalized co-catabolism of carbon substrates. Chem. Biol. 2010, 17, 1122–1131. [Google Scholar] [CrossRef] [PubMed]
- Beste, D.J.; Nöh, K.; Niedenführ, S.; Mendum, T.A.; Hawkins, N.D.; Ward, J.L.; Beale, M.H.; Wiechert, W.; McFadden, J. 13C-flux spectral analysis of host-pathogen metabolism reveals a mixed diet for intracellular Mycobacterium tuberculosis. Chem. Biol. 2013, 20, 1012–1021. [Google Scholar] [CrossRef] [PubMed]
- Bloch, H.; Segal, W. Biochemical differentiation of Mycobacterium tuberculosis grown in vivo and in vitro. J. Bacteriol. 1956, 72, 132–141. [Google Scholar] [PubMed]
- Nazarova, E.V.; Montague, C.R.; Huang, L.; La, T.; Russell, D.; VanderVen, B.C. The genetic requirements of fatty acid import by Mycobacterium tuberculosis within macrophages. eLife 2019, 8, e43621. [Google Scholar] [CrossRef] [PubMed]
- Rohde, K.H.; Veiga, D.F.; Caldwell, S.; Balazsi, G.; Russell, D.G. Linking the transcriptional profiles and the physiological states of Mycobacterium tuberculosis during an extended intracellular infection. PLoS Pathog. 2012, 8, e1002769. [Google Scholar] [CrossRef] [PubMed]
- Schnappinger, D.; Ehrt, S.; Voskuil, M.I.; Liu, Y.; Mangan, J.A.; Monahan, I.M.; Dolganov, G.; Efron, B.; Butcher, P.D.; Nathan, C.; et al. Transcriptional adaptation of Mycobacterium tuberculosis within macrophages: Insights into the phagosomal environment. J. Exp. Med. 2003, 198, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Wilburn, K.M.; Fieweger, R.A.; VanderVen, B.C. Cholesterol and fatty acids grease the wheels of Mycobacterium tuberculosis pathogenesis. Pathog. Dis. 2018, 76. [Google Scholar] [CrossRef] [PubMed]
- Wipperman, M.F.; Sampson, N.S.; Thomas, S.T. Pathogen roid rage: Cholesterol utilization by Mycobacterium tuberculosis. Crit. Rev. Biochem. Mol. Biol. 2014, 49, 269–293. [Google Scholar] [CrossRef]
- Yam, K.C.; Okamoto, S.; Roberts, J.N.; Eltis, L.D. Adventures in rhodococcus - from steroids to explosives. Can. J. Microbiol. 2011, 57, 155–168. [Google Scholar] [CrossRef]
- Bergstrand, L.H.; Cardenas, E.; Holert, J.; Van Hamme, J.D.; Mohn, W.W. Delineation of Steroid-Degrading Microorganisms through Comparative Genomic Analysis. mBio 2016, 7, e00166. [Google Scholar] [CrossRef]
- Van Wyk, R.; van Wyk, M.; Mashele, S.S.; Nelson, D.R.; Syed, K. Comprehensive Comparative Analysis of Cholesterol Catabolic Genes/Proteins in Mycobacterial Species. Int. J. Mol. Sci. 2019, 20. [Google Scholar] [CrossRef] [PubMed]
- Maxfield, F.R. Role of endosomes and lysosomes in human disease. Cold Spring Harbor Perspect. Biol. 2014, 6, a016931. [Google Scholar] [CrossRef] [PubMed]
- Remmerie, A.; Scott, C.L. Macrophages and lipid metabolism. Cell. Immunol. 2018, 330, 27–42. [Google Scholar] [CrossRef]
- Marrero, J.; Trujillo, C.; Rhee, K.Y.; Ehrt, S. Glucose phosphorylation is required for Mycobacterium tuberculosis persistence in mice. PLoS Pathog. 2013, 9, e1003116. [Google Scholar] [CrossRef] [PubMed]
- Trujillo, C.; Blumenthal, A.; Marrero, J.; Rhee, K.Y.; Schnappinger, D.; Ehrt, S. Triosephosphate isomerase is dispensable in vitro yet essential for Mycobacterium tuberculosis to establish infection. mBio 2014, 5, e00085. [Google Scholar] [CrossRef]
- Rhee, K.Y.; de Carvalho, L.P.; Bryk, R.; Ehrt, S.; Marrero, J.; Park, S.W.; Schnappinger, D.; Venugopal, A.; Nathan, C. Central carbon metabolism in Mycobacterium tuberculosis: An unexpected frontier. Trends Microbiol. 2011, 19, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Yu, J.; Russell, D.G. pckA-deficient Mycobacterium bovis BCG shows attenuated virulence in mice and in macrophages. Microbiology 2003, 149, 1829–1835. [Google Scholar] [CrossRef] [PubMed]
- Marrero, J.; Rhee, K.Y.; Schnappinger, D.; Pethe, K.; Ehrt, S. Gluconeogenic carbon flow of tricarboxylic acid cycle intermediates is critical for Mycobacterium tuberculosis to establish and maintain infection. Proc. Natl. Acad. Sci. USA 2010, 107, 9819–9824. [Google Scholar] [CrossRef]
- Garnier, T.; Eiglmeier, K.; Camus, J.C.; Medina, N.; Mansoor, H.; Pryor, M.; Duthoy, S.; Grondin, S.; Lacroix, C.; Monsempe, C.; et al. The complete genome sequence of Mycobacterium bovis. PNAS 2003, 100, 7877–7882. [Google Scholar] [CrossRef] [PubMed]
- Keating, L.A.; Wheeler, P.R.; Mansoor, H.; Inwald, J.K.; Dale, J.; Hewinson, R.G.; Gordon, S.V. The pyruvate requirement of some members of the Mycobacterium tuberculosis complex is due to an inactive pyruvate kinase: Implications for in vivo growth. Mol. Microbiol. 2005, 56, 163–174. [Google Scholar] [CrossRef]
- Griffin, J.E.; Gawronski, J.D.; Dejesus, M.A.; Ioerger, T.R.; Akerley, B.J.; Sassetti, C.M. High-Resolution Phenotypic Profiling Defines Genes Essential for Mycobacterial Growth and Cholesterol Catabolism. PLoS Pathog. 2011, 7, e1002251. [Google Scholar] [CrossRef]
- Zhang, F.; Xie, J.P. Mammalian cell entry gene family of Mycobacterium tuberculosis. Mol. Cell. Biochem. 2011, 352, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Homolka, S.; Niemann, S.; Russell, D.G.; Rohde, K.H. Functional genetic diversity among Mycobacterium tuberculosis complex clinical isolates: Delineation of conserved core and lineage-specific transcriptomes during intracellular survival. PLoS Pathog. 2010, 6, e1000988. [Google Scholar] [CrossRef]
- Casali, N.; Riley, L.W. A phylogenomic analysis of the Actinomycetales mce operons. BMC Genom. 2007, 8, 60. [Google Scholar] [CrossRef]
- Marmiesse, M.; Brodin, P.; Buchrieser, C.; Gutierrez, C.; Simoes, N.; Vincent, V.; Glaser, P.; Cole, S.T.; Brosch, R. Macro-array and bioinformatic analyses reveal mycobacterial ‘core’ genes, variation in the ESAT-6 gene family and new phylogenetic markers for the Mycobacterium tuberculosis complex. Microbiology 2004, 150, 483–496. [Google Scholar] [CrossRef] [PubMed]
- Winglee, K.; Manson McGuire, A.; Maiga, M.; Abeel, T.; Shea, T.; Desjardins, C.A.; Diarra, B.; Baya, B.; Sanogo, M.; Diallo, S.; et al. Whole Genome Sequencing of Mycobacterium africanum Strains from Mali Provides Insights into the Mechanisms of Geographic Restriction. PLoS Negl. Trop. Dis. 2016, 10, e0004332. [Google Scholar] [CrossRef]
- Boritsch, E.C.; Khanna, V.; Pawlik, A.; Honoré, N.; Navas, V.H.; Ma, L.; Bouchier, C.; Seemann, T.; Supply, P.; Stinear, T.P.; et al. Key experimental evidence of chromosomal DNA transfer among selected tuberculosis-causing mycobacteria. Proc. Natl. Acad. Sci. USA 2016, 113, 9876–9881. [Google Scholar] [CrossRef] [PubMed]
- Behr, M.A. Evolution of Mycobacterium tuberculosis. Adv. Exp. Med. Biol. 2013, 783, 81–91. [Google Scholar] [PubMed]
- Das, S.; Pettersson, B.M.; Behra, P.R.; Ramesh, M.; Dasgupta, S.; Bhattacharya, A.; Kirsebom, L.A. Characterization of Three Mycobacterium spp. with Potential Use in Bioremediation by Genome Sequencing and Comparative Genomics. Genome Biol. Evol. 2015, 7, 1871–1886. [Google Scholar] [CrossRef] [PubMed]
- Quadri, L.E. Biosynthesis of mycobacterial lipids by polyketide synthases and beyond. Crit. Rev. Biochem. Mol. Biol. 2014, 49, 179–211. [Google Scholar] [CrossRef] [PubMed]
- Krithika, R.; Marathe, U.; Saxena, P.; Ansari, M.Z.; Mohanty, D.; Gokhale, R.S. A genetic locus required for iron acquisition in Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 2006, 103, 2069–2074. [Google Scholar] [CrossRef]
- Marrakchi, H.; Laneelle, M.A.; Daffe, M. Mycolic acids: Structures, biosynthesis, and beyond. Chem. Biol. 2014, 21, 67–85. [Google Scholar] [CrossRef]
- Daniel, J.; Deb, C.; Dubey, V.S.; Sirakova, T.D.; Abomoelak, B.; Morbidoni, H.R.; Kolattukudy, P.E. Induction of a novel class of diacylglycerol acyltransferases and triacylglycerol accumulation in Mycobacterium tuberculosis as it goes into a dormancy-like state in culture. J. Bacteriol. 2004, 186, 5017–5030. [Google Scholar] [CrossRef]
- Daniel, J.; Maamar, H.; Deb, C.; Sirakova, T.D.; Kolattukudy, P.E. Mycobacterium tuberculosis uses host triacylglycerol to accumulate lipid droplets and acquires a dormancy-like phenotype in lipid-loaded macrophages. PLoS Pathog. 2011, 7, e1002093. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, N.D.; Kolattukudy, P.E. Cloning, sequencing and characterization of a fatty acid synthase-encoding gene from Mycobacterium tuberculosis var. bovis BCG. Gene 1996, 170, 95–99. [Google Scholar] [CrossRef]
- Gobin, J.; Wong, D.K.; Gibson, B.W.; Horwitz, M.A. Characterization of exochelins of the Mycobacterium bovis type strain and BCG substrains. Infect. Immun. 1999, 67, 2035–2039. [Google Scholar] [PubMed]
- Kikuchi, S.; Rainwater, D.L.; Kolattukudy, P.E. Purification and characterization of an unusually large fatty acid synthase from Mycobacterium tuberculosis var. bovis BCG. Arch. Biochem. Biophys. 1992, 295, 318–326. [Google Scholar] [CrossRef]
- Rainwater, D.L.; Kolattukudy, P.E. Synthesis of mycocerosic acids from methylmalonyl coenzyme A by cell-free extracts of Mycobacterium tuberculosis var. bovis BCG. J. Biol. Chem. 1983, 258, 2979–2985. [Google Scholar] [PubMed]
- Vergnolle, O.; Xu, H.; Blanchard, J.S. Mechanism and regulation of mycobactin fatty acyl-AMP ligase FadD33. J. Biol. Chem. 2013, 288, 28116–28125. [Google Scholar] [CrossRef] [PubMed]
- Eoh, H.; Rhee, K.Y. Methylcitrate cycle defines the bactericidal essentiality of isocitrate lyase for survival of Mycobacterium tuberculosis on fatty acids. Proc. Natl. Acad. Sci. USA 2014, 111, 4976–4981. [Google Scholar] [CrossRef]
- Muñoz-Elías, E.J.; Upton, A.M.; Cherian, J.; McKinney, J.D. Role of the methylcitrate cycle in Mycobacterium tuberculosis metabolism, intracellular growth, and virulence. Mol. Microbiol. 2006, 60, 1109–1122. [Google Scholar] [CrossRef] [PubMed]
- Savvi, S.; Warner, D.F.; Kana, B.D.; McKinney, J.D.; Mizrahi, V.; Dawes, S.S. Functional Characterization of a Vitamin B12-Dependent Methylmalonyl Pathway in Mycobacterium tuberculosis: Implications for Propionate Metabolism during Growth on Fatty Acids. J. Bacteriol. 2008, 190, 3886–3895. [Google Scholar] [CrossRef]
- Horswill, A.R.; Escalante-Semerena, J.C. In vitro conversion of propionate to pyruvate by Salmonella enterica enzymes: 2-methylcitrate dehydratase (PrpD) and aconitase Enzymes catalyze the conversion of 2-methylcitrate to 2-methylisocitrate. Biochemistry 2001, 40, 4703–4713. [Google Scholar] [CrossRef]
- Upton, A.M.; McKinney, J.D. Role of the methylcitrate cycle in propionate metabolism and detoxification in Mycobacterium smegmatis. Microbiology 2007, 153, 3973–3982. [Google Scholar] [CrossRef] [PubMed]
- Kozyraki, R.; Cases, O. Vitamin B12 absorption: Mammalian physiology and acquired and inherited disorders. Biochimie 2013, 95, 1002–1007. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, K.; Venclovas, Č.; Ioerger, T.R.; Sacchettini, J.C.; McKinney, J.D.; Mizrahi, V.; Warner, D.F. A vitamin B(1)(2) transporter in Mycobacterium tuberculosis. Open Biol. 2013, 3, 120175. [Google Scholar] [CrossRef] [PubMed]
- Boritsch, E.C.; Supply, P.; Honoré, N.; Seemann, T.; Stinear, T.P.; Brosch, R. A glimpse into the past and predictions for the future: The molecular evolution of the tuberculosis agent. Mol. Microbiol. 2014, 93, 835–852. [Google Scholar] [CrossRef]
- Griffin, J.E.; Pandey, A.K.; Gilmore, S.A.; Mizrahi, V.; McKinney, J.D.; Bertozzi, C.R.; Sassetti, C.M. Cholesterol catabolism by Mycobacterium tuberculosis requires transcriptional and metabolic adaptations. Chem. Biol. 2012, 19, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.; Petzold, C.J.; Schelle, M.W.; Leavell, M.D.; Mougous, J.D.; Bertozzi, C.R.; Leary, J.A.; Cox, J.S. Lipidomics reveals control of Mycobacterium tuberculosis virulence lipids via metabolic coupling. Proc. Natl. Acad. Sci. USA 2007, 104, 5133–5138. [Google Scholar] [CrossRef]
- Yang, X.; Nesbitt, N.M.; Dubnau, E.; Smith, I.; Sampson, N.S. Cholesterol Metabolism Increases the Metabolic Pool of Propionate in Mycobacterium tuberculosis. Biochemistry 2009, 48, 3819–3821. [Google Scholar] [CrossRef]
- Astarie-Dequeker, C.; Le Guyader, L.; Malaga, W.; Seaphanh, F.K.; Chalut, C.; Lopez, A.; Guilhot, C. Phthiocerol dimycocerosates of M. tuberculosis participate in macrophage invasion by inducing changes in the organization of plasma membrane lipids. PLoS Pathog. 2009, 5, e1000289. [Google Scholar] [CrossRef]
- Cambier, C.J.; Takaki, K.K.; Larson, R.P.; Hernandez, R.E.; Tobin, D.M.; Urdahl, K.B.; Cosma, C.L.; Ramakrishnan, L. Mycobacteria manipulate macrophage recruitment through coordinated use of membrane lipids. Nature 2014, 505, 218–222. [Google Scholar] [CrossRef]
- Day, T.A.; Mittler, J.E.; Nixon, M.R.; Thompson, C.; Miner, M.D.; Hickey, M.J.; Liao, R.P.; Pang, J.M.; Shayakhmetov, D.M.; Sherman, D.R. Mycobacterium tuberculosis strains lacking surface lipid phthiocerol dimycocerosate are susceptible to killing by an early innate host response. Infect. Immun. 2014, 82, 5214–5222. [Google Scholar] [CrossRef]
- Kirksey, M.A.; Tischler, A.D.; Siméone, R.; Hisert, K.B.; Uplekar, S.; Guilhot, C.; McKinney, J.D. Spontaneous phthiocerol dimycocerosate-deficient variants of Mycobacterium tuberculosis are susceptible to gamma interferon-mediated immunity. Infect. Immun. 2011, 79, 2829–2838. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, C.; Winter, N.; Pivert, E.; Bordat, Y.; Neyrolles, O.; Avé, P.; Huerre, M.; Gicquel, B.; Jackson, M. Production of phthiocerol dimycocerosates protects Mycobacterium tuberculosis from the cidal activity of reactive nitrogen intermediates produced by macrophages and modulates the early immune response to infection. Cell. Microbiol. 2004, 6, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Barczak, A.K.; Avraham, R.; Singh, S.; Luo, S.S. Systematic, multiparametric analysis of Mycobacterium tuberculosis intracellular infection offers insight into coordinated virulence. PLos Pathog. 2017, 13, e1006363. [Google Scholar] [CrossRef] [PubMed]
- Quigley, J.; Hughitt, V.K.; Velikovsky, C.A.; Mariuzza, R.A.; El-Sayed, N.M. The Cell Wall Lipid PDIM Contributes to Phagosomal Escape and Host Cell Exit of Mycobacterium tuberculosis. mBio 2017, 8, e00148-17. [Google Scholar] [CrossRef]
- Constant, P.; Perez, E.; Malaga, W.; Lanéelle, M.A.; Saurel, O.; Daffé, M.; Guilhot, C. Role of the pks15/1 Gene in the Biosynthesis of Phenolglycolipids in the Mycobacterium tuberculosis Complex. J. Biol. Chem. 2002, 277, 38148–38158. [Google Scholar] [CrossRef]
- Malaga, W.; Constant, P.; Euphrasie, D.; Cataldi, A.; Daffé, M.; Reyrat, J.M.; Guilhot, C. Deciphering the genetic bases of the structural diversity of phenolic glycolipids in strains of the Mycobacterium tuberculosis complex. J. Biol. Chem. 2008, 283, 15177–15184. [Google Scholar] [CrossRef]
- Reed, M.B.; Domenech, P.; Manca, C.; Su, H.; Barczak, A.K.; Kreiswirth, B.N.; Kaplan, G.; Barry, C.E., III. A glycolipid of hypervirulent tuberculosis strains that inhibits the innate immune response. Nature 2004, 431, 84–87. [Google Scholar] [CrossRef]
- Cambier, C.J.; O’Leary, S.M.; O’Sullivan, M.P.; Keane, J.; Ramakrishnan, L. Phenolic Glycolipid Facilitates Mycobacterial Escape from Microbicidal Tissue-Resident Macrophages. Immunity 2017, 47, 552–565.e554. [Google Scholar] [CrossRef]
- Manca, C.; Reed, M.B.; Freeman, S.; Mathema, B.; Kreiswirth, B.; Barry, C.E., III; Kaplan, G. Differential monocyte activation underlies strain-specific Mycobacterium tuberculosis pathogenesis. Infect. Immun. 2004, 72, 5511–5514. [Google Scholar] [CrossRef]
- Ginhoux, F.; Guilliams, M. Tissue-Resident Macrophage Ontogeny and Homeostasis. Immunuity 2016, 44, 439–449. [Google Scholar] [CrossRef]
- Ginhoux, F.; Jung, S. Monocytes and macrophages: Developmental pathways and tissue homeostasis. Nat. Rev. Immunol. 2014, 14, 392–404. [Google Scholar] [CrossRef]
- Huang, L.; Nazarova, E.V.; Tan, S.; Liu, Y.; Russell, D.G. Growth of Mycobacterium tuberculosis in vivo segregates with host macrophage metabolism and ontogeny. J. Exp. Med. 2018, 215, 1135–1152. [Google Scholar] [CrossRef] [PubMed]
- Mattila, J.T.; Ojo, O.O.; Kepka-Lenhart, D.; Marino, S.; Kim, J.H.; Eum, S.Y.; Via, L.E.; Barry, C.E., III; Klein, E.; Kirschner, D.E.; et al. Microenvironments in tuberculous granulomas are delineated by distinct populations of macrophage subsets and expression of nitric oxide synthase and arginase isoforms. J. Immunol. 2013, 191, 773–784. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, L.A.; Pearce, E.J. Immunometabolism governs dendritic cell and macrophage function. J. Exp. Med. 2016, 213, 15–23. [Google Scholar] [CrossRef]
- Billig, S.; Schneefeld, M.; Huber, C.; Grassl, G.A.; Eisenreich, W.; Bange, F.C. Lactate oxidation facilitates growth of Mycobacterium tuberculosis in human macrophages. Sci. Rep. 2017, 7, 6484. [Google Scholar] [CrossRef] [PubMed]
- Agapova, A.; Serafini, A.; Petridis, M.; Hunt, D.M.; Garza-Garcia, A.; Sohaskey, C.D.; de Carvalho, L.P.S. Flexible nitrogen utilisation by the metabolic generalist pathogen Mycobacterium tuberculosis. eLife 2019, 8, e41129. [Google Scholar] [CrossRef] [PubMed]
- Cunningham-Bussel, A.; Zhang, T.; Nathan, C.F. Nitrite produced by Mycobacterium tuberculosis in human macrophages in physiologic oxygen impacts bacterial ATP consumption and gene expression. Proc. Natl. Acad. Sci. USA 2013, 110, E4256–E4265. [Google Scholar] [CrossRef]
- Jung, J.Y.; Madan-Lala, R.; Georgieva, M.; Rengarajan, J.; Sohaskey, C.D.; Bange, F.C.; Robinson, C.M. The intracellular environment of human macrophages that produce nitric oxide promotes growth of mycobacteria. Infect. Immun. 2013, 81, 3198–3209. [Google Scholar] [CrossRef]
- Cook, G.M.; Hards, K.; Vilcheze, C.; Hartman, T.; Berney, M. Energetics of Respiration and Oxidative Phosphorylation in Mycobacteria. Microbiol. Spectr. 2014, 2. [Google Scholar] [CrossRef]
- Gouzy, A.; Larrouy-Maumus, G.; Bottai, D.; Levillain, F.; Dumas, A.; Wallach, J.B.; Caire-Brandli, I.; de Chastellier, C.; Wu, T.D.; Poincloux, R.; et al. Mycobacterium tuberculosis exploits asparagine to assimilate nitrogen and resist acid stress during infection. PLoS Pathog. 2014, 10, e1003928. [Google Scholar] [CrossRef] [PubMed]
- Gouzy, A. Mycobacterium tuberculosis nitrogen assimilation and host colonization require aspartate. Nat. Chem. Biol. 2013, 9, 674–676. [Google Scholar] [CrossRef] [PubMed]
- Stermann, M.; Bohrssen, A.; Diephaus, C.; Maass, S.; Bange, F.C. Polymorphic nucleotide within the promoter of nitrate reductase (NarGHJI) is specific for Mycobacterium tuberculosis. J. Clin. Microbiol. 2003, 41, 3252–3259. [Google Scholar] [CrossRef]
- Goh, K.S.; Rastogi, N.; Berchel, M.; Huard, R.C.; Sola, C. Molecular evolutionary history of tubercle bacilli assessed by study of the polymorphic nucleotide within the nitrate reductase (narGHJI) operon promoter. J. Clin. Microbiol. 2005, 43, 4010–4014. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.P.; Sequeira, P.; Lin, W.W.; Phong, W.Y.; Cliff, P.; Ng, S.H.; Lee, B.H.; Camacho, L.; Schnappinger, D.; Ehrt, S.; et al. Nitrate respiration protects hypoxic Mycobacterium tuberculosis against acid- and reactive nitrogen species stresses. PLoS ONE 2010, 5, e13356. [Google Scholar] [CrossRef] [PubMed]
- Levillain, F.; Poquet, Y.; Mallet, L.; Mazères, S.; Marceau, M.; Brosch, R.; Bange, F.C.; Supply, P.; Magalon, A.; Neyrolles, O. Horizontal acquisition of a hypoxia-responsive molybdenum cofactor biosynthesis pathway contributed to Mycobacterium tuberculosis pathoadaptation. PLoS Pathog. 2017, 13, e1006752. [Google Scholar] [CrossRef]
- Williams, M.J.; Shanley, C.A.; Zilavy, A.; Peixoto, B.; Manca, C.; Kaplan, G.; Orme, I.M.; Mizrahi, V.; Kana, B.D. bis-Molybdopterin guanine dinucleotide is required for persistence of Mycobacterium tuberculosis in guinea pigs. Infect. Immun. 2015, 83, 544–550. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fang, Z.; Sampson, S.L.; Warren, R.M.; Gey van Pittius, N.C.; Newton-Foot, M. Iron acquisition strategies in mycobacteria. Tuberculosis 2015, 95, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Rohde, K.H.; Abramovitch, R.B.; Russell, D.G. Mycobacterium tuberculosis invasion of macrophages: Linking bacterial gene expression to environmental cues. Cell Host Microbe 2007, 2, 352–364. [Google Scholar] [CrossRef]
- Tullius, M.V.; Harmston, C.A.; Owens, C.P.; Chim, N.; Morse, R.P.; McMath, L.M.; Iniguez, A.; Kimmey, J.M.; Sawaya, M.R.; Whitelegge, J.P.; et al. Discovery and characterization of a unique mycobacterial heme acquisition system. Proc. Natl. Acad. Sci. USA 2011, 108, 5051–5056. [Google Scholar] [CrossRef]
- Wagner, D.; Maser, J.; Lai, B.; Cai, Z.; Barry, C.E., III; Höner Zu Bentrup, K.; Russell, D.G.; Bermudez, L.E. Elemental analysis of Mycobacterium avium-, Mycobacterium tuberculosis-, and Mycobacterium smegmatis-containing phagosomes indicates pathogen-induced microenvironments within the host cell’s endosomal system. J. Immunol. 2005, 174, 1491–1500. [Google Scholar] [CrossRef]
- Megehee, J.A.; Hosler, J.P.; Lundrigan, M.D. Evidence for a cytochrome bcc-aa3 interaction in the respiratory chain of Mycobacterium smegmatis. Microbiology 2006, 152, 823–829. [Google Scholar] [CrossRef][Green Version]
- Mendel, R.R. The molybdenum cofactor. J. Biol. Chem. 2013, 288, 13165–13172. [Google Scholar] [CrossRef]
- Williams, M.J.; Kana, B.D.; Mizrahi, V. Functional analysis of molybdopterin biosynthesis in mycobacteria identifies a fused molybdopterin synthase in Mycobacterium tuberculosis. J. Bacteriol. 2011, 193, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.; Mizrahi, V.; Kana, B.D. Molybdenum cofactor: A key component of Mycobacterium tuberculosis pathogenesis? Crit. Rev. Microbiol. 2014, 40, 18–29. [Google Scholar] [CrossRef] [PubMed]
- McGuire, A.M.; Weiner, B.; Park, S.T.; Wapinski, I.; Raman, S.; Dolganov, G.; Peterson, M.; Riley, R.; Zucker, J.; Abeel, T.; et al. Comparative analysis of mycobacterium and related actinomycetes yields insight into the evolution of Mycobacterium tuberculosis pathogenesis. BMC Genom. 2012, 13, 120. [Google Scholar] [CrossRef]
- Becq, J.; Gutierrez, M.C.; Rosas-Magallanes, V.; Rauzier, J.; Gicquel, B.; Neyrolles, O.; Deschavanne, P. Contribution of horizontally acquired genomic islands to the evolution of the Tubercle bacilli. Mol. Biol. Evol. 2007, 24, 1861–1871. [Google Scholar] [CrossRef] [PubMed]
- Stinear, T.P.; Seemann, T.; Harrison, P.F.; Jenkin, G.A.; Davies, J.K.; Johnson, P.D.; Abdellah, Z.; Arrowsmith, C.; Chillingworth, T.; Churcher, C.; et al. Insights from the complete genome sequence of Mycobacterium marinum on the evolution of Mycobacterium tuberculosis. Genome Res. 2008, 18, 729–741. [Google Scholar] [CrossRef]
- Veyrier, F.; Pletzer, D.; Turenne, C.; Behr, M.A. Phylogenetic detection of horizontal gene transfer during the step-wise genesis of Mycobacterium tuberculosis. BMC Evol. Biol. 2009, 9, 196. [Google Scholar] [CrossRef]
- Van Ingen, J.; Rahim, Z.; Mulder, A.; Boeree, M.J.; Simeone, R.; Brosch, R.; van Soolingen, D. Characterization of mycobacterium orygis as M. tuberculosis complex subspecies. Emerg. Infect. Dis. 2012, 18, 653–655. [Google Scholar] [CrossRef]
- Michelucci, A.; Cordes, T.; Ghelfi, J.; Pailot, A.; Reiling, N.; Goldmann, O.; Binz, T.; Wegner, A.; Tallam, A.; Rausell, A.; et al. Immune-responsive gene 1 protein links metabolism to immunity by catalyzing itaconic acid production. Proc. Natl. Acad. Sci. USA 2013, 110, 7820–7825. [Google Scholar] [CrossRef]
- Lampropoulou, V.; Sergushichev, A.; Bambouskova, M.; Nair, S.; Vincent, E.E.; Loginicheva, E.; Cervantes-Barragan, L.; Ma, X.; Huang, S.C.; Griss, T.; et al. Itaconate Links Inhibition of Succinate Dehydrogenase with Macrophage Metabolic Remodeling and Regulation of Inflammation. Cell Metab. 2016, 24, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.J. Amino acid auxotrophy as a system of immunological control nodes. Nat. Immunol 2016, 17, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, A.; Nagalingam, G.; Huch, J.H.; Walker, L.; Guillemin, G.J.; Smythe, G.A.; Ehrt, S.; Britton, W.J.; Saunders, B.M. M. tuberculosis induces potent activation of IDO-1, but this is not essential for the immunological control of infection. PLoS ONE 2012, 7, e37314. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.J.; Reddy, M.C.; Ioerger, T.R.; Rothchild, A.C.; Dartois, V.; Schuster, B.M.; Trauner, A.; Wallis, D.; Galaviz, S.; Huttenhower, C.; et al. Tryptophan biosynthesis protects mycobacteria from CD4 T-cell-mediated killing. Cell 2013, 155, 1296–1308. [Google Scholar] [CrossRef] [PubMed]
- Moreau, M.; Lestage, J.; Verrier, D.; Mormede, C.; Kelley, K.W.; Dantzer, R.; Castanon, N. Bacille Calmette-Guerin inoculation induces chronic activation of peripheral and brain indoleamine 2,3-dioxygenase in mice. J. Infect. Dis. 2005, 192, 537–544. [Google Scholar] [CrossRef]
- Djoko, K.Y.; Ong, C.L.; Walker, M.J.; McEwan, A.G. The Role of Copper and Zinc Toxicity in Innate Immune Defense against Bacterial Pathogens. J. Biol. Chem. 2015, 290, 18954–18961. [Google Scholar] [CrossRef] [PubMed]
- White, C.; Lee, J.; Kambe, T.; Fritsche, K.; Petris, M.J. A role for the ATP7A copper-transporting ATPase in macrophage bactericidal activity. J. Biol. Chem. 2009, 284, 33949–33956. [Google Scholar] [CrossRef] [PubMed]
- Marcus, S.A.; Sidiropoulos, S.W.; Steinberg, H.; Talaat, A.M. CsoR Is Essential for Maintaining Copper Homeostasis in Mycobacterium tuberculosis. PLoS ONE 2016, 11, e0151816. [Google Scholar] [CrossRef]
- Wolschendorf, F.; Ackart, D.; Shrestha, T.B.; Hascall-Dove, L.; Nolan, S.; Lamichhane, G.; Wang, Y.; Bossmann, S.H.; Basaraba, R.J.; Niederweis, M. Copper resistance is essential for virulence of Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 2011, 108, 1621–1626. [Google Scholar] [CrossRef] [PubMed]
- Gold, B.; Deng, H.; Bryk, R.; Vargas, D.; Eliezer, D.; Roberts, J.; Jiang, X.; Nathan, C. Identification of a copper-binding metallothionein in pathogenic mycobacteria. Nat. Chem. Biol. 2008, 4, 609–616. [Google Scholar] [CrossRef]
- Bin, B.H.; Seo, J.; Kim, S.T. Function, Structure, and Transport Aspects of ZIP and ZnT Zinc Transporters in Immune Cells. J. Immunol. Res. 2018, 2018, 9365747. [Google Scholar] [CrossRef] [PubMed]
- Botella, H.; Peyron, P.; Levillain, F.; Poincloux, R.; Poquet, Y.; Brandli, I.; Wang, C.; Tailleux, L.; Tilleul, S.; Charrière, G.M.; et al. Mycobacterial p(1)-type ATPases mediate resistance to zinc poisoning in human macrophages. Cell Host Microbe 2011, 10, 248–259. [Google Scholar] [CrossRef] [PubMed]
- Abramovitch, R.B.; Rohde, K.H.; Hsu, F.-F.; Russell, D.G. aprABC: A Mycobacterium tuberculosis complex-specific locus that modulates pH-driven adaptation to the macrophage phagosome. Mol. Microbiol. 2011, 80, 678–694. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.J.; Johnson, B.K.; Abramovitch, R.B. Slow growth of Mycobacterium tuberculosis at acidic pH is regulated by phoPR and host-associated carbon sources. Mol. Microbiol. 2014, 94, 56–69. [Google Scholar] [CrossRef]
- Chesne-Seck, M.L.; Barilone, N.; Boudou, F.; Gonzalo Asensio, J.; Kolattukudy, P.E.; Martín, C.; Cole, S.T.; Gicquel, B.; Gopaul, D.N.; Jackson, M. A point mutation in the two-component regulator PhoP-PhoR accounts for the absence of polyketide-derived acyltrehaloses but not that of phthiocerol dimycocerosates in Mycobacterium tuberculosis H37Ra. J. Bacteriol. 2008, 190, 1329–1334. [Google Scholar] [CrossRef]
- Frigui, W.; Bottai, D.; Majlessi, L.; Monot, M.; Josselin, E.; Brodin, P.; Garnier, T.; Gicquel, B.; Martin, C.; Leclerc, C.; et al. Control of M. tuberculosis ESAT-6 secretion and specific T cell recognition by PhoP. PLoS Pathog. 2008, 4, e33. [Google Scholar] [CrossRef]
- Walters, S.B.; Dubnau, E.; Kolesnikova, I.; Laval, F.; Daffe, M.; Smith, I. The Mycobacterium tuberculosis PhoPR two-component system regulates genes essential for virulence and complex lipid biosynthesis. Mol. Microbiol. 2006, 60, 312–330. [Google Scholar] [CrossRef]
- Gonzalo-Asensio, J.; Malaga, W.; Pawlik, A.; Astarie-Dequeker, C.; Passemar, C.; Moreau, F.; Laval, F.; Daffé, M.; Martin, C.; Brosch, R.; et al. Evolutionary history of tuberculosis shaped by conserved mutations in the PhoPR virulence regulator. Proc. Natl. Acad. Sci. USA 2014, 111, 11491–11496. [Google Scholar] [CrossRef]
- Broset, E.; Martin, C.; Gonzalo-Asensio, J. Evolutionary landscape of the Mycobacterium tuberculosis complex from the viewpoint of PhoPR: Implications for virulence regulation and application to vaccine development. mBio 2015, 6, e01289-15. [Google Scholar] [CrossRef]
- Ates, L.S.; Dippenaar, A.; Sayes, F.; Pawlik, A.; Bouchier, C.; Ma, L.; Warren, R.M.; Sougakoff, W.; Majlessi, L.; van Heijst, J.W.J.; et al. Unexpected Genomic and Phenotypic Diversity of Mycobacterium africanum Lineage 5 Affects Drug Resistance, Protein Secretion, and Immunogenicity. Genome Biol. Evol. 2018, 10, 1858–1874. [Google Scholar] [CrossRef]
- Jackson, M.; Stadthagen, G.; Gicquel, B. Long-chain multiple methyl-branched fatty acid-containing lipids of Mycobacterium tuberculosis: Biosynthesis, transport, regulation and biological activities. Tuberculosis 2007, 87, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Rivero, A.; Márquez, M.; Santos, J.; Pinedo, A.; Sánchez, M.A.; Esteve, A.; Samper, S.; Martín, C. High rate of tuberculosis reinfection during a nosocomial outbreak of multidrug-resistant tuberculosis caused by Mycobacterium bovis strain B. Clin. Infect. Dis. 2001, 32, 159–161. [Google Scholar] [CrossRef] [PubMed]
- Soto, C.Y.; Menéndez, M.C.; Pérez, E.; Samper, S.; Gómez, A.B.; García, M.J.; Martín, C. IS6110 mediates increased transcription of the phoP virulence gene in a multidrug-resistant clinical isolate responsible for tuberculosis outbreaks. J. Clin. Microbiol. 2004, 42, 212–219. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fieweger, R.A.; Wilburn, K.M.; VanderVen, B.C. Comparing the Metabolic Capabilities of Bacteria in the Mycobacterium tuberculosis Complex. Microorganisms 2019, 7, 177. https://doi.org/10.3390/microorganisms7060177
Fieweger RA, Wilburn KM, VanderVen BC. Comparing the Metabolic Capabilities of Bacteria in the Mycobacterium tuberculosis Complex. Microorganisms. 2019; 7(6):177. https://doi.org/10.3390/microorganisms7060177
Chicago/Turabian StyleFieweger, Rachael A., Kaley M. Wilburn, and Brian C. VanderVen. 2019. "Comparing the Metabolic Capabilities of Bacteria in the Mycobacterium tuberculosis Complex" Microorganisms 7, no. 6: 177. https://doi.org/10.3390/microorganisms7060177
APA StyleFieweger, R. A., Wilburn, K. M., & VanderVen, B. C. (2019). Comparing the Metabolic Capabilities of Bacteria in the Mycobacterium tuberculosis Complex. Microorganisms, 7(6), 177. https://doi.org/10.3390/microorganisms7060177




