Microbial Eukaryotes in Oil Sands Environments: Heterotrophs in the Spotlight
Abstract
1. Introduction
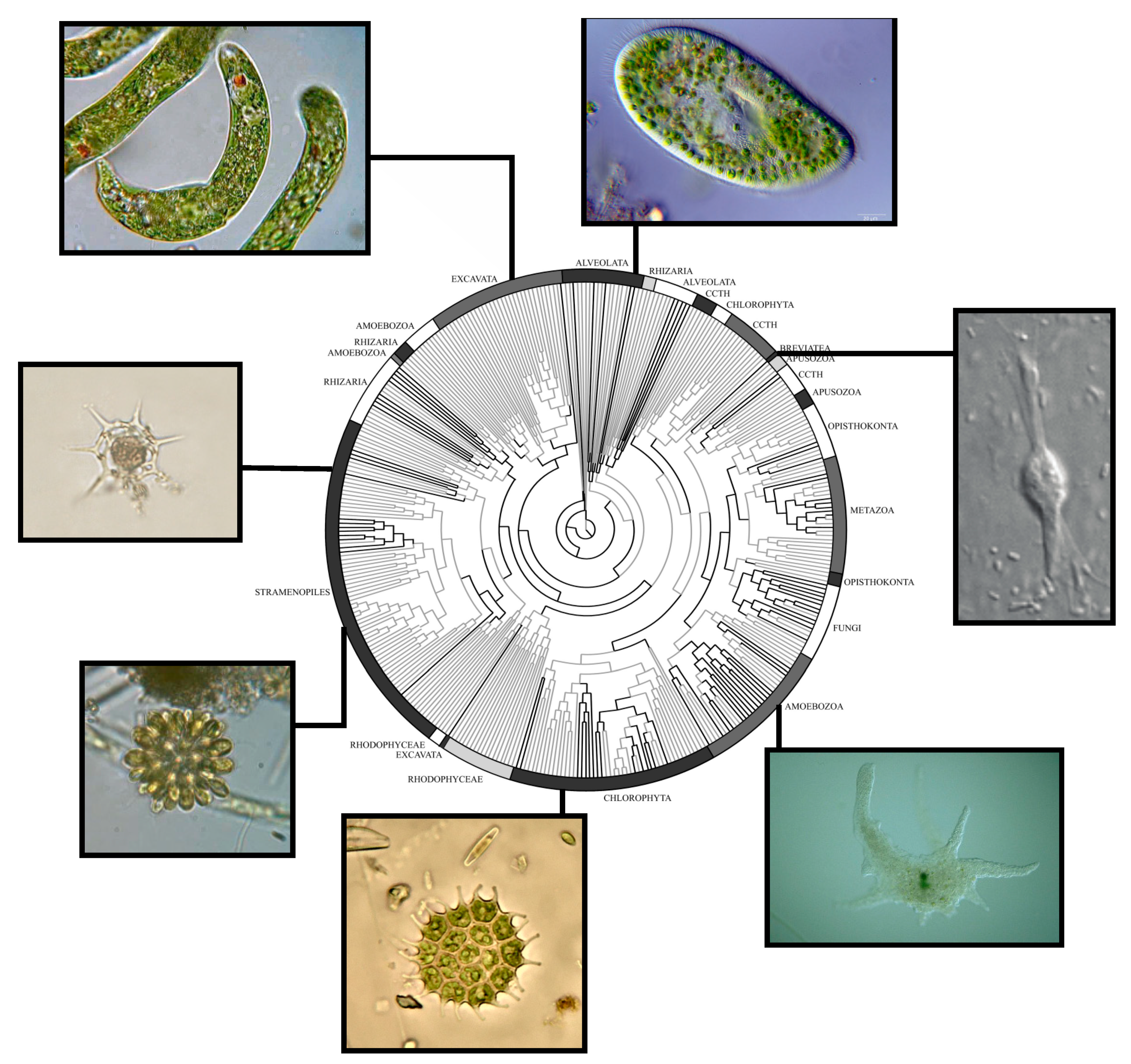
2. Protist Diversity and Environmental Abundance: New Technology Uncover New Realities
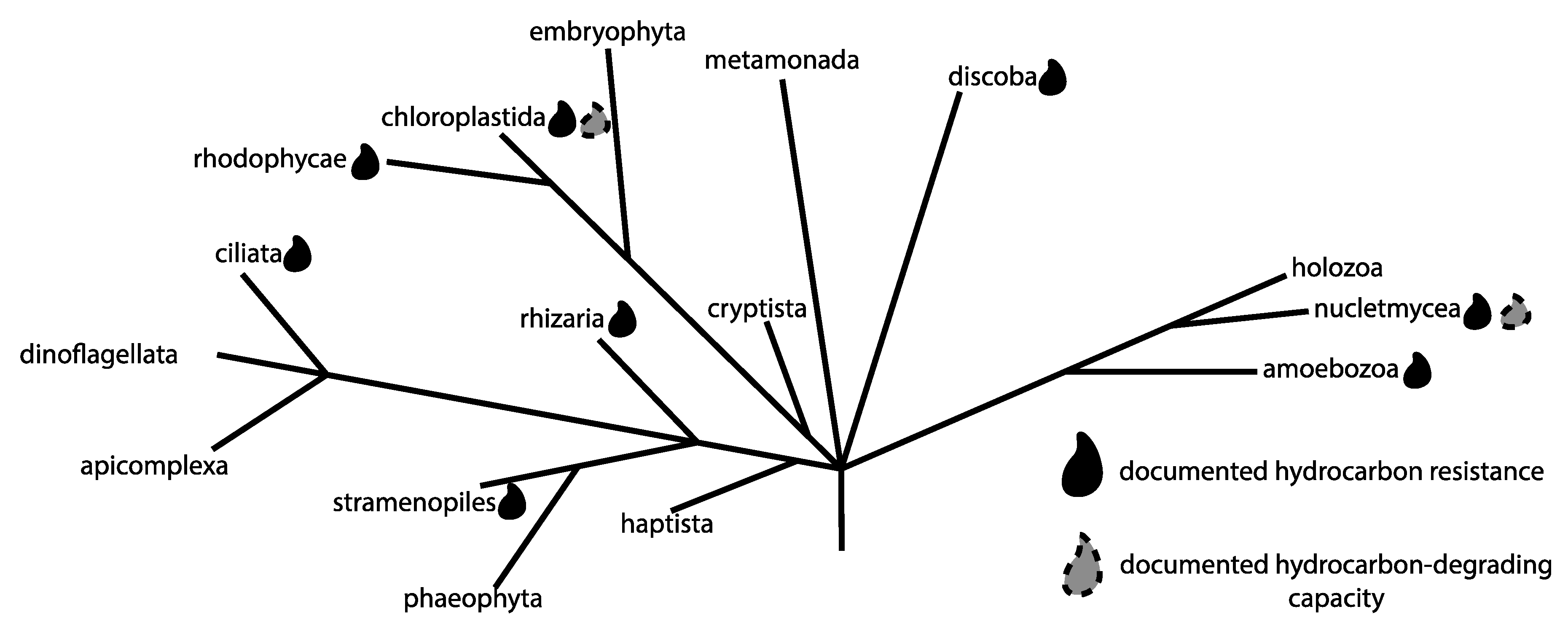
3. Protists and Anthropogenically Impacted Environments
4. The Albertan Oil Sands
5. Protists in the Oil Sands Region
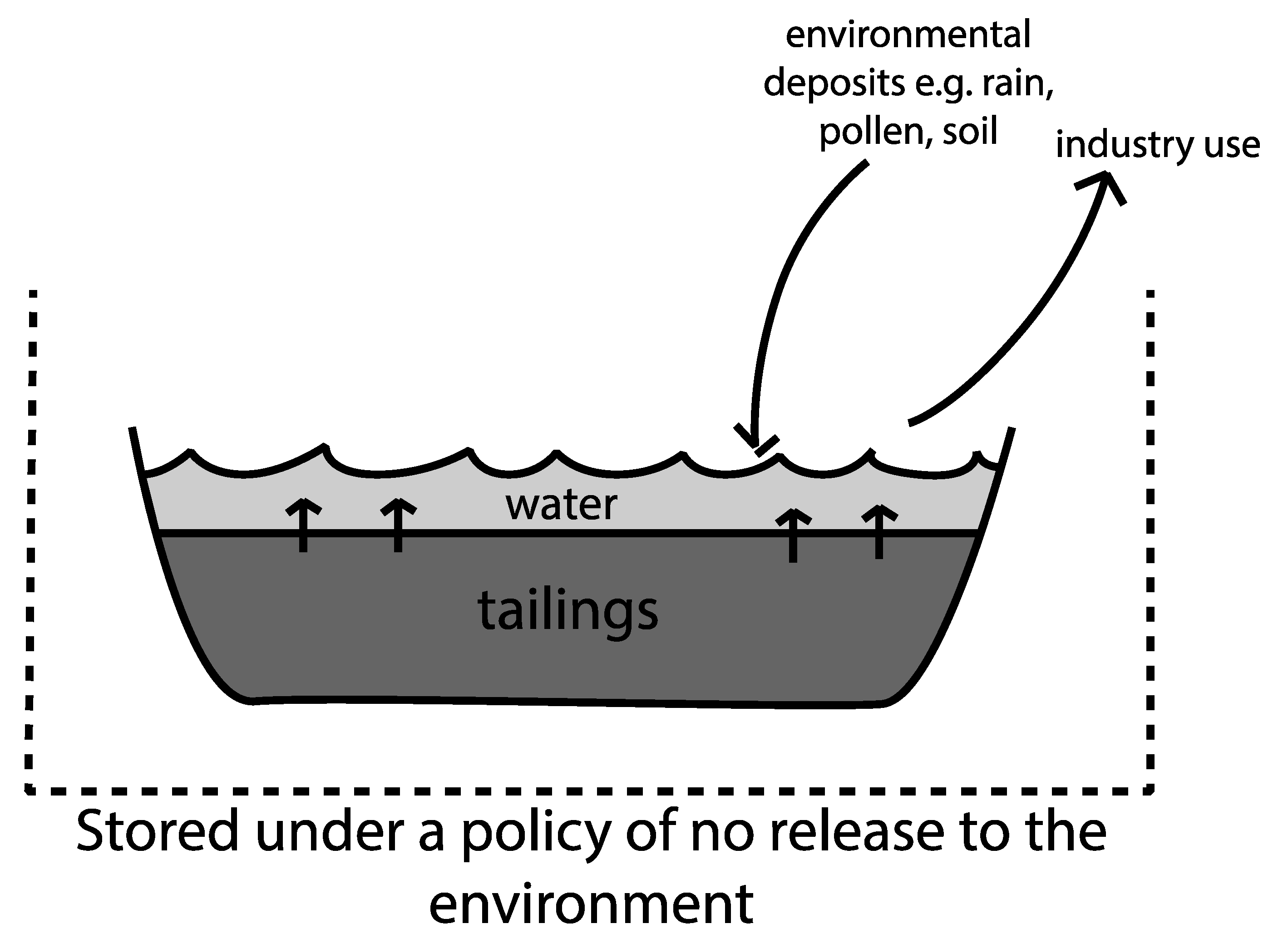
6. Tailings Ponds
7. In Vitro and In Situ Assessments of Protists in Reclamation Environments
8. Protists and Oil Sands Reclamation
9. Final Remarks
Funding
Acknowledgments
Conflicts of Interest
References
- Adl, S.M.; Bass, D.; Lane, C.E.; Lukeš, J.; Schoch, C.L.; Smirnov, A.; Agatha, S.; Berney, C.; Brown, M.W.; Burki, F.; et al. Revisions to the Classification, Nomenclature, and Diversity of Eukaryotes. J. Eukaryot. Microbiol. 2018, 66, jeu.12691. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, P.F.; Willerslev, E. Environmental DNA—An emerging tool in conservation for monitoring past and present biodiversity. Biol. Conserv. 2014, 183, 4–18. [Google Scholar] [CrossRef]
- Bik, H.M.; Porazinska, D.L.; Creer, S.; Caporaso, J.G.; Knight, R.; Thomas, W.K. Sequencing our way towards understanding global eukaryotic biodiversity. Trends Ecol. Evol. 2012, 27, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Bik, H.M.; Sung, W.; de Ley, P.; Baldwin, J.G.; Sharma, J.; Rocha-Olivares, A.; Thomas, W.K. Metagenetic community analysis of microbial eukaryotes illuminates biogeographic patterns in deep-sea and shallow water sediments. Mol. Ecol. 2012, 21, 1048–1059. [Google Scholar] [CrossRef] [PubMed]
- Obbels, D.; Verleyen, E.; Mano, M.-J.; Namsaraev, Z.; Sweetlove, M.; Tytgat, B.; Fernandez-Carazo, R.; De Wever, A.; D’hondt, S.; Ertz, D.; et al. Bacterial and eukaryotic biodiversity patterns in terrestrial and aquatic habitats in the Sør Rondane Mountains, Dronning Maud Land, East Antarctica. FEMS Microbiol. Ecol. 2016, 92, fiw041. [Google Scholar] [CrossRef]
- Mesa, V.; Gallego, J.L.R.; González-Gil, R.; Lauga, B.; Sánchez, J.; Méndez-García, C.; Peláez, A.I. Bacterial, Archaeal, and Eukaryotic Diversity across Distinct Microhabitats in an Acid Mine Drainage. Front. Microbiol. 2017, 8, 1756. [Google Scholar] [CrossRef]
- Bates, S.T.; Clemente, J.C.; Flores, G.E.; Walters, W.A.; Parfrey, L.W.; Knight, R.; Fierer, N. Global biogeography of highly diverse protistan communities in soil. ISME J. 2013, 7, 652–659. [Google Scholar] [CrossRef]
- del Campo, J.; Kolisko, M.; Boscaro, V.; Santoferrara, L.F.; Nenarokov, S.; Massana, R.; Guillou, L.; Simpson, A.; Berney, C.; de Vargas, C.; et al. EukRef: Phylogenetic curation of ribosomal RNA to enhance understanding of eukaryotic diversity and distribution. PLoS Biol. 2018, 16, e2005849. [Google Scholar] [CrossRef]
- Berney, C.; Ciuprina, A.; Bender, S.; Brodie, J.; Edgcomb, V.; Kim, E.; Rajan, J.; Parfrey, L.W.; Adl, S.; Audic, S.; et al. UniEuk: Time to Speak a Common Language in Protistology! J. Eukaryot. Microbiol. 2017, 64, 407–411. [Google Scholar] [CrossRef]
- Massana, R. Protistan Diversity in Environmental Molecular Surveys. In Marine Protists; Springer: Tokyo, Japan, 2015; pp. 3–21. [Google Scholar]
- Geisen, S. Thorough high-throughput sequencing analyses unravels huge diversities of soil parasitic protists. Environ. Microbiol. 2016, 18, 1669–1672. [Google Scholar] [CrossRef]
- Flegontova, O.; Flegontov, P.; Malviya, S.; Audic, S.; Wincker, P.; de Vargas, C.; Bowler, C.; Lukeš, J.; Horák, A. Extreme Diversity of Diplonemid Eukaryotes in the Ocean. Curr. Biol. 2016, 26, 3060–3065. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, J.A.; Jansson, J.K.; Knight, R. The Earth Microbiome project: Successes and aspirations. BMC Biol. 2014, 12, 69. [Google Scholar] [CrossRef] [PubMed]
- OPEC. OPEC: World Oil Outlook; OPEC: Vienna, Austria, 2018. [Google Scholar]
- Colwell, R.R.; Leinen, M.; Benoit, D.S.; Brewer, P.G.; Dodge, R.E.; Farrington, J.W.; Halanych, K.M.; Halpern, D.; Hogarth, W.T.; Mauritzen, C.; et al. The Gulf of Mexico Research Initiative: A New Research Paradigm. In Proceedings of the International Oil Spill Conference Proceedings, Savannah, GA, USA, 5–8 May 2014; p. 300123. [Google Scholar]
- Bretherton, L.; Williams, A.; Genzer, J.; Hillhouse, J.; Kamalanathan, M.; Finkel, Z.V.; Quigg, A. Physiological response of 10 phytoplankton species exposed to macondo oil and the dispersant, Corexit. J. Phycol. 2018, 54, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Özhan, K.; Miles, S.M.; Gao, H.; Bargu, S. Relative phytoplankton growth responses to physically and chemically dispersed South Louisiana sweet crude oil. Environ. Monit. Assess. 2014, 186, 3941–3956. [Google Scholar] [CrossRef] [PubMed]
- Lara, E.; Berney, C.; Harms, H.; Chatzinotas, A. Cultivation-independent analysis reveals a shift in ciliate 18S rRNA gene diversity in a polycyclic aromatic hydrocarbon-polluted soil. FEMS Microbiol. Ecol. 2007, 62, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Lanzén, A.; Lekang, K.; Jonassen, I.; Thompson, E.M.; Troedsson, C. High-throughput metabarcoding of eukaryotic diversity for environmental monitoring of offshore oil-drilling activities. Mol. Ecol. 2016, 25, 4392–4406. [Google Scholar] [CrossRef]
- Rahsepar, S.; Smit, M.P.J.; Murk, A.J.; Rijnaarts, H.H.M.; Langenhoff, A.A.M. Chemical dispersants: Oil biodegradation friend or foe? Mar. Pollut. Bull. 2016, 108, 113–119. [Google Scholar] [CrossRef]
- Snyder, R.A.; Ederington-Hagy, M.; Hileman, F.; Moss, J.A.; Amick, L.; Carruth, R.; Head, M.; Marks, J.; Tominack, S.; Jeffrey, W.H. Polycyclic aromatic hydrocarbon concentrations across the Florida Panhandle continental shelf and slope after the BP MC 252 well failure. Mar. Pollut. Bull. 2014, 89, 201–208. [Google Scholar] [CrossRef]
- Rodriguez-R, L.M.; Overholt, W.A.; Hagan, C.; Huettel, M.; Kostka, J.E.; Konstantinidis, K.T. Microbial community successional patterns in beach sands impacted by the Deepwater Horizon oil spill. ISME J. 2015, 9, 1928–1940. [Google Scholar] [CrossRef]
- Canadian Association of Petroleum Producers. Canada’s Oil Sands Fact Book; Canadian Association of Petroleum Producers: Calgary, AB, Canada, 2018. [Google Scholar]
- Brandt, J.P.; Flannigan, M.D.; Maynard, D.G.; Thompson, I.D.; Volney, W.J.A. An introduction to Canada’s boreal zone: Ecosystem processes, health, sustainability, and environmental issues. Environ. Rev. 2013, 21, 207–226. [Google Scholar] [CrossRef]
- Government of Alberta. Responsible Energy Development Act; Government of Alberta: Alberta, AB, Canada, 2014.
- Mossop, G.D. Geology of the Athabasca Oil Sands. Science (80-) 1980, 207, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Yergeau, E.; Lawrence, J.R.; Sanschagrin, S.; Waiser, M.J.; Korber, D.R.; Greer, C.W. Next-generation sequencing of microbial communities in the Athabasca River and its tributaries in relation to oil sands mining activities. Appl. Environ. Microbiol. 2012, 78, 7626–7637. [Google Scholar] [CrossRef] [PubMed]
- Reid, T.; Chaganti, S.R.; Droppo, I.G.; Weisener, C.G. Novel insights into freshwater hydrocarbon-rich sediments using metatranscriptomics: Opening the black box. Water Res. 2018, 136, 1–11. [Google Scholar] [CrossRef]
- Wong, M.-L.; An, D.; Caffrey, S.M.; Soh, J.; Dong, X.; Sensen, C.W.; Oldenburg, T.B.P.; Larter, S.R.; Voordouw, G. Roles of Thermophiles and Fungi in Bitumen Degradation in Mostly Cold Oil Sands Outcrops. Appl. Environ. Microbiol. 2015, 81, 6825–6838. [Google Scholar] [CrossRef] [PubMed]
- Aranda, E. Promising approaches towards biotransformation of polycyclic aromatic hydrocarbons with Ascomycota fungi. Curr. Opin. Biotechnol. 2016, 38, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Al-Hawash, A.B.; Alkooranee, J.T.; Abbood, H.A.; Zhang, J.; Sun, J.; Zhang, X.; Ma, F. Isolation and characterization of two crude oil-degrading fungi strains from Rumaila oil field, Iraq. Biotechnol. Rep. 2018, 17, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Foght, J.M.; Gieg, L.M.; Siddique, T. The microbiology of oil sands tailings: Past, present, future. FEMS Microbiol. Ecol. 2017, 93. [Google Scholar] [CrossRef]
- Gieg, L.M. Microbial Communities in Oil Shales, Biodegraded and Heavy Oil Reservoirs, and Bitumen Deposits. In Microbial Communities Utilizing Hydrocarbons and Lipids: Members, Metagenomics and Ecophysiology; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–21. [Google Scholar]
- Ridley, C.M.; Voordouw, G. Aerobic microbial taxa dominate deep subsurface cores from the Alberta oil sands. FEMS Microbiol. Ecol. 2018, 94. [Google Scholar] [CrossRef]
- An, D.; Caffrey, S.M.; Soh, J.; Agrawal, A.; Brown, D.; Budwill, K.; Dong, X.; Dunfield, P.F.; Foght, J.; Gieg, L.M.; et al. Metagenomics of Hydrocarbon Resource Environments Indicates Aerobic Taxa and Genes to be Unexpectedly Common. Environ. Sci. Technol. 2013, 47, 10708–10717. [Google Scholar] [CrossRef]
- Yi, Z.; Berney, C.; Hartikainen, H.; Mahamdallie, S.; Gardner, M.; Boenigk, J.; Cavalier-Smith, T.; Bass, D. High-throughput sequencing of microbial eukaryotes in Lake Baikal reveals ecologically differentiated communities and novel evolutionary radiations. FEMS Microbiol. Ecol. 2017, 93. [Google Scholar] [CrossRef]
- Bielewicz, S.; Bell, E.; Kong, W.; Friedberg, I.; Priscu, J.C.; Morgan-Kiss, R.M. Protist diversity in a permanently ice-covered Antarctic lake during the polar night transition. ISME J. 2011, 5, 1559–1564. [Google Scholar] [CrossRef] [PubMed]
- Amato, P.; Joly, M.; Besaury, L.; Oudart, A.; Taib, N.; Moné, A.I.; Deguillaume, L.; Delort, A.-M.; Debroas, D. Active microorganisms thrive among extremely diverse communities in cloud water. PLoS ONE 2017, 12, e0182869. [Google Scholar] [CrossRef] [PubMed]
- Charette, T.; Castendyk, D.; Hrynyshyn, J.; Kupper, A.; McKenna, G.; Mooder, B. End Pit Lakes Guidance Document 2012; Cumulative Environmental Management Association: Fort McMurray, AB, Canada, 2015. [Google Scholar]
- Alberta Energy Regulator. Directive 085: Fluid Tailings Management for Oil Sands Mining Projects; Alberta Energy Regulator: Alberta, AB, Canada, 2017. [Google Scholar]
- Siddique, T.; Stasik, S.; Mohamad Shahimin, M.F.; Wendt-Potthoff, K. Microbial Communities in Oil Sands Tailings: Their Implications in Biogeochemical Processes and Tailings Management. In Microbial Communities Utilizing Hydrocarbons and Lipids: Members, Metagenomics and Ecophysiology; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–33. [Google Scholar]
- Mahaffey, M.A.; Dube, D.M. Review of the composition and toxicity of oil sands process-affected water. Environ. Rev. 2016, 25, 97–114. [Google Scholar] [CrossRef]
- Saidi-Mehrabad, A.; He, Z.; Tamas, I.; Sharp, C.E.; Brady, A.L.; Rochman, F.F.; Bodrossy, L.; Abell, G.C.; Penner, T.; Dong, X.; et al. Methanotrophic bacteria in oilsands tailings ponds of northern Alberta. ISME J. 2013, 7, 908–921. [Google Scholar] [CrossRef] [PubMed]
- Dykova, I.; Veverkova Fialova, M.; Fiala, I.; Dvorakova, H. Protacanthamoeba bohemica sp. n., isolated from the liver of tench, Tinca tinca (Linnaeus, 1758). Acta Protozool. 2005, 44, 369–376. [Google Scholar]
- Buse, H.Y.; Lu, J.; Struewing, I.T.; Ashbolt, N.J. Eukaryotic diversity in premise drinking water using 18S rDNA sequencing: Implications for health risks. Environ. Sci. Pollut. Res. Int. 2013, 20, 6351–6366. [Google Scholar] [CrossRef] [PubMed]
- Schuster, F.L.; Visvesvara, G.S. Free-living amoebae as opportunistic and non-opportunistic pathogens of humans and animals. Int. J. Parasitol. 2004, 34, 1001–1027. [Google Scholar] [CrossRef] [PubMed]
- Meyer, C.; Desalme, D.; Bernard, N.; Binet, P.; Toussaint, M.-L.; Gilbert, D. Using testate amoeba as potential biointegrators of atmospheric deposition of phenanthrene (polycyclic aromatic hydrocarbon) on “moss/soil interface-testate amoeba community” microecosystems. Ecotoxicology 2013, 22, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, M.; Richardson, E.; Tan, B.; Walker, G.; Dunfield, P.; Bass, D.; Nesbø, C.; Foght, J.; Dacks, J.B. Next-generation Sequencing Assessment of Eukaryotic Diversity in Oil Sands Tailings Ponds Sediments and Surface Water. J. Eukaryot. Microbiol. 2016, 63, 732–743. [Google Scholar] [CrossRef]
- Walker, G.; Dacks, J.B.; Martin Embley, T. Ultrastructural Description of Breviata anathema, N. Gen., N. Sp., the Organism Previously Studied as “Mastigamoeba invertens”. J. Eukaryot. Microbiol. 2006, 53, 65–78. [Google Scholar] [CrossRef]
- Bass, D.; Czech, L.; Williams, B.; Berney, C.; Dunthorn, M.; Mahé, F.; Torruella, G.; Stentiford, G.D.; Williams, T.A. Clarifying the Relationships between Microsporidia and Cryptomycota. J. Eukaryot. Microbiol. 2018, 65, 773–782. [Google Scholar] [CrossRef] [PubMed]
- Howe, A.T.; Bass, D.; Vickerman, K.; Chao, E.E.; Cavalier-Smith, T. Phylogeny, Taxonomy, and Astounding Genetic Diversity of Glissomonadida ord. nov., The Dominant Gliding Zooflagellates in Soil (Protozoa: Cercozoa). Protist 2009, 160, 159–189. [Google Scholar] [CrossRef]
- Rogerson, A.; Berger, J. Ultrastructural Modification of the Ciliate Protozoan, Colpidium colpoda, Following Chronic Exposure to Partially Degraded Crude Oil on JSTOR. BioScience 1982, 32, 534–535. [Google Scholar] [CrossRef]
- Kota, S.; Borden, R.C.; Barlaz, M.A. Influence of protozoan grazing on contaminant biodegradation. FEMS Microbiol. Ecol. 1999, 29, 179–189. [Google Scholar] [CrossRef]
- Tso, S.-F.; Taghon, G.L. Protozoan grazing increases mineralization of naphthalene in marine sediment. Microb. Ecol. 2006, 51, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, D.; Jakobsen, H.H.; Winding, A.; Mayer, P. Co-transport of polycyclic aromatic hydrocarbons by motile microorganisms leads to enhanced mass transfer under diffusive conditions. Environ. Sci. Technol. 2014, 48, 4368–4375. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Marentette, J.R.; Sarty, K.; Cowie, A.M.; Frank, R.A.; Hewitt, L.M.; Parrott, J.L.; Martyniuk, C.J. Molecular responses of Walleye (Sander vitreus) embryos to naphthenic acid fraction components extracted from fresh oil sands process-affected water. Aquat. Toxicol. 2017, 182, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, H.A.; Alcorn, J.; Al-Mousa, A.; Giesy, J.P.; Wiseman, S.B. Toxicokinetics and toxicodynamics of chlorpyrifos is altered in embryos of Japanese medaka exposed to oil sands process-affected water: Evidence for inhibition of P-glycoprotein. J. Appl. Toxicol. 2017, 37, 591–601. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Sun, J.; Alharbi, H.A.; Jones, P.D.; Giesy, J.P.; Wiseman, S.B. Peroxisome Proliferator-Activated Receptor γ is a Sensitive Target for Oil Sands Process-affected Water: Effects on Adipogenesis and Identification of Ligands. Environ. Sci. Technol. 2016, 50, 7816–7824. [Google Scholar] [CrossRef] [PubMed]
- Leung, S.S.; MacKinnon, M.D.; Smith, R.E.H. The ecological effects of naphthenic acids and salts on phytoplankton from the Athabasca oil sands region. Aquat. Toxicol. 2003, 62, 11–26. [Google Scholar] [CrossRef]
- Woodward, F.I. Global primary production. Curr. Biol. 2007, 17, R269–R273. [Google Scholar] [CrossRef] [PubMed]
- Ruffell, S.E.; Frank, R.A.; Woodworth, A.P.; Bragg, L.M.; Bauer, A.E.; Deeth, L.E.; Müller, K.M.; Farwell, A.J.; Dixon, D.G.; Servos, M.R.; et al. Assessing the bioremediation potential of algal species indigenous to oil sands process-affected waters on mixtures of oil sands acid extractable organics. Ecotoxicol. Environ. Saf. 2016, 133, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Graham, L.E.; Graham, J.M.; Cook, M.E.; Wilcox, L.W. Algae; Benjamin Cummings: San Francisco, CA, USA, 2009; ISBN 9780986393532. [Google Scholar]
- Dompierre, K.A.; Barbour, S.L. Characterization of physical mass transport through oil sands fluid fine tailings in an end pit lake: A multi-tracer study. J. Contam. Hydrol. 2016, 189, 12–26. [Google Scholar] [CrossRef] [PubMed]
- White, K.B.; Liber, K. Early chemical and toxicological risk characterization of inorganic constituents in surface water from the Canadian oil sands first large-scale end pit lake. Chemosphere 2018, 211, 745–757. [Google Scholar] [CrossRef] [PubMed]
- Tedford, E.; Halferdahl, G.; Pieters, R.; Lawrence, G.A. Temporal variations in turbidity in an oil sands pit lake. Environ. Fluid Mech. 2018, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Risacher, F.F.; Morris, P.K.; Arriaga, D.; Goad, C.; Nelson, T.C.; Slater, G.F.; Warren, L.A. The interplay of methane and ammonia as key oxygen consuming constituents in early stage development of Base Mine Lake, the first demonstration oil sands pit lake. Appl. Geochem. 2018, 93, 49–59. [Google Scholar] [CrossRef]
- Rekik, A.; Denis, M.; Dugenne, M.; Maalej, S.; Ayadi, H. Journal of Oceanography, Research and Data; Observatoire Océanologique de Villefranche-sur-Mer: Villefranche-sur-Mer, France, 2015. [Google Scholar]
- Stoeck, T.; Edgcomb, V. Role of Protists in Microbial Interactions with Hydrocarbons. In Handbook of Hydrocarbon and Lipid Microbiology; Springer: Berlin/Heidelberg, Germany, 2010; pp. 2423–2434. [Google Scholar]
- Caron, D.A. Mixotrophy stirs up our understanding of marine food webs. Proc. Natl. Acad. Sci. USA 2016, 113, 2806–2808. [Google Scholar] [CrossRef] [PubMed]
- Foissner, W. Protists as bioindicators in activated sludge: Identification, ecology and future needs. Eur. J. Protistol. 2016, 55, 75–94. [Google Scholar] [CrossRef] [PubMed]
- Howe, A.T.; Bass, D.; Chao, E.E.; Cavalier-Smith, T. New Genera, Species, and Improved Phylogeny of Glissomonadida (Cercozoa). Protist 2011, 162, 710–722. [Google Scholar] [CrossRef]
- Alberta Agriculture and Forestry. A Review of the 2016 Horse River Wildfire Alberta Agriculture and Forestry Preparedness and Response; Alberta Agriculture and Forestry: Edmonton, AB, Canada, 2017.
- Zhang, Q.; Carroll, J.J.; Dixon, A.J.; Anastasio, C. Aircraft Measurements of Nitrogen and Phosphorus in and around the Lake Tahoe Basin: Implications for Possible Sources of Atmospheric Pollutants to Lake Tahoe. Environ. Sci. Technol. 2002, 36, 4981–4989. [Google Scholar] [CrossRef]
- Rust, A.J.; Hogue, T.S.; Saxe, S.; McCray, J. Post-fire water-quality response in the western United States. Int. J. Wildl. Fire 2018, 27, 203–216. [Google Scholar] [CrossRef]
- Vicars, W.C.; Sickman, J.O.; Ziemann, P.J. Atmospheric phosphorus deposition at a montane site: Size distribution, effects of wildfire, and ecological implications. Atmos. Environ. 2010, 44, 2813–2821. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Richardson, E.; Dacks, J.B. Microbial Eukaryotes in Oil Sands Environments: Heterotrophs in the Spotlight. Microorganisms 2019, 7, 178. https://doi.org/10.3390/microorganisms7060178
Richardson E, Dacks JB. Microbial Eukaryotes in Oil Sands Environments: Heterotrophs in the Spotlight. Microorganisms. 2019; 7(6):178. https://doi.org/10.3390/microorganisms7060178
Chicago/Turabian StyleRichardson, Elisabeth, and Joel B. Dacks. 2019. "Microbial Eukaryotes in Oil Sands Environments: Heterotrophs in the Spotlight" Microorganisms 7, no. 6: 178. https://doi.org/10.3390/microorganisms7060178
APA StyleRichardson, E., & Dacks, J. B. (2019). Microbial Eukaryotes in Oil Sands Environments: Heterotrophs in the Spotlight. Microorganisms, 7(6), 178. https://doi.org/10.3390/microorganisms7060178





