Inactivation of Escherichia coli O157:H7 by High Hydrostatic Pressure Combined with Gas Packaging
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strain and Culture Conditions
2.2. Packing and HHP Processing
2.3. Determination of Viable Cells
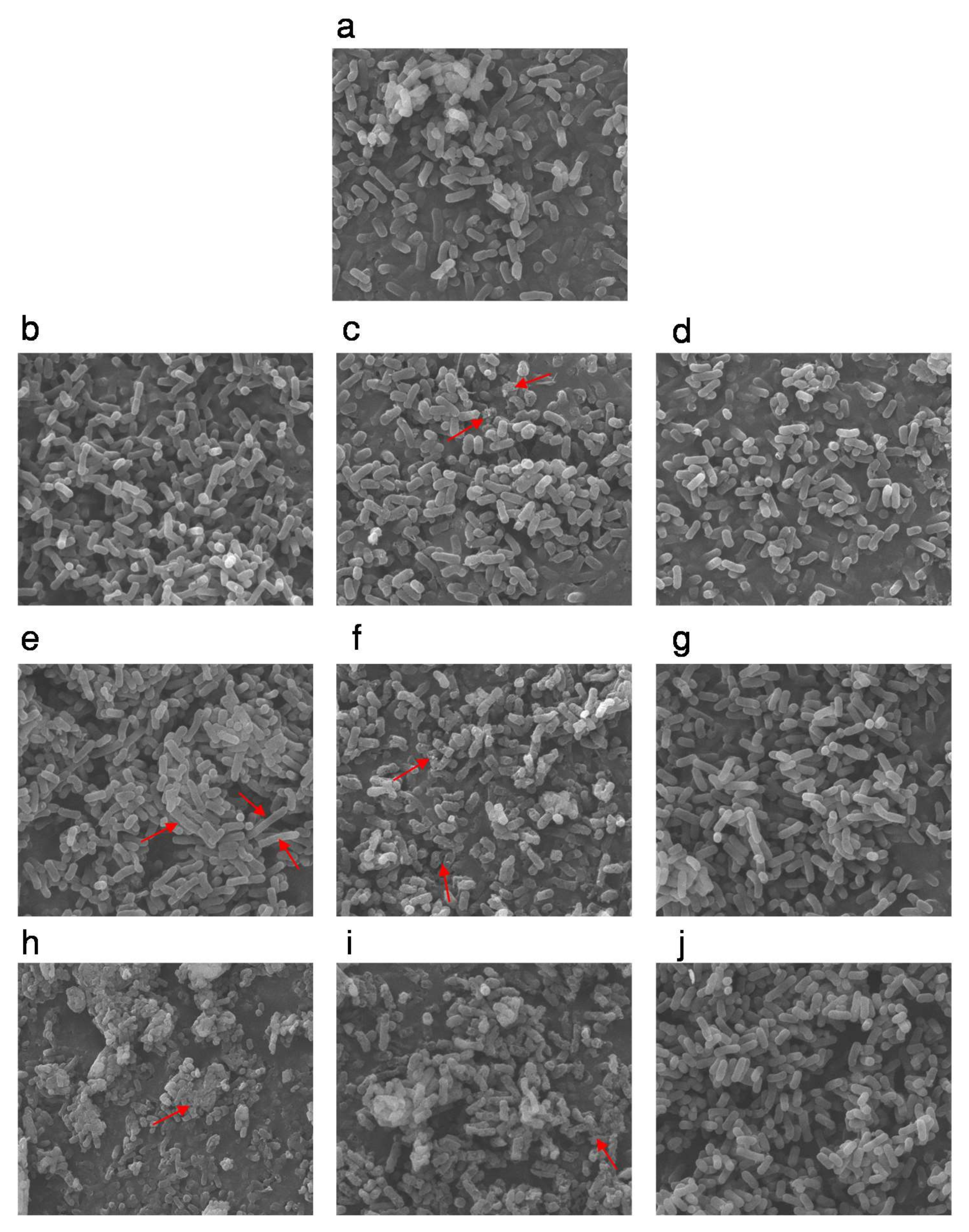
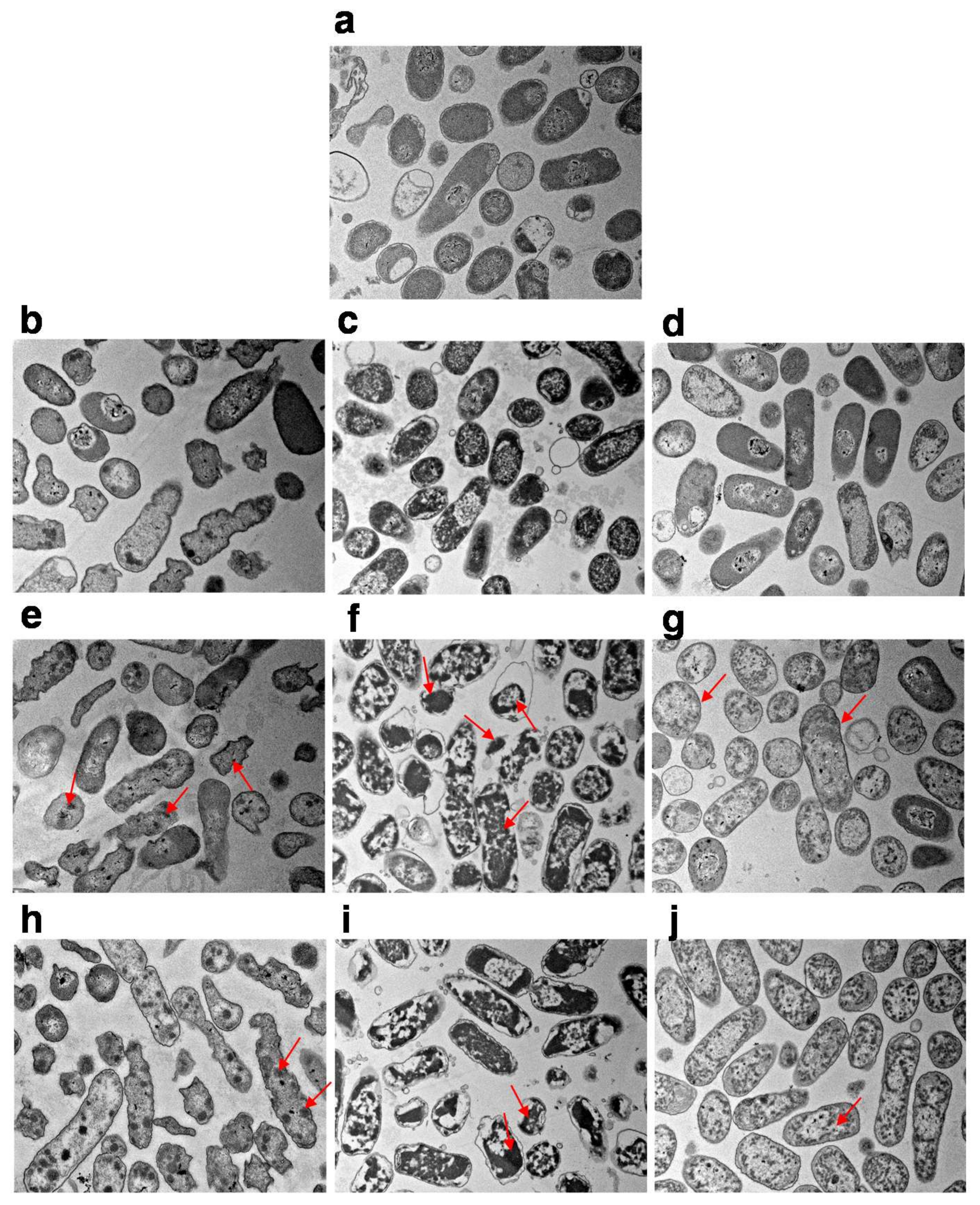
2.4. SEM and TEM Analysis
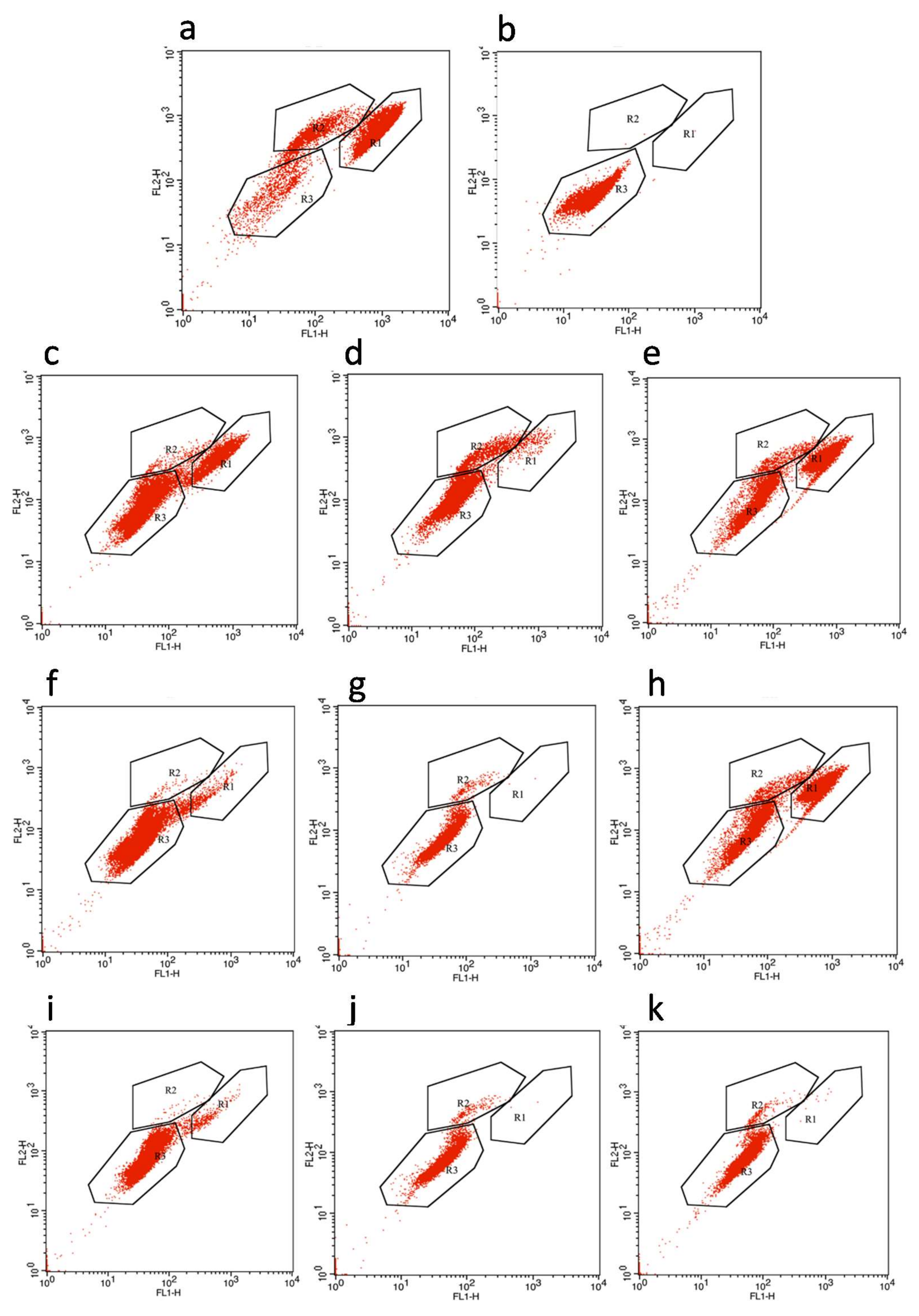
2.5. FCM Analysis
2.6. Statistical Analysis
3. Results and Discussion
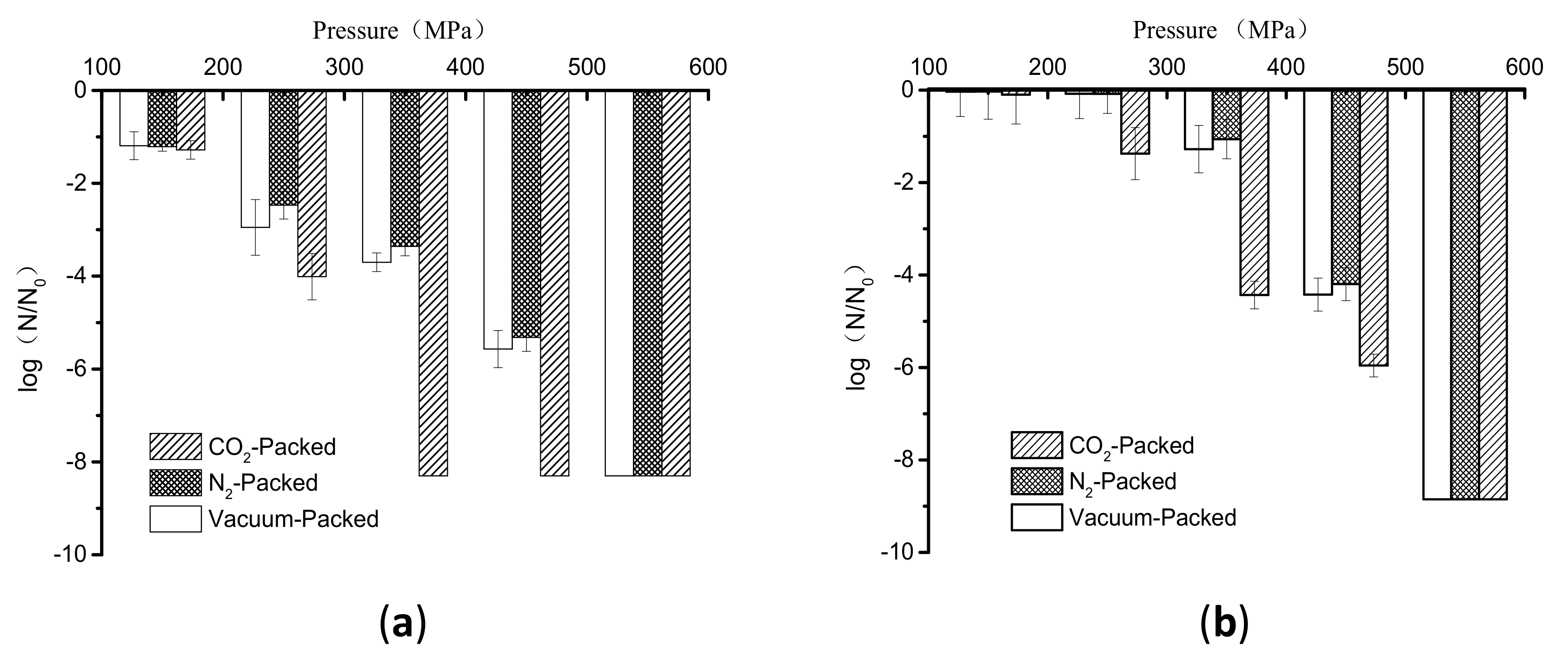
3.1. Inactivation of E. coli in Buffer and Lotus Root Suspension
3.2. The Morphology and Intracellular Structure Changes of E. coli Cells Treated with High Pressure Combined with Gas
3.3. Membrane Permeability of E. coli Cells
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ortega-Rivas, E.; Salmeron-Ochoa, I. Nonthermal Food Processing Alternatives and Their Effects on Taste and Flavor Compounds of Beverages. Crit. Rev. Food Sci. 2014, 54, 190–207. [Google Scholar] [CrossRef] [PubMed]
- Fonberg-Broczek, M.; Windyga, B.; Szczawinski, J.; Szczawinska, M.; Pietrzak, D.; Prestamo, G. High pressure processing for food safety. Acta. Biochim. Pol. 2005, 52, 721–724. [Google Scholar]
- Van Impe, J.; Smet, C.; Tiwari, B.; Greiner, R.; Ojha, S.; Stulic, V.; Vukusic, T.; Jambrak, A.R. State of the art of nonthermal and thermal processing for inactivation of micro-organisms. J. Appl. Microbiol. 2018, 125, 16–35. [Google Scholar] [CrossRef] [PubMed]
- Lou, F.F.; Neetoo, H.; Chen, H.Q.; Li, J.R. High Hydrostatic Pressure Processing: A Promising Nonthermal Technology to Inactivate Viruses in High-Risk Foods. Annu. Rev. Food Sci. Technol. 2015, 6, 389–409. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.W.; Lung, H.M.; Yang, B.B.; Wang, C.Y. Responses of microorganisms to high hydrostatic pressure processing. Food Control. 2014, 40, 250–259. [Google Scholar] [CrossRef]
- Cruz-Romero, M.; Kelly, A.L.; Kerry, J.P. Influence of packaging strategy on microbiological and biochemical changes in high-pressure-treated oysters (Crassostrea gigas). J. Sci. Food Agric. 2008, 88, 2713–2723. [Google Scholar] [CrossRef]
- Black, E.P.; Setlow, P.; Hocking, A.D.; Stewart, C.M.; Kelly, A.L.; Hoover, D.G. Response of spores to high-pressure processing. Compr. Rev. Food Sci. Food Saf. 2007, 6, 103–119. [Google Scholar] [CrossRef]
- Sarker, M.R.; Akhtar, S.; Torres, J.A.; Paredes-Sabja, D. High hydrostatic pressure-induced inactivation of bacterial spores. Crit. Rev. Food Sci. 2015, 41, 18–26. [Google Scholar] [CrossRef] [PubMed]
- San Martin, M.F.; Barbosa-Canovas, G.V.; Swanson, B.G. Food processing by high hydrostatic pressure. Crit. Rev. Food Sci. 2002, 42, 627–645. [Google Scholar] [CrossRef]
- Queiros, R.P.; Gouveia, S.; Saraiva, J.A.; Lopes-da-Silva, J.A. Impact of pH on the high-pressure inactivation of microbial transglutaminase. Food Res. Int. 2019, 115, 73–82. [Google Scholar] [CrossRef]
- Pyatkovskyy, T.I.; Shynkaryk, M.V.; Mohamed, H.M.; Yousef, A.E.; Sastry, S.K. Effects of combined high pressure (HPP), pulsed electric field (PEF) and sonication treatments on inactivation of Listeria innocua. J. Food Eng. 2018, 233, 49–56. [Google Scholar] [CrossRef]
- Ross, A.I.V.; Griffiths, M.W.; Mittal, G.S.; Deeth, H.C. Combining nonthermal technologies to control foodborne microorganisms. Int J. Food Microbiol. 2003, 89, 125–138. [Google Scholar] [CrossRef]
- Roberts, C.M.; Hoover, D.G. Sensitivity of Bacillus coagulans spores to combinations of high hydrostatic pressure, heat, acidity and nisin. J. Appl. Bacteriol. 1996, 81, 363–368. [Google Scholar]
- Rodriguez-Calleja, J.M.; Cruz-Romero, M.C.; O’Sullivan, M.G.; Garcia-Lopez, M.L.; Kerry, J.P. High-pressure-based hurdle strategy to extend the shelf-life of fresh chicken breast fillets. Food Control. 2012, 25, 516–524. [Google Scholar] [CrossRef]
- Arao, T.; Hara, Y.; Suzuki, Y.; Tamura, K. Effect of high-pressure gas on yeast growth. Biosci. Biotech. Bioch. 2005, 69, 1365–1371. [Google Scholar] [CrossRef] [PubMed]
- Debs-Louka, E.; Louka, N.; Abraham, G.; Chabot, V.; Allaf, K. Effect of compressed carbon dioxide on microbial cell viability. Appl. Environ. Microbiol. 1999, 65, 626–631. [Google Scholar] [PubMed]
- Amanatidou, A.; Slump, R.A.; Gorris, L.G.M.; Smid, E.J. High oxygen and high carbon dioxide modified atmospheres for shelf-life extension of minimally processed carrots. J. Food Sci. 2000, 65, 61–66. [Google Scholar] [CrossRef]
- Garcia-Gonzalez, L.; Geeraerd, A.H.; Spilimbergo, S.; Elst, K.; Van Ginneken, L.; Debevere, J.; Van Impe, J.F.; Devlieghere, F. High pressure carbon dioxide inactivation of microorganisms in foods: The past, the present and the future. Int. J. Food Microbiol. 2007, 117, 1–28. [Google Scholar] [CrossRef]
- Lerasle, M.; Federighi, M.; Simonin, H.; Anthoine, V.; Reee, S.; Cheret, R.; Guillou, S. Combined use of modified atmosphere packaging and high pressure to extend the shelf-life of raw poultry sausage. Innov. Food Sci. Emerg. Technol. 2014, 23, 54–60. [Google Scholar] [CrossRef]
- Al-Nehlawi, A.; Guri, S.; Guamis, B.; Saldo, J. Synergistic effect of carbon dioxide atmospheres and high hydrostatic pressure to reduce spoilage bacteria on poultry sausages. LWT-Food Sci. Technol. 2014, 58, 404–411. [Google Scholar] [CrossRef]
- Wang, L.; Pan, J.A.; Xie, H.M.; Yang, Y.; Lin, C.M. Inactivation of Staphylococcus aureus and Escherichia coli by the synergistic action of high hydrostatic pressure and dissolved CO2. Int. J. Food Microbiol. 2010, 144, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Deng, K.; Serment-Moreno, V.; Welti-Chanes, J.; Paredes-Sabja, D.; Fuentes, C.; Wu, X.; Torres, J.A. Inactivation model and risk-analysis design for apple juice processing by high-pressure CO2. J. Food Sci. Technol. 2018, 55, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xu, Z.Z.; Zhao, F.; Wang, Y.T.; Liao, X.J. Synergetic effects of high-pressure carbon dioxide and nisin on the inactivation of Escherichia coli and Staphylococcus aureus. Innov. Food Sci. Emerg. Technol. 2016, 33, 180–186. [Google Scholar] [CrossRef]
- Liao, H.M.; Zhang, F.S.; Liao, X.J.; Hu, X.S.; Chen, Y.; Deng, L. Analysis of Escherichia coli cell damage induced by HPCD using microscopies and fluorescent staining. Int. J. Food Microbiol. 2010, 144, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.M.; Zhang, F.S.; Hu, X.S.; Liao, X.J. Effects of high-pressure carbon dioxide on proteins and DNA in Escherichia coli. Microbiology-Sgm 2011, 157, 709–720. [Google Scholar] [CrossRef] [PubMed]
- Amor, K.B.; Breeuwer, P.; Verbaarschot, P.; Rombouts, F.M.; Akkermans, A.D.; De Vos, W.M.; Abee, T. Multiparametric flow cytometry and cell sorting for the assessment of viable, injured, and dead bifidobacterium cells during bile salt stress. Appl. Environ. Microbiol. 2002, 68, 5209–5216. [Google Scholar] [CrossRef]
- Erkmen, O.; Dogan, C. Kinetic analysis of Escherichia coli inactivation by high hydrostatic pressure in broth and foods. Food Microbiol. 2004, 21, 181–185. [Google Scholar] [CrossRef]
- Chen, H.Q.; Hoover, D.G. Pressure inactivation kinetics of Yersinia enterocolitica ATCC 35669. Int. J. Food Microbiol. 2003, 87, 161–171. [Google Scholar] [CrossRef]
- Abe, F. Exploration of the effects of high hydrostatic pressure on microbial growth, physiology and survival: Perspectives from piezophysiology. Biosci. Biotech. Bioch. 2007, 71, 2347–2357. [Google Scholar] [CrossRef]
- Lo, R.; Xue, T.; Weeks, M.; Turner, M.S.; Bansal, N. Inhibition of bacterial growth in sweet cheese whey by carbon dioxide as determined by culture-independent community profiling. Int. J. Food Microbiol. 2016, 217, 20–28. [Google Scholar] [CrossRef]
- Jakobsen, M.; Bertelsen, G. Solubility of carbon dioxide in fat and muscle tissue. J. Muscle Food 2006, 17, 9–19. [Google Scholar] [CrossRef]
- Zhao, F.; Wang, Y.T.; An, H.R.; Hao, Y.L.; Hu, X.S.; Liao, X.J. New Insights into the Formation of Viable but Nonculturable Escherichia coli O157:H7 Induced by High-Pressure CO2. Mbio 2016, 7, e00961-16. [Google Scholar] [CrossRef] [PubMed]
- Provincial, L.; Guillen, E.; Alonso, V.; Gil, M.; Roncales, P.; Beltran, J.A. Survival of Vibrio parahaemolyticus and Aeromonas hydrophila in sea bream (Sparus aurata) fillets packaged under enriched CO2 modified atmospheres. Int. J. Food Microbiol. 2013, 166, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.I.; Pyun, Y.R. Membrane damage and enzyme inactivation of Lactobacillus plantarum by high pressure CO2 treatment. Int. J. Food Microbiol. 2001, 63, 19–28. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, B.; Zhang, L.; Wang, X.; Dong, P.; Hu, X.; Zhang, Y. Inactivation of Escherichia coli O157:H7 by High Hydrostatic Pressure Combined with Gas Packaging. Microorganisms 2019, 7, 154. https://doi.org/10.3390/microorganisms7060154
Zhou B, Zhang L, Wang X, Dong P, Hu X, Zhang Y. Inactivation of Escherichia coli O157:H7 by High Hydrostatic Pressure Combined with Gas Packaging. Microorganisms. 2019; 7(6):154. https://doi.org/10.3390/microorganisms7060154
Chicago/Turabian StyleZhou, Bing, Luyao Zhang, Xiao Wang, Peng Dong, Xiaosong Hu, and Yan Zhang. 2019. "Inactivation of Escherichia coli O157:H7 by High Hydrostatic Pressure Combined with Gas Packaging" Microorganisms 7, no. 6: 154. https://doi.org/10.3390/microorganisms7060154
APA StyleZhou, B., Zhang, L., Wang, X., Dong, P., Hu, X., & Zhang, Y. (2019). Inactivation of Escherichia coli O157:H7 by High Hydrostatic Pressure Combined with Gas Packaging. Microorganisms, 7(6), 154. https://doi.org/10.3390/microorganisms7060154




