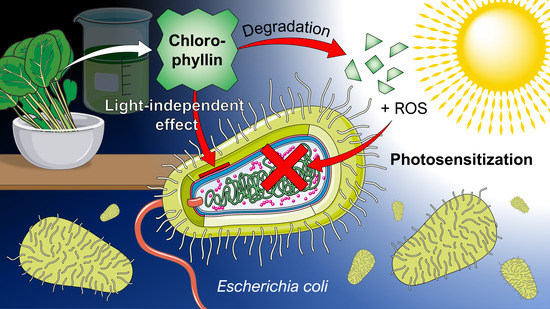
What an Escherichia coli Mutant Can Teach Us About the Antibacterial Effect of Chlorophyllin
Abstract
Share and Cite
Krüger, M.; Richter, P.; Strauch, S.M.; Nasir, A.; Burkovski, A.; Antunes, C.A.; Meißgeier, T.; Schlücker, E.; Schwab, S.; Lebert, M. What an Escherichia coli Mutant Can Teach Us About the Antibacterial Effect of Chlorophyllin. Microorganisms 2019, 7, 59. https://doi.org/10.3390/microorganisms7020059
Krüger M, Richter P, Strauch SM, Nasir A, Burkovski A, Antunes CA, Meißgeier T, Schlücker E, Schwab S, Lebert M. What an Escherichia coli Mutant Can Teach Us About the Antibacterial Effect of Chlorophyllin. Microorganisms. 2019; 7(2):59. https://doi.org/10.3390/microorganisms7020059
Chicago/Turabian StyleKrüger, Marcus, Peter Richter, Sebastian M. Strauch, Adeel Nasir, Andreas Burkovski, Camila A. Antunes, Tina Meißgeier, Eberhard Schlücker, Stefan Schwab, and Michael Lebert. 2019. "What an Escherichia coli Mutant Can Teach Us About the Antibacterial Effect of Chlorophyllin" Microorganisms 7, no. 2: 59. https://doi.org/10.3390/microorganisms7020059
APA StyleKrüger, M., Richter, P., Strauch, S. M., Nasir, A., Burkovski, A., Antunes, C. A., Meißgeier, T., Schlücker, E., Schwab, S., & Lebert, M. (2019). What an Escherichia coli Mutant Can Teach Us About the Antibacterial Effect of Chlorophyllin. Microorganisms, 7(2), 59. https://doi.org/10.3390/microorganisms7020059