Dietary Saccharomyces cerevisiae boulardii CNCM I-1079 Positively Affects Performance and Intestinal Ecosystem in Broilers during a Campylobacter jejuni Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Experimental Design
2.2. Feed Analyses
2.3. Experimental Challenge
2.4. Microbiological Analyses
2.5. Histo-Morphometrical Analyses
2.6. Gut Microbiota Sampling and DNA Analysis
2.7. Bioinformatic Analyses
2.8. Biostatistical Analyses
3. Results
3.1. Animal Performances
3.2. Microbiological Analyses
3.3. Histo-Morphometrical Analyses
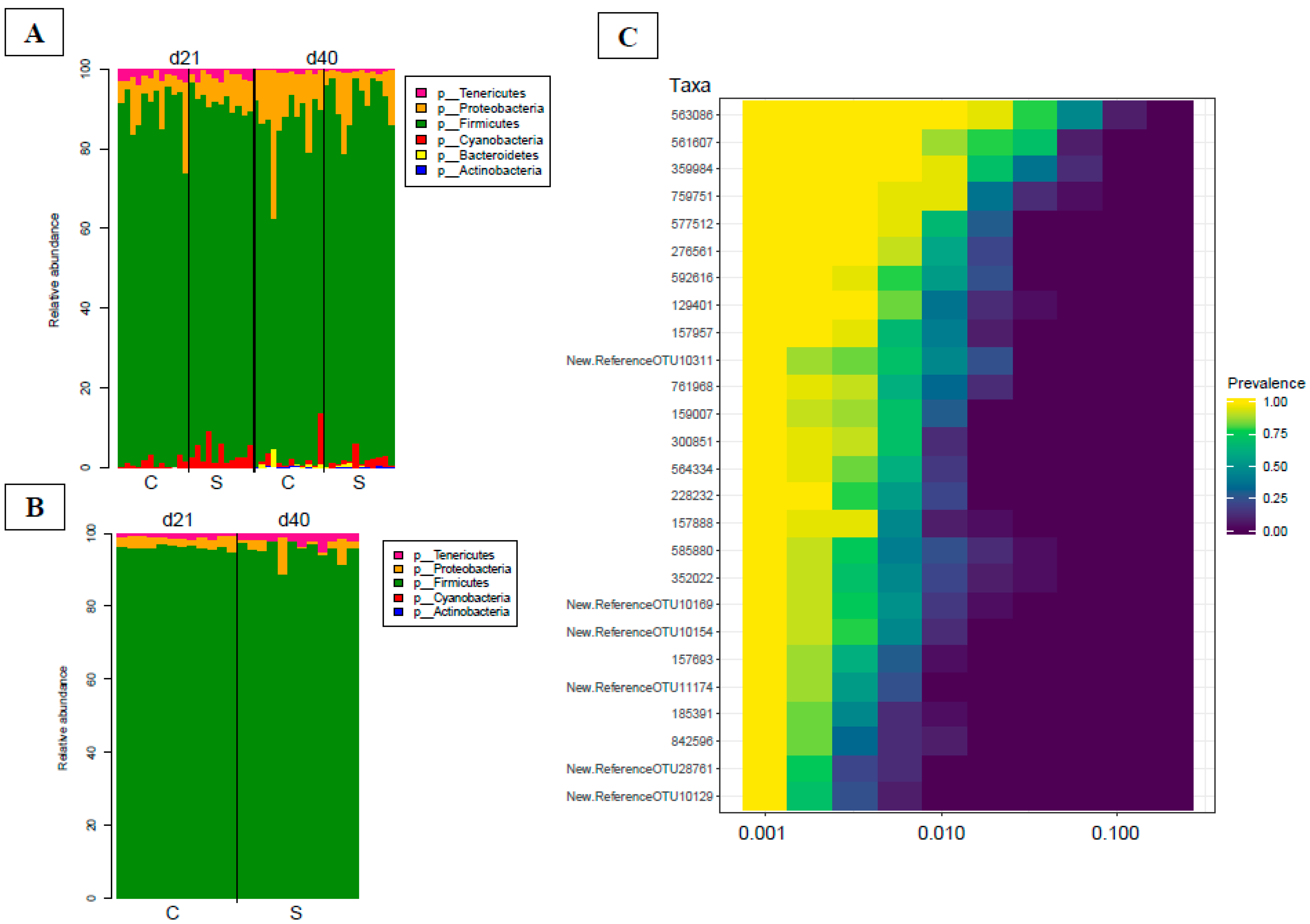
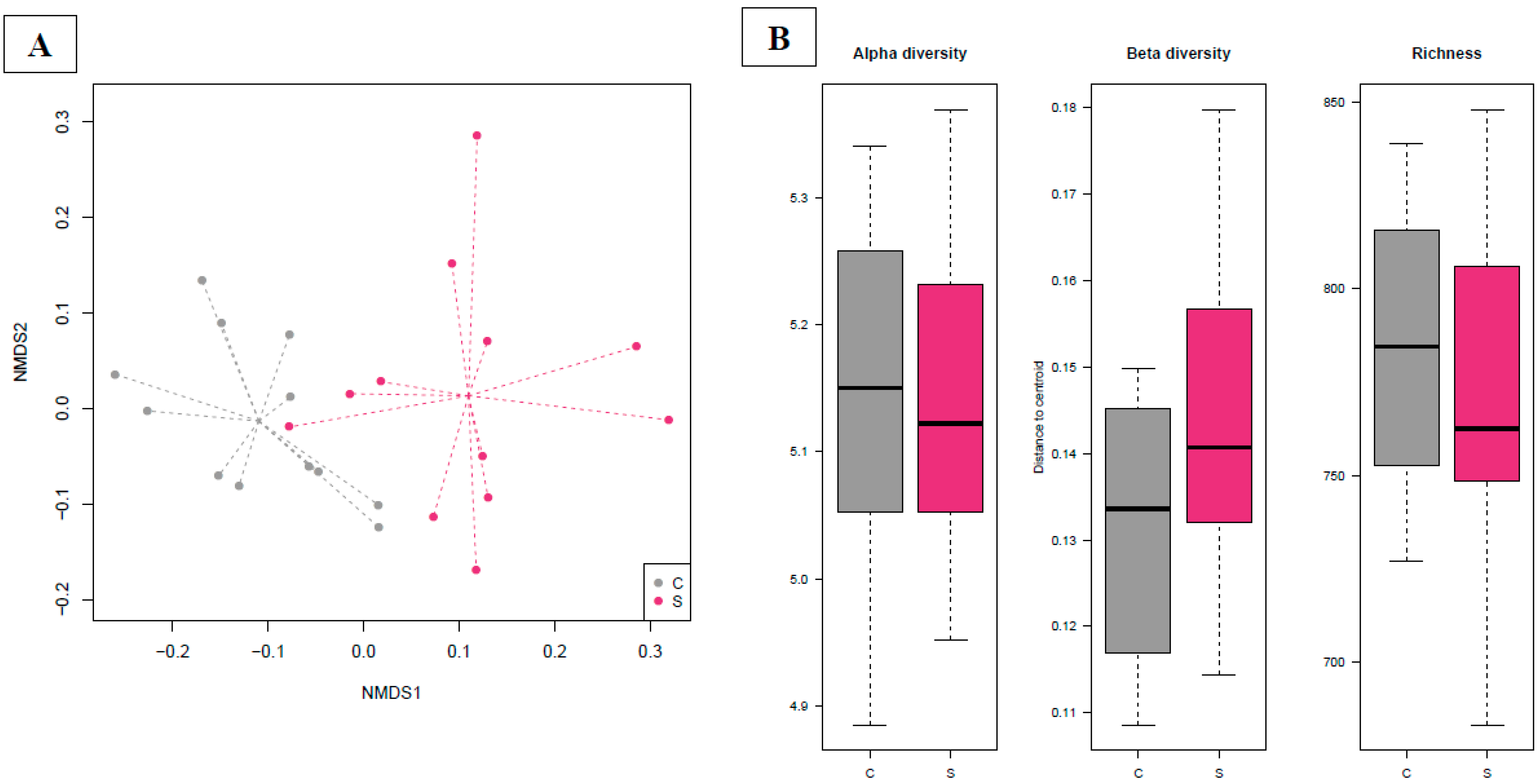
3.4. Gut Microbiota Analyses
3.4.1. Bacterial Phylogenetic Composition and Cecal Core Microbiota in Broilers
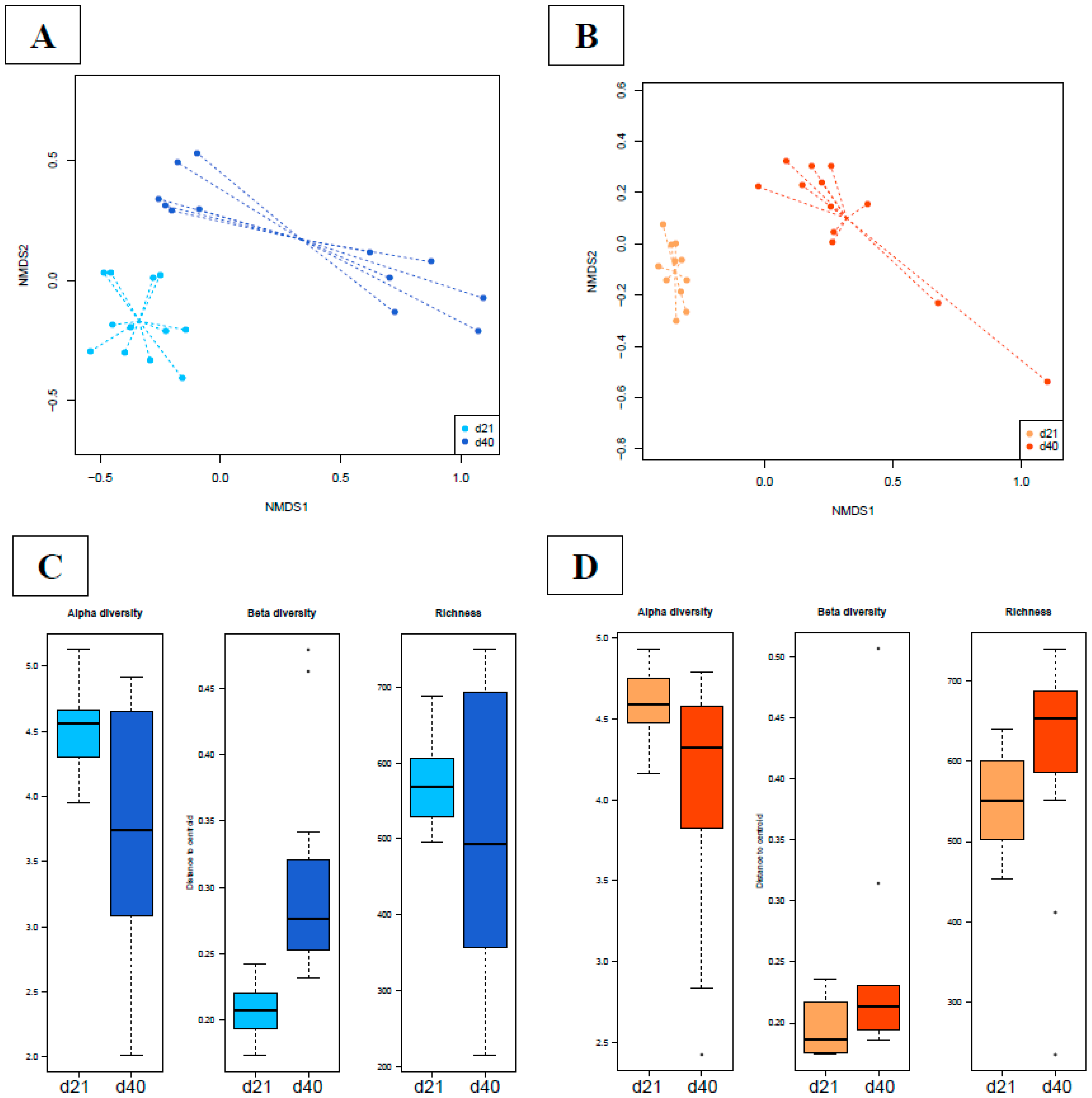
3.4.2. Time Dependency of Fecal Microbiota Composition and Diversity
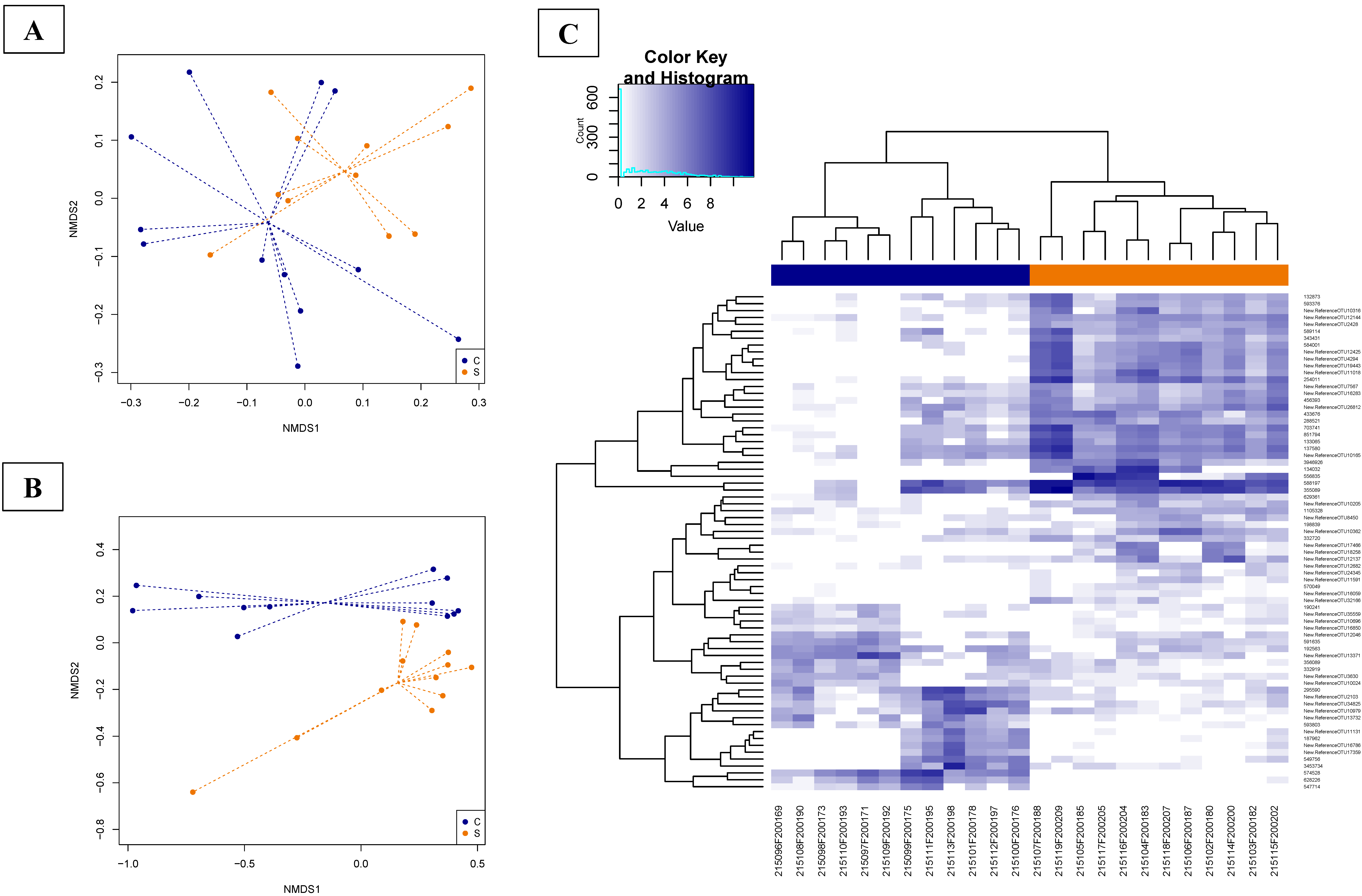
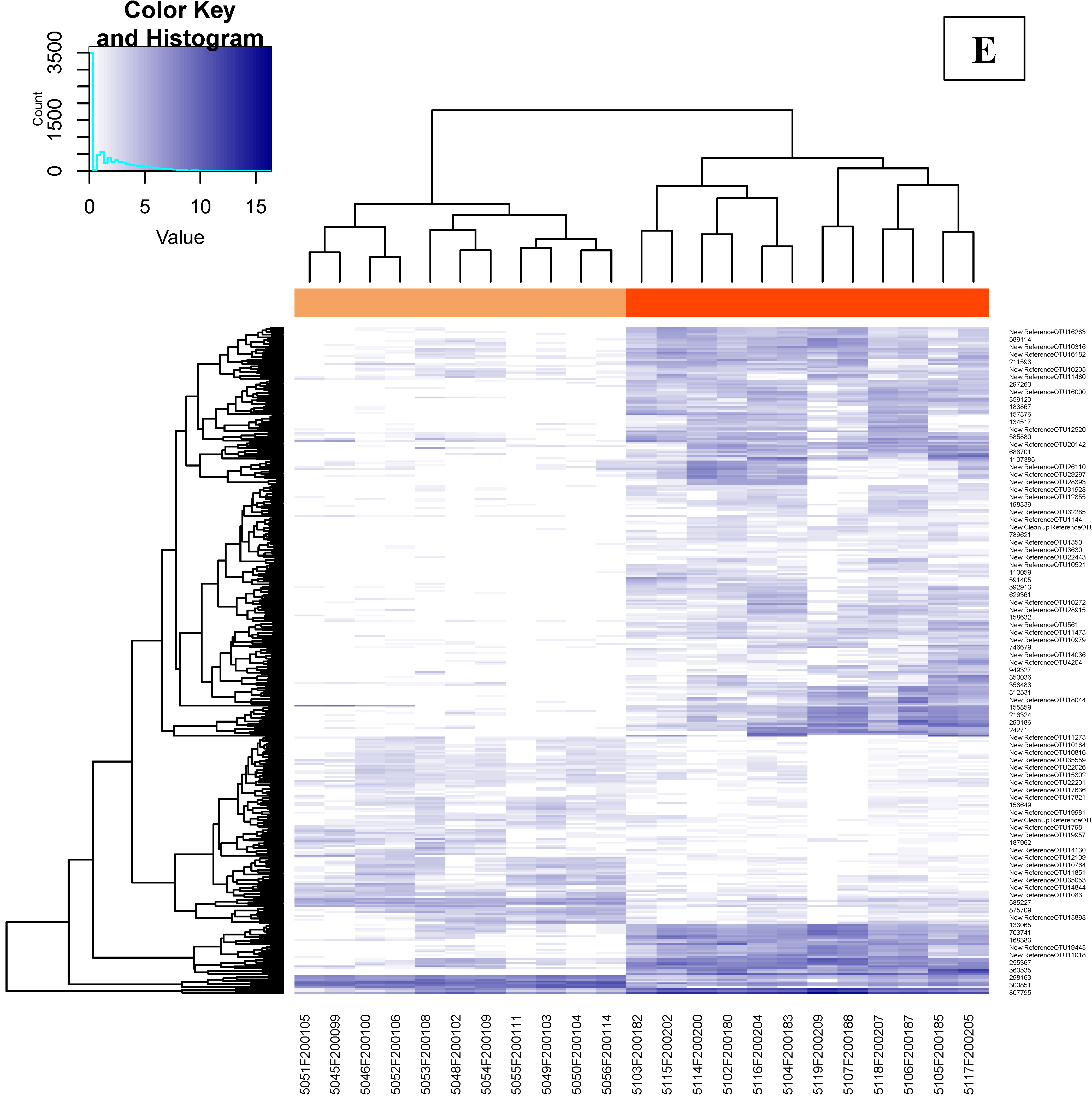
3.4.3. Effects of Supplementation in Fecal Microbiota Composition and Diversity
3.4.4. Time Dependency of Cecal Microbiota Composition and Diversity
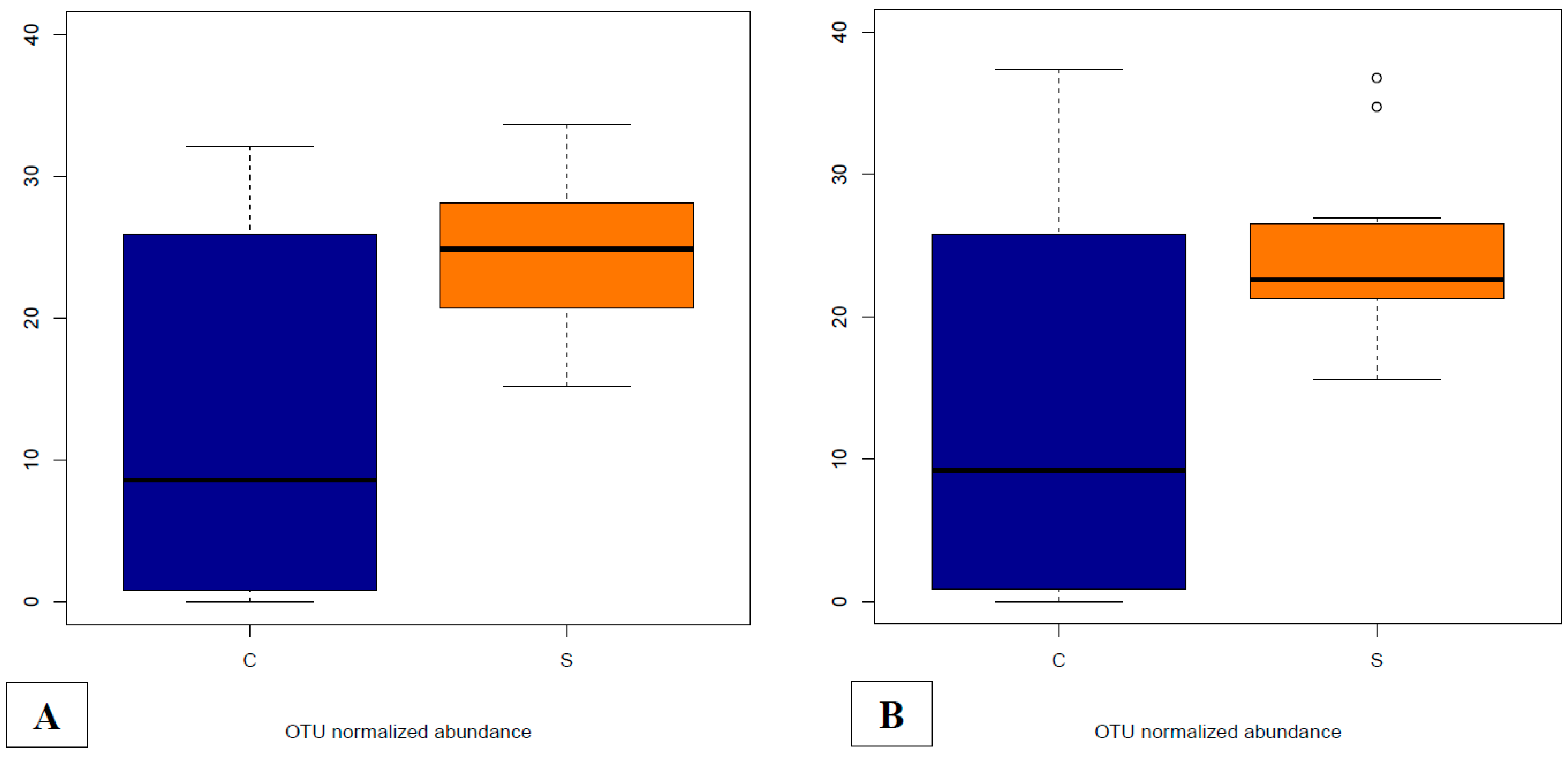
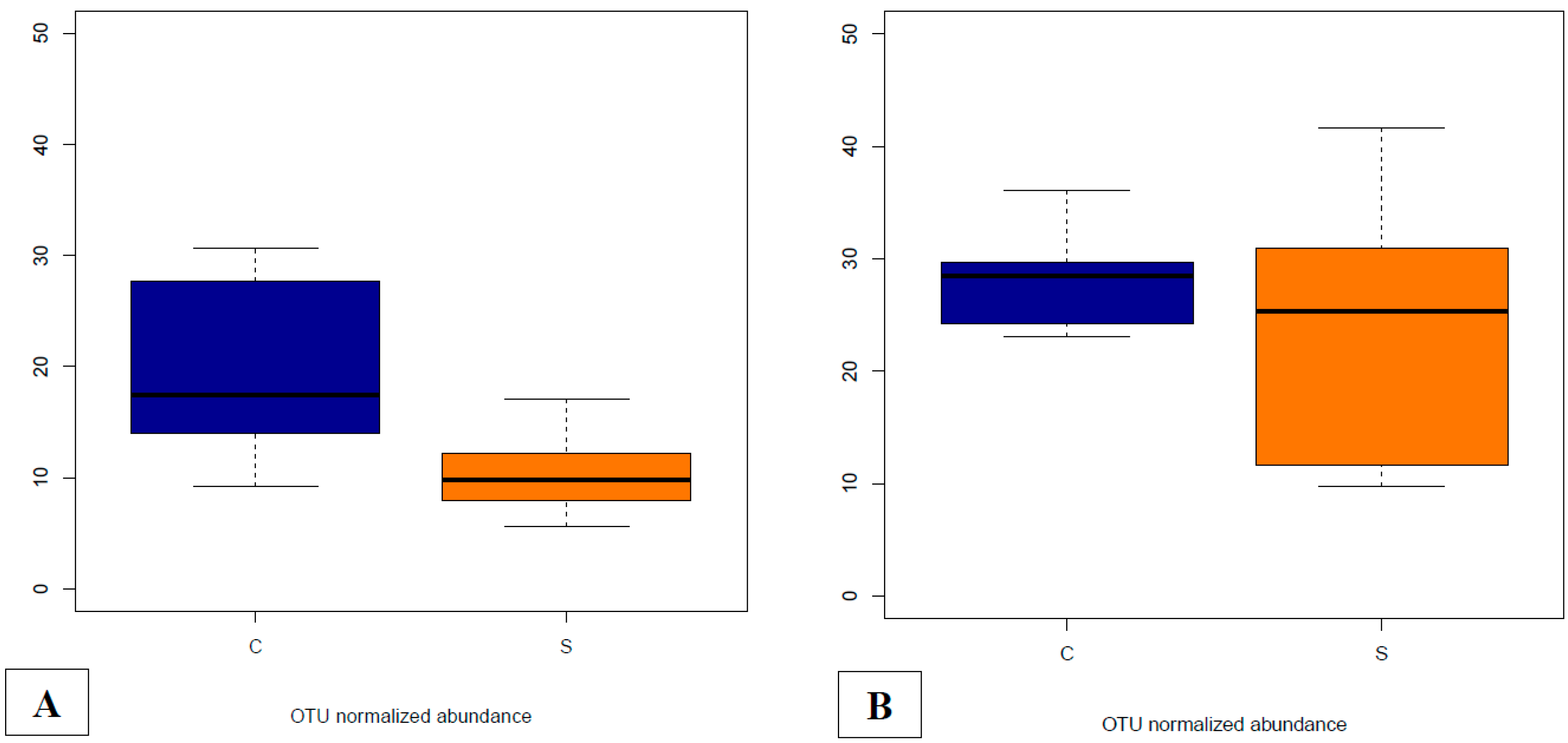
3.4.5. Abundances of OTUs Related to F. prausnitzii in Fecal and Cecal Contents According to Groups
3.4.6. Supplementation-Related Effects on Abundances of OTUs Related to Campylobacter spp. in Fecal and Cecal Contents
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- EFSA. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2017. EFSA J. 2018, 16, 5500. [Google Scholar]
- EFSA. Panel on Biological Hazards (BIOHAZ). Scientific opinion on Campylobacter in broiler meat production: Control options and performance objectives and/or targets at different stages of the food chain. EFSA J. 2011, 9, 2105–2246. [Google Scholar] [CrossRef]
- Luber, P.; Brynestad, S.; Topsch, D.; Scherer, K.; Bartelt, E. Quantification of campylobacter species cross-contamination during handling of contaminated fresh chicken parts in kitchens. Appl. Environ. Microbiol. 2006, 72, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, S.; Hughes, R.J.; Van, T.T.H.; Moore, R.J.; Stanley, D. At-hatch administration of probiotic to chickens can introduce beneficial changes in gut microbiota. PLoS ONE 2018, 13, e0194825. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.C.; Yan, F.F.; Hu, J.Y.; Amen, O.A.; Cheng, H.W. Supplementation of Bacillus subtilis-based probiotic reduces heat stress-related behaviors and inflammatory response in broiler chickens. J. Anim. Sci. 2018, 96, 1654–1666. [Google Scholar] [CrossRef]
- Ezema, C.; Ugwu, C.C. Yeast (Saccharomyces cerevisiae) as a Probiotic of Choice for Broiler Production. In Beneficial Microorganisms in Agriculture, Aquaculture and Other Areas. Microbiology Monographs; Springer: Berlin, Germany, 2015. [Google Scholar]
- Panda, A.K.; Reddy, M.R.; Rao, S.V.R.; Raju, M.V.L.N.; Praharaj, N.K. Growth, carcass characteristics, immunocompetence and response to Escherichia coli of broilers fed diets with various levels of probiotic. Arch. Geflügelk. 2000, 64, 152–156. [Google Scholar]
- Branciari, R.; Ranucci, D.; Forte, C.; Acuti, G.; Miraglia, D.; Olivieri, O.; Trabalza-Marinucci, M. Effects of Saccharomyces cerevisiae in broiler diets: 2. Meat rheological properties and consumer perception. Prog. Nutr. 2014, 16, 303–309. [Google Scholar]
- Acuti, G.; Forte, C.; Miraglia, D.; Branciari, R.; Ranucci, D.; Trabalza-Marinucci, M.; Frega, N.G. Effects of Saccharomyces cerevisiae in broiler diets: 1. Performance and meat composition. Prog. Nutr. 2014, 16, 292–302. [Google Scholar]
- Line, E.J.; Bailey, J.S.; Cox, N.A.; Stern, N.J.; Tompkins, T. Effect of yeast-supplemented feed on Salmonella and Campylobacter populations in broilers. Poult. Sci. 1998, 77, 405–410. [Google Scholar] [CrossRef]
- Mountzouris, K.C.; Dalaka, E.; Palamidi, I.; Paraskeuas, V.; Demey, V.; Theodoropoulos, G.; Fegeros, K. Evaluation of yeast dietary supplementation in broilers challenged or not with Salmonella on growth performance, cecal microbiota composition and Salmonella in ceca, cloacae and carcass skin. Poult. Sci. 2015, 94, 2445–2455. [Google Scholar] [CrossRef]
- Al-Khalaifa, H.; Al-Nasser, A.; Al-Surayee, T.; Al-Kandari, S.; Al-Enzi, N.; Al-Sharrah, T.; Ragheb, G.; Al-Qalaf, S.; Mohammed, A. Effect of dietary probiotics and prebiotics on the performance of broiler chickens. Poult. Sci. 2019, 98, 4465–4479. [Google Scholar] [CrossRef] [PubMed]
- Froebel, L.K.; Jalukar, S.; Lavergne, T.A.; Lee, J.T.; Duong, T. Administration of dietary prebiotics improves growth performance and reduces pathogen colonization in broiler chickens. Poult. Sci. 2019. [Google Scholar] [CrossRef] [PubMed]
- Fanelli, A.; Agazzi, A.; Pilotto, A.; Bontempo, V.; Dell’Orto, V.; Caputo, J.M.; Savoini, G.; Alborali, G.L.; Demey, V. Prevalence reduction of pathogens in poultry fed with Saccharomyces cerevisiae. Biotechnol. Agron. Soc. Environ. 2015, 19, 3–10. [Google Scholar]
- Muthusamy, N.; Haldar, S.; Ghosh, T.K.; Bedford, M.R. Effects of hydrolysed Saccharomyces cerevisiae yeast and yeast cell wall components on live performance, intestinal histo-morphology and humoral immune response of broilers. Br. Poult. Sci. 2011, 52, 694–703. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Ren, W.; Li, Z.; Yue, Y.; Guo, Y. Effects of live yeast on immune responses and intestinal morphological structure in lipopolysaccharide-challenged broilers. Can. J. Anim. Sci. 2017, 97, 136–144. [Google Scholar] [CrossRef]
- Simmering, R.; Blaut, M. Pro- and prebiotics—the tasty guardian angels? Appl. Microbiol. Biotechnol. 2001, 55, 19–28. [Google Scholar] [CrossRef]
- Yadav, S.; Jha, R. Strategies to modulate the intestinal microbiota and their effects on nutrient utilization, performance, and health of poultry. J. Anim. Sci. Biotechnol. 2019, 10. [Google Scholar] [CrossRef]
- Li, Z.; Wang, W.; Liu, D.; Guo, Y. Effects of Lactobacillus acidophilus on gut microbiota composition in broilers challenged with Clostridium perfringens. PLoS ONE 2017, 12, e0188634. [Google Scholar] [CrossRef]
- Peng, Q.; Zeng, X.F.; Zhu, J.L.; Wang, S.; Liu, X.T.; Hou, C.L.; Thacker, P.A.; Qiao, S.Y. Effects of dietary Lactobacillus plantarum B1 on growth performance, intestinal microbiota, and short chain fatty acid profiles in broiler chickens. Poult. Sci. 2016, 95, 893–900. [Google Scholar] [CrossRef]
- Markowiak, P.; Śliżewska, K. The role of probiotics, prebiotics and synbiotics in animal nutrition. Gut Pathog. 2018, 10. [Google Scholar] [CrossRef]
- Qin, C.; Gong, L.; Zhang, X.; Wang, Y.; Wang, Y.; Wang, B.; Li, Y.; Li, W. Effect of Saccharomyces boulardii and Bacillus subtilis B10 on gut microbiota modulation in broilers. Anim. Nutr. 2018, 4, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Mountzouris, K.C.; Palamidi, I.; Paraskeuas, V.; Griela, E.; Fegeros, K. Dietary probiotic form modulates broiler gut microbiota indices and expression of gut barrier genes including essential components for gut homeostasis. J. Anim. Physiol. Anim. Nutr. 2019, 103, 1143–1159. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Ma, C.; Sun, Z.; Wang, L.; Huang, S.; Su, X.; Xu, J.; Zhang, H. Feed-additive probiotics accelerate yet antibiotics delay intestinal microbiota maturation in broiler chicken. Microbiome 2017, 5, 91. [Google Scholar] [CrossRef] [PubMed]
- Forte, C.; Acuti, G.; Manuali, E.; Casagrande Proietti, P.; Pavone, S.; Trabalza-Marinucci, M.; Moscati, L.; Onofri, A.; Lorenzetti, C.; Franciosini, M.P. Effects of two different probiotics on microflora, morphology, and morphometry of gut in organic laying hens. Poult. Sci. 2016, 95, 2528–2535. [Google Scholar] [CrossRef] [PubMed]
- Aviagen. Ross308 Management Handbook; Huntsville, Group: Huntsville, AL, USA, 2014. [Google Scholar]
- Methods 925.10, 65.17, 974.24, 992.16. In AOAC Official Methods of Analysis, 17th ed.; The Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2000.
- Methods 932.06, 925.09, 985.29, 923.03. In AOAC Official Methods of Analysis of the AOAC, 15th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 1990.
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- AOAC Official Method of Analysis, 15th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1998.
- AOAC Official Method of Analysis of the Association of Official Analytical Chemists; AOAC International: Arlington, VA, USA, 1996.
- Wang, G.; Clark, C.G.; Taylor, T.M.; Pucknell, C.; Barton, C.; Price, L.; Woodward, D.L.; Rodgers, F.G. Colony multiplex PCR assay for identification and differentiation of Campylobacter jejuni, C. coli, C. lari, C. upsaliensis, and C. fetus subsp. fetus. J. Clin. Microbiol. 2002, 40, 4744–4747. [Google Scholar] [CrossRef]
- International-Standars UNI EN ISO 6887–1 Microbiology of the Food Chain—Preparation of Test Samples, Initial Suspension and Decimal Dilutions for Microbiological Examination; ISO 2017; ISO: Geneva, Switzerland, 2017.
- International-Standars ISO 10272-2:2017 Microbiology of the Food Chain—Horizontal Method for Detection and Enumeration of Campylobacter Spp. Colony-Count Technique; ISO 2017; ISO: Geneva, Switzerland, 2017.
- International-Standars ISO 21527-1:2008 Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Yeasts and Moulds; ISO 2008; ISO: Geneva, Switzerland, 2008.
- Aliakbarpour, H.R.M.; Chamani, G.; Rahimi, A.; Sadeghi, A.; Qujeq, D. The Bacillus subtilis and lactic acid bacteria probiotics influences intestinal mucin gene expression, histomorphology and growth performance in broilers. Asian-Australas. J. Anim. Sci. 2012, 25, 1285–1293. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Rideout, J.R.; He, Y.; Navas-Molina, J.A.; Walters, W.A.; Ursell, L.K.; Gibbons, S.M.; Chase, J.; McDonald, D.; Gonzalez, A.; Robbins-Pianka, A.; et al. Subsampled open-reference clustering creates consistent, comprehensive OTU definitions and scales to billions of sequences. PeerJ 2014, 2, e545. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. J. 2011, 17, 10. [Google Scholar] [CrossRef]
- Navas-Molina, J.A.; Peralta-Sánchez, J.M.; González, A.; McMurdie, P.J.; Vázquez-Baeza, Y.; Xu, Z.; Ursell, L.K.; Lauber, C.; Zhou, H.; Song, S.J.; et al. Advancing our understanding of the human microbiome using QIIME. Methods Enzymol. 2013, 531, 371–444. [Google Scholar] [PubMed]
- DeSantis, T.Z.; Hugenholtz, P.; Larsen, N.; Rojas, M.; Brodie, E.L.; Keller, K.; Huber, T.; Dalevi, D.; Hu, P.; Andersen, G.L. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl. Environ. Microbiol. 2006, 72, 5069–5072. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef] [PubMed]
- Bokulich, N.A.; Subramanian, S.; Faith, J.J.; Gevers, D.; Gordon, J.I.; Knight, R.; Mills, D.A.; Caporaso, J.G. Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat. Methods 2012, 10, 57–59. [Google Scholar] [CrossRef] [PubMed]
- Price, M.N.; Dehal, P.S.; Arkin, A.P. FastTree 2–Approximately maximum-likelihood trees for large alignments. PLoS ONE 2010, 5, e9490. [Google Scholar] [CrossRef]
- SAS. JMP Statistics and Graphics Guide; Version 4; SAS, Inc.: Cary, NC, USA, 2001. [Google Scholar]
- McMurdie, P.J.; Holmes, S. Phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, B.R.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. R Package. Version 2.5-5. 2019. Available online: https://CRAN.R-project.org/package=vegan (accessed on 21 November 2019).
- Lahti, L.; Shetty, S. Microbiome R Package. 2019. Available online: http://microbiome.github.io (accessed on 21 November 2019).
- Paulson, J.N.; Stine, O.C.; Bravo, H.C.; Pop, M. Differential abundance analysis for microbial marker-gene surveys. Nat. Methods 2013, 10, 1200–1202. [Google Scholar] [CrossRef]
- Hansson, I.; Sandberg, M.; Habib, I.; Lowman, R.; Engvall, E.O. Knowledge gaps in control of Campylobacter for prevention of campylobacteriosis. Transbound. Emerg. Dis. 2018, 65, 30–48. [Google Scholar] [CrossRef]
- Clavijo, V.; Flórez, M.J.V. The gastrointestinal microbiome and its association with the control of pathogens in broiler chicken production: A review. Poult. Sci. 2018, 97, 1006–1021. [Google Scholar] [CrossRef]
- Micciche, A.C.; Foley, S.L.; Pavlidis, H.O.; McIntyre, D.R.; Ricke, S.C. A review of prebiotics against Salmonella in poultry: Current and future potential for microbiome research applications. Front. Vet. Sci. 2018, 5, 1–11. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, J.; Zhong, H.; Li, N.; Xu, H.; Zhu, Q.; Liu, Y. Effect of probiotics on the meat flavour and gut microbiota of chicken. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Borda-Molina, D.; Seifert, J.; Camarinha-Silva, A. Current Perspectives of the Chicken Gastrointestinal Tract and Its Microbiome. Comput. Struct. Biotechnol. J. 2018, 16, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.; Bortoluzzi, C.; Durrer, A.; Fagundes, N.S.; Pedroso, A.A.; Rafael, J.M.; Perim, J.E.L.; Zavarize, K.C.; Napty, G.S.; Andreote, F.D.; et al. Performance and intestinal microbiota of chickens receiving probiotic in the feed and submitted to antibiotic therapy. J. Anim. Physiol. Anim. Nutr. 2019, 103, 72–86. [Google Scholar] [CrossRef] [PubMed]
- Li, C.L.; Wang, J.; Zhang, H.J.; Wu, S.G.; Hui, Q.R.; Yang, C.B.; Fang, R.J.; Qi, G.H. Intestinal morphologic and microbiota responses to dietary Bacillus spp. in a broiler chicken model. Front. Physiol. 2019, 10, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Baidya, N.; Mandal, L.; Banerjee, G.C. Efficiency of feeding antibiotic and probiotics in broilers. Vet. Anim. Sci. 1993, 24, 120–124. [Google Scholar]
- Kumprechtova, D.; Zobač, P.; Kumprecht, I. The effect of Saccharomyces cerevisiae Sc47 on chicken broiler performance and nitrogen output. Czech J. Anim. Sci. 2001, 45, 169–177. [Google Scholar]
- Santin, E.; Paulillo, A.P.; Mariorka, A.; Nakaghi, L.S.O.; Macari, M.; Fischer, A.V.; da Silva Alessi, A.C. Evaluation of efficacy of Saccharomyces cerevisiae cell wall to ameliorate the toxic effects of aflatoxin in broilers. Int. J. Poult. Sci. 2003, 2, 341–344. [Google Scholar]
- Seifi, S.; Sayrafi, R.; Khoshbakht, R.; Gilani, A.; Goudarzi, B. Acta Scientiarum Evaluation of yeast culture and direct-fed microbial on gut histology and serum components of broilers challenged with suboptimal diets under heat stress. Acta Sci. Anim. Sci. 2019, 42, 1–7. [Google Scholar]
- Gao, J.; Zhang, H.J.; Yu, S.H.; Wu, S.G.; Yoon, I.; Quigley, J.; Gao, Y.P.; Qi, G.H. Effects of yeast culture in broiler diets on performance and immunomodulatory functions. Poult. Sci. 2008, 87, 1377–1384. [Google Scholar] [CrossRef]
- Zhang, A.W.; Lee, B.D.; Lee, S.K.; Lee, K.W.; An, G.H.; Song, K.B.; Lee, C.H. Effects of yeast (Saccharomyces cerevisiae) cell components on growth performance, meat quality, and ileal mucosa development of broiler chicks. Poult. Sci. 2005, 84, 1015–1021. [Google Scholar] [CrossRef]
- MacMillan, J.L.; Vicaretti, S.D.; Noyovitz, B.; Xing, X.; Low, K.E.; Inglis, G.D.; Zaytsoff, S.J.M.; Boraston, A.B.; Smith, S.P.; Uwiera, R.R.E.; et al. Structural analysis of broiler chicken small intestinal mucin O-glycan modification by Clostridium perfringens. Poult. Sci. 2019, 98, 5074–5088. [Google Scholar] [CrossRef]
- Awad, W.A.; Mann, E.; Dzieciol, M.; Hess, C.; Schmitz-Esser, S.; Wagner, M.; Hess, M. Age-related differences in the luminal and mucosa-associated gut microbiome of broiler chickens and shifts associated with Campylobacter jejuni infection. Front. Cell. Infect. Microbiol. 2016, 6, 1–17. [Google Scholar] [CrossRef]
- Johnson, T.J.; Youmans, B.P.; Noll, S.; Cardona, C.; Evans, N.P.; Peter Karnezos, T.; Ngunjiri, J.M.; Abundo, M.C.; Lee, C.W. A consistent and predictable commercial broiler chicken bacterial microbiota in antibiotic-free production displays strong correlations with performance. Appl. Environ. Microbiol. 2018, 84, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, E.E.; Stanley, D.; Hughes, R.J.; Moore, R.J. The time-course of broiler intestinal microbiota development after administration of cecal contents to incubating eggs. PeerJ 2017, 5, e3587. [Google Scholar] [CrossRef]
- Lourenco, J.M.; Rothrock, M.J., Jr.; Fluharty, F.L.; Callaway, T.R. The Successional Changes in the Gut Microbiome of Pasture-Raised Chickens Fed Soy-Containing and Soy-Free Diets. Front. Sustain. Food Syst. 2019, 3. [Google Scholar] [CrossRef]
- Wang, L.; Lilburn, M.; Yu, Z.; Waite, D.W. Intestinal Microbiota of Broiler Chickens As Affected by Litter Management Regimens. Front. Microbiol. 2016, 7, 1–12. [Google Scholar] [CrossRef]
- Gusils, C.; Oppezzo, O.; Pizarro, R.; Gonzalez, S. Adhesion of probiotic lactobacilli to chick intestinal mucus. Can. J. Microbiol. 2003, 49, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Ocejo, M.; Oporto, B.; Hurtado, A. 16S rRNA amplicon sequencing characterization of caecal microbiome composition of broilers and free-range slow-growing chickens throughout their productive lifespan. Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef]
- Sakaridis, I.; Ellis, R.J.; Cawthraw, S.A.; van Vliet, A.H.M.; Stekel, D.J.; Penell, J.; Chambers, M.; La Ragione, R.M.; Cook, A.J. Investigating the association between the caecal microbiomes of broilers and Campylobacter burden. Front. Microbiol. 2018, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Casas, I.; Edens, F.; Parkhurst, C. Probiotic Administrations of Lactobacillus reuteri Moderate Avian Growth Depression in Turkeys. Biosci. Microfl. 1998, 17, 125–131. [Google Scholar] [CrossRef]
- Nakphaichit, M.; Thanomwongwattana, S.; Phraephaisarn, C.; Sakamoto, N.; Keawsompong, S.; Nakayama, J.; Nitisinprasert, S. The effect of including Lactobacillus reuteri KUB-AC5 during post-hatch feeding on the growth and ileum microbiota of broiler chickens. Poult. Sci. 2011, 90, 2753–2765. [Google Scholar] [CrossRef] [PubMed]
- Miquel, S.; Martín, R.; Rossi, O.; Bermúdez-Humarán, L.G.; Chatel, J.M.; Sokol, H.; Thomas, M.; Wells, J.M.; Langella, P. Faecalibacterium prausnitzii and human intestinal health. Curr. Opin. Microbiol. 2013, 16, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Sokol, H.; Pigneur, B.; Watterlot, L.; Lakhdari, O.; Bermudez-Humaràn, L.G.; Gratadoux, J.-J.; Blugeon, S.; Bridonneau, C.; Furet, J.; Corthier, G.; et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc. Natl. Acad. Sci. USA 2008, 105, 16731–16736. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Shen, J.; Ran, Z.H. Association between Faecalibacterium prausnitzii Reduction and Inflammatory Bowel Disease: A Meta-Analysis and Systematic Review of the Literature. Gastroenterol. Res. Pract. 2014, 2014, 872725. [Google Scholar] [CrossRef]
- Dave, M.; Gorospe, E.C.; Luther, J. Faecalibacterium Prausnitzii in Crohn’s Disease: Hope or Hype? A Systematic Review. Gastroenterology 2011, 140, S-329. [Google Scholar] [CrossRef]
- Lopez-Siles, M.; Enrich-Capó, N.; Aldeguer, X.; Sabat-Mir, M.; Duncan, S.H.; Garcia-Gil, L.J.; Martinez-Medina, M. Alterations in the Abundance and Co-occurrence of Akkermansia muciniphila and Faecalibacterium prausnitzii in the Colonic Mucosa of Inflammatory Bowel Disease Subjects. Front. Cell. Infect. Microbiol. 2018, 8. [Google Scholar] [CrossRef]
- Lopez-Siles, M.; Duncan, S.H.; Garcia-Gil, L.J.; Martinez-Medina, M. Faecalibacterium prausnitzii: From microbiology to diagnostics and prognostics. ISME J. 2017, 11, 841–852. [Google Scholar] [CrossRef]
- Lopez-Siles, M.; Martinez-Medina, M.; Busquets, D.; Sabat-Mir, M.; Duncan, S.H.; Flint, H.J.; Aldeguer, X.; Garcia-Gil, L.J. Mucosa-associated Faecalibacterium prausnitzii and Escherichia coli co-abundance can distinguish Irritable Bowel Syndrome and Inflammatory Bowel Disease phenotypes. Int. J. Med. Microbiol. 2014, 304, 464–475. [Google Scholar] [CrossRef]
- Lopez-Siles, M.; Martinez-medina, M.; Abellà, C.; Busquets, D.; Sabat-mir, M.; Duncan, S.H.; Aldeguer, X.; Flint, H.J.; Garcia-gil, L.J. Reduced in Patients with Inflammatory Bowel Disease. Appl. Environ. Microbiol. 2015, 81, 7582–7592. [Google Scholar] [CrossRef]
- Martín, R.; Miquel, S.; Benevides, L.; Bridonneau, C.; Robert, V.; Hudault, S.; Chain, F.; Berteau, O.; Azevedo, V.; Chatel, J.M.; et al. Functional Characterization of Novel Faecalibacterium prausnitzii Strains Isolated from Healthy Volunteers: A Step Forward in the Use of F. prausnitzii as a Next-Generation Probiotic Isolation of Novel Extremely Oxygen. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef]
- Miquel, S.; Martín, R.; Lashermes, A.; Gillet, M.; Meleine, M.; Gelot, A.; Eschalier, A.; Ardid, D.; Bermúdez-Humarán, L.G.; Sokol, H.; et al. Anti-nociceptive effect of Faecalibacterium prausnitzii in non-inflammatory IBS-like models. Sci. Rep. 2016, 6, 19399. [Google Scholar] [CrossRef] [PubMed]
- Konstantinov, S.; Favier, C.; Zhu, W.Y.; Williams, B.; Souffrant, W.-B.; De Vos, W.; Akkermans, A.; Konstantinov, S.; Smidt, H. Microbial diversity studies of the porcine gastrointestinal ecosystem during weaning transition To cite this version: Review article. Anim. Res. 2004, 53, 317–324. [Google Scholar] [CrossRef]
- Massacci, F.R.; Berri, M.; Lemonnier, G.; Guettier, E.; Blanc, F.; Jardet, D.; Rossignol, M.N.; Mercat, M.J.; Doré, J.; Lepage, P.; et al. Late weaning is associated with increased microbial diversity and higher F. prausnitzii abundance in piglet’s gut microbiota. Anim. Microbiome J. 2019. [Google Scholar]
- Singh, K.M.; Shah, T.; Deshpande, S.; Jakhesara, S.J.; Koringa, P.G.; Rank, D.N.; Joshi, C.G. High through put 16S rRNA gene-based pyrosequencing analysis of the fecal microbiota of high FCR and low FCR broiler growers. Mol. Biol. Rep. 2012, 39, 10595–10602. [Google Scholar] [CrossRef]
- Rinttilä, T.; Apajalahti, J. Intestinal microbiota and metabolites-Implications for broiler chicken health and performance. J. Appl. Poult. Res. 2013, 22, 647–658. [Google Scholar] [CrossRef]
- Torok, V.A.; Hughes, R.J.; Mikkelsen, L.L.; Perez-Maldonado, R.; Balding, K.; MacAlpine, R.; Percy, N.J.; Ophel-Keller, K. Identification and characterization of potential performance-related gut microbiotas in broiler chickens across various feeding trials. Appl. Environ. Microbiol. 2011, 77, 5868–5878. [Google Scholar] [CrossRef]
| Items | Diet x Time | Effects | p | ||||
| Live weight | Day 1 | Day 21 | Day 28 | Day 40 | |||
| C | 40.74 G | 464.45 E | 824.23 C | 1791.46 B | Diet | <0.0001 | |
| S | 36.14 G | 667.11 F | 1078.67 D | 2021.03 A | Time | <0.0001 | |
| Diet × time | <0.0001 | ||||||
| SEM | 17.980 | 17.980 | 17.980 | 24.24 | |||
| ADG | Day 0–21 | Day 21–27 | Day 28–40 | Overall | |||
| C | 21.18 D | 59.96 B | 74.66 A | 51.94 | Diet | 0.0008 | |
| S | 31.53 C | 68.75 A | 72.84 A | 57.71 | Time | <0.0001 | |
| Diet × time | 0.0062 | ||||||
| SEM | 1.901 | 1.098 | |||||
| FCR | Day 0–21 | Day 22–27 | Day 28–40 |
|---|---|---|---|
| C | 1.87 A | 1.95 A | 2.08 |
| S | 1.72 B | 1.85 B | 1.92 |
| SEM | 0.046 | 0.050 | 0.061 |
| p | 0.0423 | 0.024 | 0.0837 |
| Group | Morphometrical Analyses | PAS positive cells | |||
|---|---|---|---|---|---|
| Villi length (µ) | Crypt depth (µ) | V/C ratio | Villi | Crypt | |
| C | 2688.92 B | 352.66 B | 7.965 | 136.99 | 19.48 |
| S | 3870.82 A | 428.78 A | 9.714 | 188.18 | 20.76 |
| SEM | 111.844 | 20.504 | 0.540 | 27.748 | 0.808 |
| p | <0.0001 | 0.0333 | 0.0617 | 0.1943 | 0.265 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Massacci, F.R.; Lovito, C.; Tofani, S.; Tentellini, M.; Genovese, D.A.; De Leo, A.A.P.; Papa, P.; Magistrali, C.F.; Manuali, E.; Trabalza-Marinucci, M.; et al. Dietary Saccharomyces cerevisiae boulardii CNCM I-1079 Positively Affects Performance and Intestinal Ecosystem in Broilers during a Campylobacter jejuni Infection. Microorganisms 2019, 7, 596. https://doi.org/10.3390/microorganisms7120596
Massacci FR, Lovito C, Tofani S, Tentellini M, Genovese DA, De Leo AAP, Papa P, Magistrali CF, Manuali E, Trabalza-Marinucci M, et al. Dietary Saccharomyces cerevisiae boulardii CNCM I-1079 Positively Affects Performance and Intestinal Ecosystem in Broilers during a Campylobacter jejuni Infection. Microorganisms. 2019; 7(12):596. https://doi.org/10.3390/microorganisms7120596
Chicago/Turabian StyleMassacci, Francesca Romana, Carmela Lovito, Silvia Tofani, Michele Tentellini, Domenica Anna Genovese, Alessia Arcangela Pia De Leo, Paola Papa, Chiara Francesca Magistrali, Elisabetta Manuali, Massimo Trabalza-Marinucci, and et al. 2019. "Dietary Saccharomyces cerevisiae boulardii CNCM I-1079 Positively Affects Performance and Intestinal Ecosystem in Broilers during a Campylobacter jejuni Infection" Microorganisms 7, no. 12: 596. https://doi.org/10.3390/microorganisms7120596
APA StyleMassacci, F. R., Lovito, C., Tofani, S., Tentellini, M., Genovese, D. A., De Leo, A. A. P., Papa, P., Magistrali, C. F., Manuali, E., Trabalza-Marinucci, M., Moscati, L., & Forte, C. (2019). Dietary Saccharomyces cerevisiae boulardii CNCM I-1079 Positively Affects Performance and Intestinal Ecosystem in Broilers during a Campylobacter jejuni Infection. Microorganisms, 7(12), 596. https://doi.org/10.3390/microorganisms7120596







