A Comparison of Constitutive and Inducible Non-Endogenous Keto-Carotenoids Biosynthesis in Synechocystis sp. PCC 6803
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Growth Conditions
2.2. Plasmid Construction for Chromosomal Integration
2.3. Synechocystis Transformation
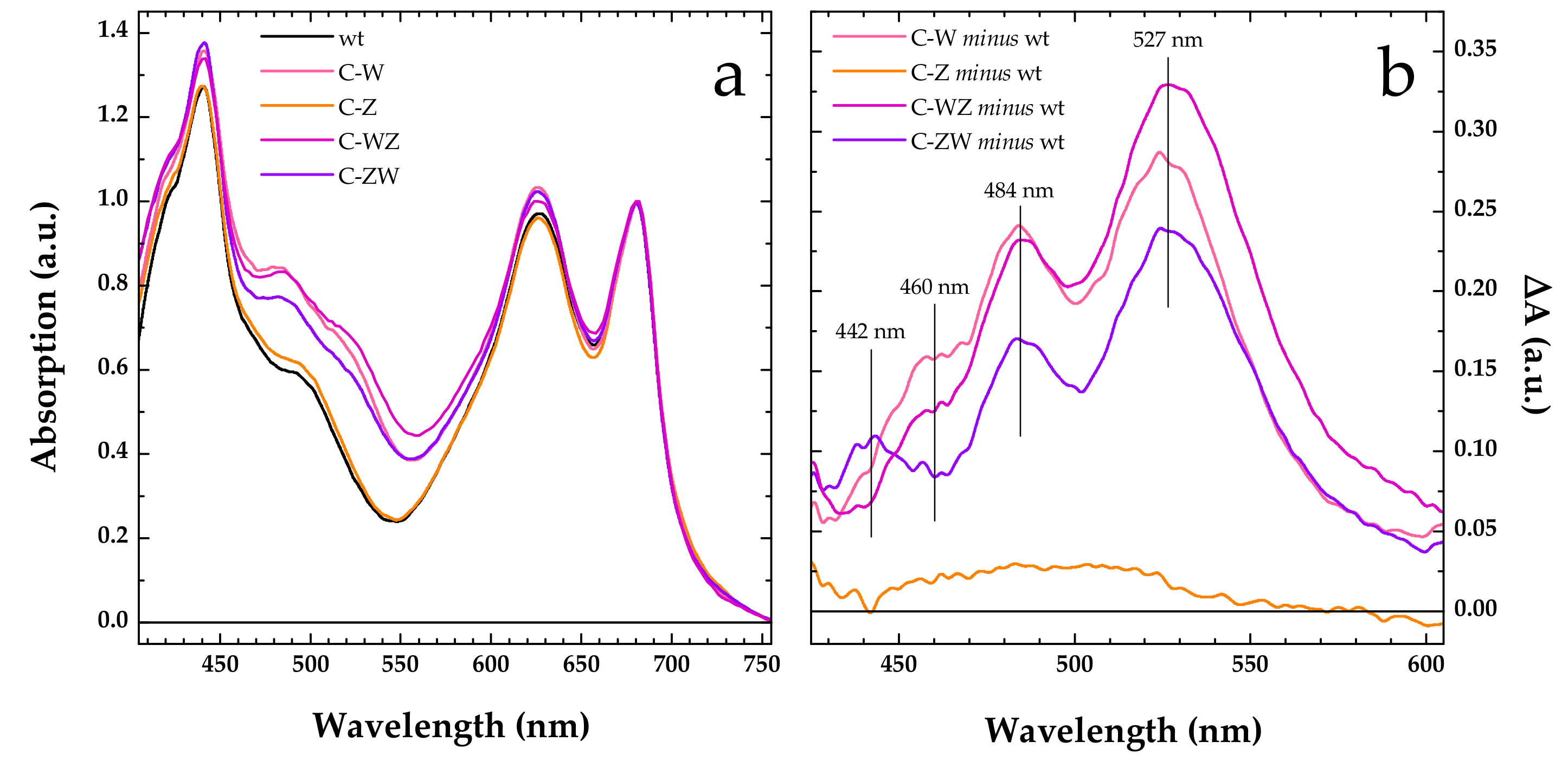
2.4. Absorption Spectroscopy
2.5. Pigments Extraction and Analysis
3. Results
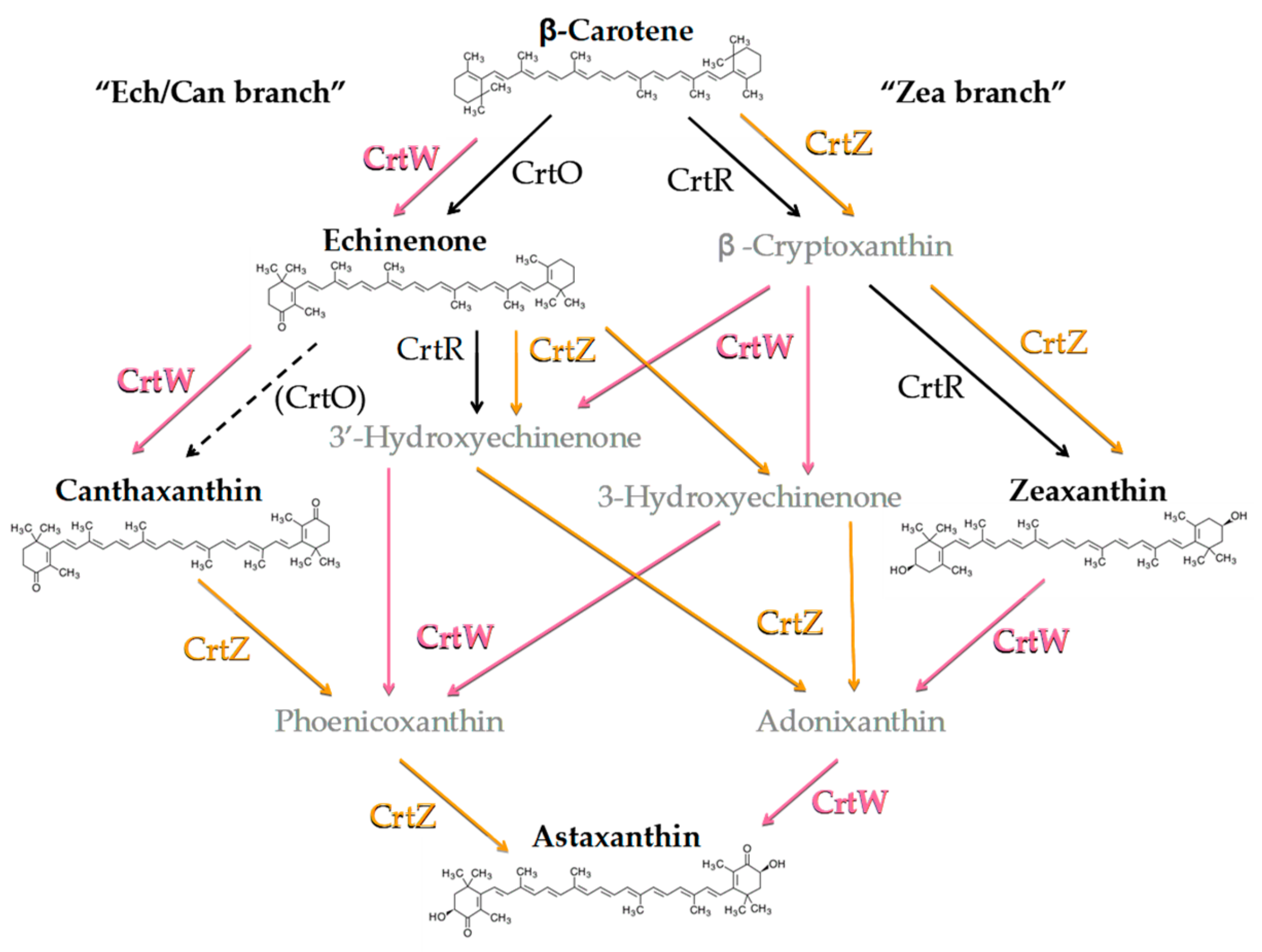
3.1. Generation of Synechocystis Engineered Strains Constitutively Expressing Exogenous CrtW and CrtZ
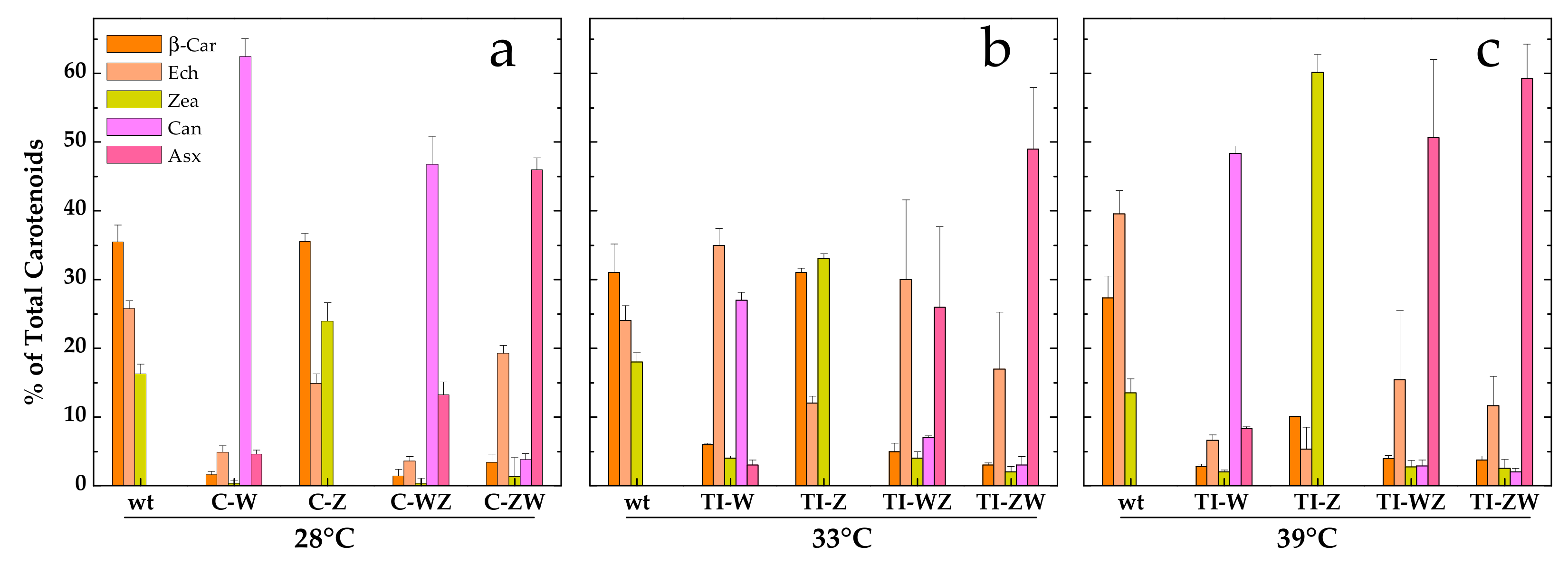
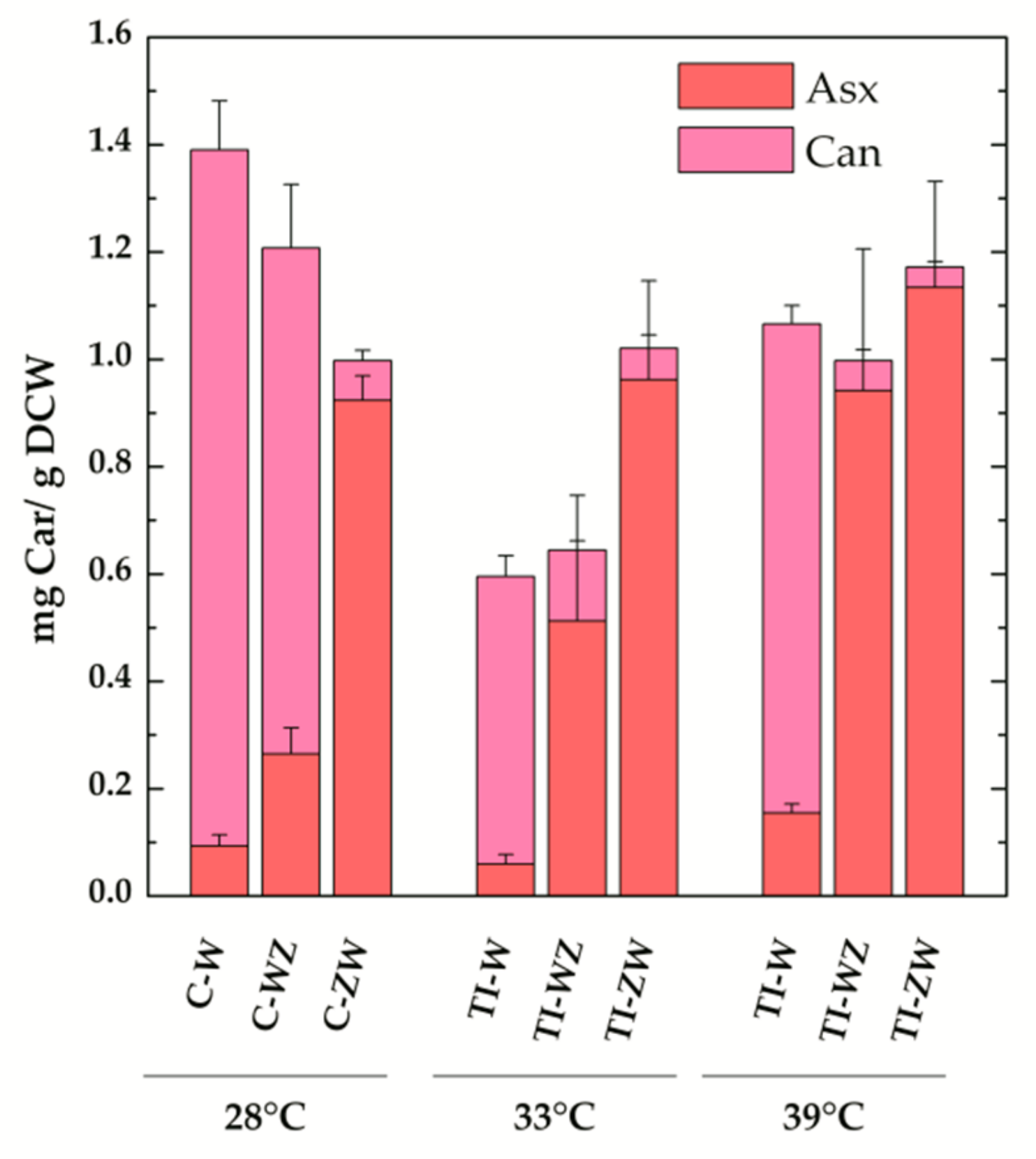
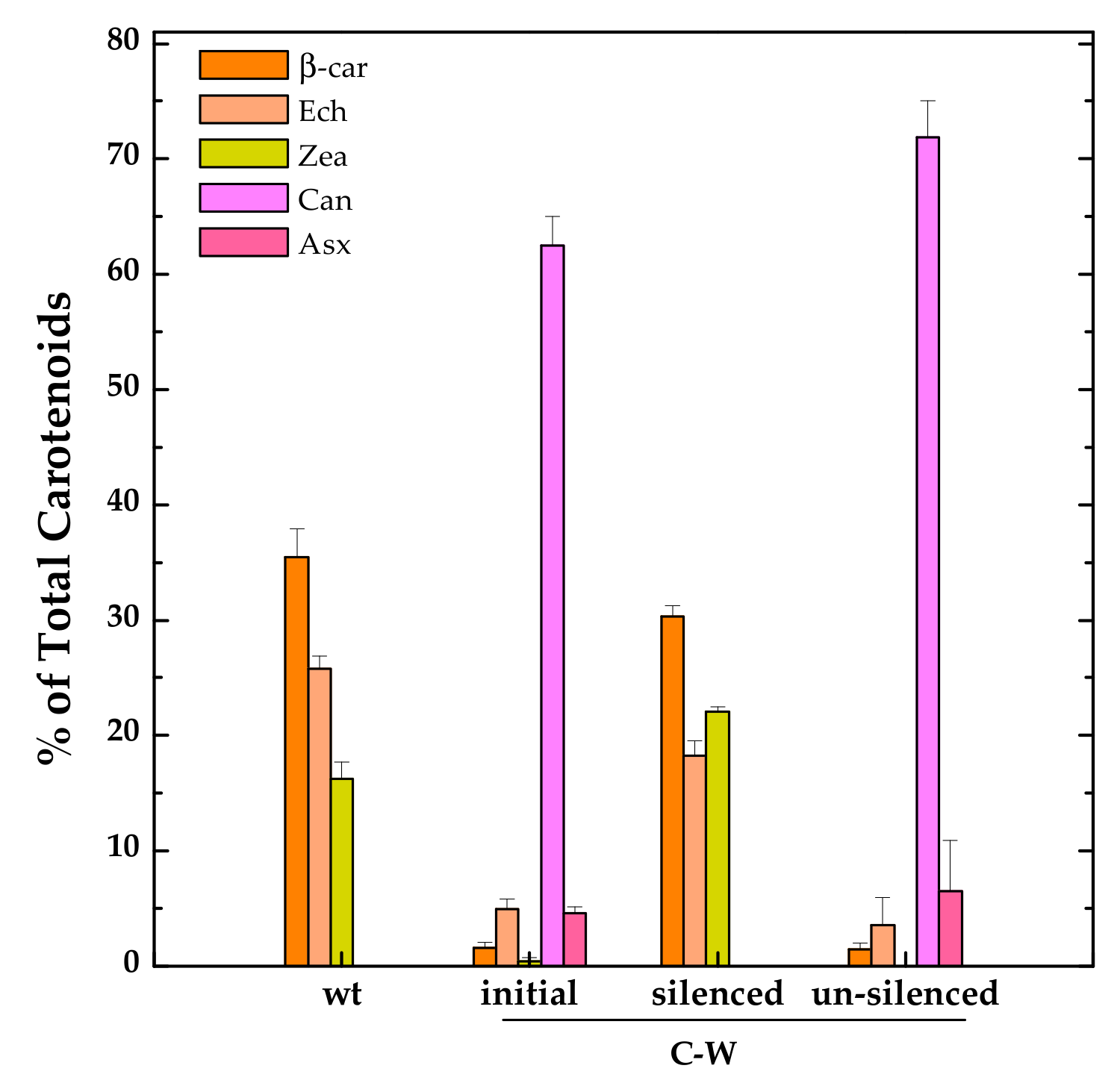
3.2. Constitutive Expression of Exogenous CrtW and CrtZ
3.3. Temperature Induction of Exogenous CrtW and CrtZ
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Grand View Research, Inc. “Carotenoids Market Analysis By Source (Natural, Synthetic), By Product (Beta-Carotene, Lutein, Lycopene, Astaxanthin, Zeaxanthin, Canthaxanthin), By Application (Food, Supplements, Feed, Pharmaceuticals, Cosmetics), And Segment Forecasts, 2018–2025” Report ID: GVR-1-68038-321-8, 90 pages. 2016. Available online: https://www.grandviewresearch.com/industry-analysis/carotenoids-market (accessed on 21 June 2019).
- Grand View Research, Inc. “Astaxanthin Market Analysis By Source (Natural [Yeast, Krill/Shrimp, Microalgae] And Synthetic), By Product (Dried Biomass/Powder, Oil, Soft gels, Liquid), By Application, And Segment Forecasts, 2018–2025” Report ID: GVR-1-68038-957-9, 96 pages. 2017. Available online: https://www.grandviewresearch.com/industry-analysis/global-astaxanthin-market (accessed on 21 June 2019).
- Lim, K.C.; Yusoff, F.M.; Shariff, M.; Kamarudin, M.S. Astaxanthin as feed supplement in aquatic animals. Rev. Aquacult. 2018, 10, 738–773. [Google Scholar] [CrossRef]
- Johnson, E.A.; Lewis, M.J.; Grau, C.R. Pigmentation of egg yolks with astaxanthin from the yeast Phaffia rhodozyma. Poultry Science 2003, 59, 1777–1782. [Google Scholar] [CrossRef]
- Higuera-Ciapara, I.; Félix-Valenzuela, L.; Goycoolea, F.M. Astaxanthin: A Review of its Chemistry and Applications. Crit. Rev. Food Sci. Nutr. 2006, 46, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Ambati, R.R.; Phang, S.M.; Ravi, S.; Aswathanarayana, R.G. Astaxanthin: Sources, Extraction, Stability, Biological Activities and Its Commercial Applications—A Review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef] [PubMed]
- Gong, M.; Bassi, A. Carotenoids from microalgae: A review of recent developments. Biotechnol. Adv. 2016, 34, 1396–1412. [Google Scholar] [CrossRef] [PubMed]
- Khoo, K.S.; Lee, S.Y.; Ooi, C.W.; Fu, X.; Miao, X.; Ling, T.C.; Show, P.L. Recent advances in biorefinery of astaxanthin from Haematococcus pluvialis. Bioresour. Technol. 2019, 121606. [Google Scholar] [CrossRef]
- Rodríguez-Sáiz, M.; de la Fuente, J.L.; Barredo, J.L. Xanthophyllomyces dendrorhous for the industrial production of astaxanthin. Appl. Microbiol. Biotechnol. 2010, 88, 645–658. [Google Scholar] [CrossRef]
- Misawa, N.; Satomi, Y.; Kondo, K.; Yokoyama, A.; Kajiwara, S.; Saito, T.; Ohtani, T.; Miki, W. Structure and functional analysis of a marine bacterial carotenoid biosynthesis gene cluster and astaxanthin biosynthetic pathway proposed at the gene level. J. Bacteriol. 1995, 177, 6575–6584. [Google Scholar] [CrossRef]
- Zhou, P.; Ye, L.; Xie, W.; Lv, X.; Yu, H. Highly efficient biosynthesis of astaxanthin in Saccharomyces cerevisiae by integration and tuning of algal crtZ and bkt. Appl. Microbiol. Biotechnol. 2015, 99, 8419–8428. [Google Scholar] [CrossRef]
- Zhou, P.P.; Xie, W.P.; Li, A.P.; Wang, F.; Yao, Z.; Bian, Q.; Zhu, Y.Q.; Yu, H.W.; Ye, L.D. Alleviation of metabolic bottleneck by combinatorial engineering enhanced astaxanthin synthesis in Saccharomyces cerevisiae. Enzyme Microb. Technol. 2017, 100, 28–36. [Google Scholar] [CrossRef]
- Lu, Q.; Bu, Y.F.; Liu, J.Z. Metabolic Engineering of Escherichia coli for Producing Astaxanthin as the Predominant Carotenoid. Mar. Drugs 2017, 15, 296. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Binkley, R.M.; Kim, W.J.; Lee, M.H.; Lee, S.Y. Metabolic engineering of Escherichia coli for high-level astaxanthin production with high productivity. Metab. Eng. 2018, 49, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Chou, Y.; Ko, C.; Yen, C.; Chen, L.O.; Shaw, J. Multiple promoters driving the expression of astaxanthin biosynthesis genes can enhance free-form astaxanthin production. J. Microbiol. Methods 2019, 160, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Branco Dos Santos, F.; Du, W.; Hellingwerf, K.J. Synechocystis: Not Just a Plug-Bug for CO2, but a Green E. coli. Front. Bioeng. Biotechnol. 2014, 2, 36. [Google Scholar] [CrossRef]
- Berla, B.M.; Saha, R.; Immethun, C.M.; Maranas, C.D.; Moon, T.S.; Pakrasi, H.B. Synthetic biology of cyanobacteria: unique challenges and opportunities. Front. Microbiol. 2013, 4, 246. [Google Scholar] [CrossRef]
- Vasudevan, R.; Gale, G.A.R.; Schiavon, A.A.; Puzorjov, A.; Malin, J.; Gillespie, M.D.; Vavitsas, K.; Zulkower, V.; Wang, B.; Howe, C.J.; et al. CyanoGate: A Modular Cloning Suite for Engineering Cyanobacteria Based on the Plant MoClo Syntax. Plant Physiol. 2019, 180, 39–55. [Google Scholar] [CrossRef]
- Oliver, N.J.; Rabinovitch-Deere, C.A.; Carroll, A.L.; Nozzi, N.E.; Case, A.E.; Atsumi, S. Cyanobacterial metabolic engineering for biofuel and chemical production. Curr. Opin. Chem. Biol. 2016, 35, 43–50. [Google Scholar] [CrossRef]
- Katayama, N.; Iijima, H.; Osanai, T. Production of Bioplastic Compounds by Genetically Manipulated and Metabolic Engineered Cyanobacteria. In Synthetic Biology of Cyanobacteria; Advances in Experimental Medicine and Biology 1080; Zhang, W., Song, X., Eds.; Springer: Singapore, 2018; pp. 155–169. [Google Scholar] [CrossRef]
- Harker, M.; Hirschberg, J. Biosynthesis of ketocarotenoids in transgenic cyanobacteria expressing the algal gene for β-C-4-oxygenase, crtO. FEBS Lett. 1997, 404, 129–134. [Google Scholar] [CrossRef]
- Menin, B.; Santabarbara, S.; Lami, A.; Musazzi, S.; Villafiorita Monteleone, F.; Casazza, A.P. Non-endogenous ketocarotenoid accumulation in engineered Synechocystis sp. PCC 6803. Physiol. Plant. 2019, 166, 403–412. [Google Scholar] [CrossRef]
- Mermet-Bouvier, P.; Chauvat, F. A conditional expression vector for the cyanobacteria Synechocystis sp. strains PCC6803 and PCC6714 or Synechococcus sp. strains PCC7942 and PCC6301. Curr. Microbiol. 1994, 28, 145–148. [Google Scholar] [CrossRef]
- Hollingshead, S.; Kopecná, J.; Jackson, P.J.; Canniffe, D.P.; Davison, P.A.; Dickman, M.J.; Sobotka, R.; Hunter, C.N. Conserved chloroplast open-reading frame ycf54 is required for activity of the magnesium protoporphyrin monomethylester oxidative cyclase in Synechocystis PCC 6803. J. Biol. Chem. 2012, 287, 27823–27833. [Google Scholar] [CrossRef] [PubMed]
- Lami, A.; Musazzi, S.; Marchetto, A.; Buchaca, T.; Kernan, M.; Jeppesen, E.; Guilizzoni, P. Sedimentary pigments in 308 alpine lakes and their relation to environmental gradients. Adv. Limnol. 2009, 62, 217–238. [Google Scholar] [CrossRef]
- Davies, B.H. Carotenoids. In Chemistry and Biochemistry of Plant Pigments, 2nd ed.; Goodwin, T.W., Ed.; Academic Press: London, UK; New York, NY, USA; San Francisco, CA, USA, 1976; Volume 2, (Analytical Methods); pp. 38–165. [Google Scholar]
- Egeland, E.S.; Garrido, J.L.; Clementson, L.; Andresen, K.; Thomas, C.S.; Zapata, M.; Airs, R.; Llewellyn, C.A.; Newman, G.L.; Rodrìguez, F.; et al. Part VII: Data sheets aiding identification of phytoplankton carotenoids and chlorophylls. In Phytoplankton Pigments: Characterization, Chemotaxonomy and Applications in Oceanography, 1st ed.; Roy, S., Llewellyn, C.A., Egeland, E.S., Johnsen, G., Eds.; Cambridge University Press: Cambridge, UK, 2011; pp. 675–822. [Google Scholar]
- Mohamed, A.; Jansson, C. Influence of light on accumulation of photosynthesis-specific transcripts in the cyanobacterium Synechocystis 6803. Plant Mol. Biol. 1989, 13, 693–700. [Google Scholar] [CrossRef]
- Marteyn, B.; Sakr, S.; Farci, S.; Bedhomme, M.; Chardonnet, S.; Decottignies, P.; Lemaire, S.D.; Cassier-Chauvat, C.; Chauvat, F. The Synechocystis PCC6803 MerA-like enzyme operates in the reduction of both mercury and uranium under the control of the glutaredoxin 1 enzyme. J. Bacteriol. 2013, 195, 4138–4145. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Ramos, M.; Jittawuttipoka, T.; Saenkham, P.; Czarnecka-Kwasiborski, A.; Bottin, H.; Cassier-Chauvat, C.; Chauvat, F. Engineering Synechocystis PCC6803 for hydrogen production: influence on the tolerance to oxidative and sugar stresses. PLoS ONE 2014, 9, e89372. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, M.; Enfissi, E.M.A.; Welsch, R.; Beyer, P.; Zurbriggen, M.D.; Fraser, P.D. Construction of a fusion enzyme for astaxanthin formation and its characterisation in microbial and plant hosts: A new tool for engineering ketocarotenoids. Metab. Eng. 2019, 52, 243–252. [Google Scholar] [CrossRef]
- Mutalik, V.K.; Guimaraes, J.C.; Cambray, G.; Mai, Q.; Christoffersen, M.J.; Martin, L.; Yu, A.; Lam, C.; Rodriguez, C.; Bennett, G.; et al. Quantitative estimation of activity and quality for collections of functional genetic elements. Nat. Methods 2013, 10, 347–353. [Google Scholar] [CrossRef]
- Englund, E.; Liang, F.; Lindberg, P. Evaluation of promoters and ribosome binding sites for biotechnological applications in the unicellular cyanobacterium Synechocystis sp. PCC 6803. Sci. Rep. 2016, 6, 36640. [Google Scholar] [CrossRef]
- Hasunuma, T.; Miyazawa, S.; Yoshimura, S.; Shinzaki, Y.; Tomizawa, K.; Shindo, K.; Choi, S.K.; Misawa, N.; Miyake, C. Biosynthesis of astaxanthin in tobacco leaves by transplastomic engineering. Plant J. 2008, 55, 857–868. [Google Scholar] [CrossRef]
- Jones, P.R. Genetic Instability in Cyanobacteria—An Elephant in the Room? Front. Bioeng. Biotechnol. 2014, 2, 12. [Google Scholar] [CrossRef]
- Cassier-Chauvat, C.; Veaudor, T.; Chauvat, F. Comparative Genomics of DNA Recombination and Repair in Cyanobacteria: Biotechnological Implications. Front. Microbiol. 2016, 7, 1809. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menin, B.; Lami, A.; Musazzi, S.; Petrova, A.A.; Santabarbara, S.; Casazza, A.P. A Comparison of Constitutive and Inducible Non-Endogenous Keto-Carotenoids Biosynthesis in Synechocystis sp. PCC 6803. Microorganisms 2019, 7, 501. https://doi.org/10.3390/microorganisms7110501
Menin B, Lami A, Musazzi S, Petrova AA, Santabarbara S, Casazza AP. A Comparison of Constitutive and Inducible Non-Endogenous Keto-Carotenoids Biosynthesis in Synechocystis sp. PCC 6803. Microorganisms. 2019; 7(11):501. https://doi.org/10.3390/microorganisms7110501
Chicago/Turabian StyleMenin, Barbara, Andrea Lami, Simona Musazzi, Anastasia A. Petrova, Stefano Santabarbara, and Anna Paola Casazza. 2019. "A Comparison of Constitutive and Inducible Non-Endogenous Keto-Carotenoids Biosynthesis in Synechocystis sp. PCC 6803" Microorganisms 7, no. 11: 501. https://doi.org/10.3390/microorganisms7110501
APA StyleMenin, B., Lami, A., Musazzi, S., Petrova, A. A., Santabarbara, S., & Casazza, A. P. (2019). A Comparison of Constitutive and Inducible Non-Endogenous Keto-Carotenoids Biosynthesis in Synechocystis sp. PCC 6803. Microorganisms, 7(11), 501. https://doi.org/10.3390/microorganisms7110501





