Characterizing the Potential of the Non-Conventional Yeast Saccharomycodes ludwigii UTAD17 in Winemaking
Abstract
1. Introduction
2. Materials and Methods
2.1. Yeast Strains and Maintenance Conditions
2.2. Phenotypic Characterization
2.3. Grape Juice and Inocula Preparation
2.4. Fermentation Trials
2.5. Determination of Growth and Fermentation Parameters
2.6. Analytical Determinations
2.7. Statistical Analysis
2.8. Comparison of S. ludwigii UTAD17 ‘ORFeome’ with S. cerevisiae s288c
3. Results and Discussion
3.1. Phenotypic Characterization of S. ludwigii UTAD17
3.2. Yeast Growth Kinetics and Fermentation Profiles
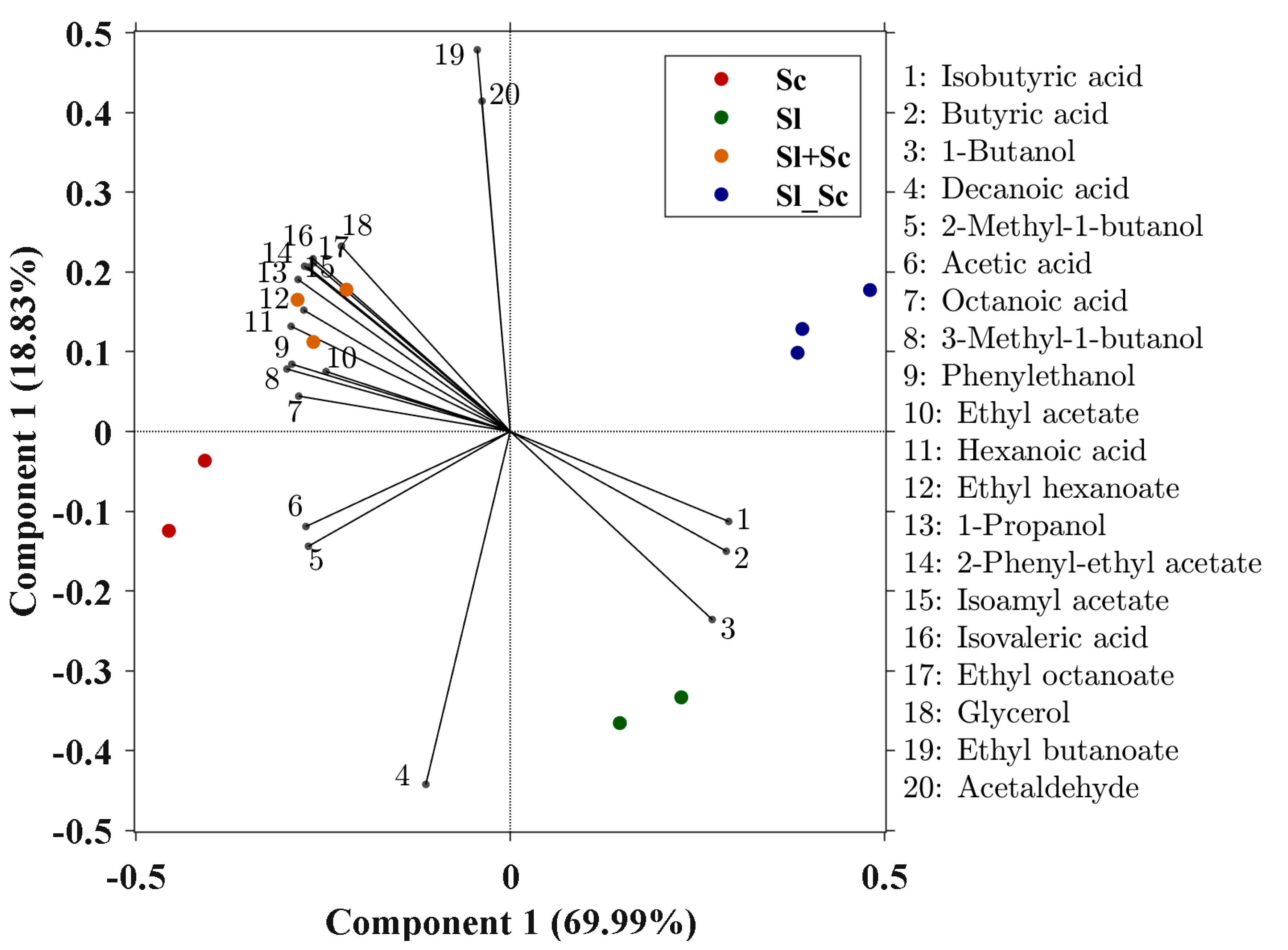
3.3. Effect of S. ludwigii UTAD17 on Wine Composition and Aroma Profile
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fleet, G.H.; Heard, G.M. Yeast growth during fermentation. In Wine Microbiology and Biotechnology (Fleet GH); Harwood Academic: Chur, Switzerland, 1993; pp. 27–54. [Google Scholar]
- Egli, C.M.; Edinger, W.D.; Mitrakul, C.M.; Henick-Kling, T.; Henick-Kling, T. Dynamics of indigenous and inoculated yeast populations and their effect on the sensory character of Riesling and Chardonnay wines. J. Appl. Microbiol. 1998, 85, 779–789. [Google Scholar] [CrossRef] [PubMed]
- Henick-Kling, T.; Edinger, W.; Daniel, P.; Monk, P. Selective effects of sulfur dioxide and yeast starter culture addition on indigenous yeast populations and sensory characteristics of wine. J. Appl. Microbiol. 1998, 84, 865–876. [Google Scholar] [CrossRef]
- Ciani, M.; Mannazzu, I.; Marinangeli, P.; Clementi, F.; Martini, A. Contribution of winery-resident Saccharomyces cerevisiae strains to spontaneous grape must fermentation. Antonie Leeuwenhoek Int. J. Gen. Mol. Microbiol. 2004, 85, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Scholl, C.M.; Morgan, S.C.; Stone, M.L.; Tantikachornkiat, M.; Neuner, M.; Durall, D.M. Composition of Saccharomyces cerevisiae strains in spontaneous fermentations of Pinot Noir and Chardonnay. Aust. J. Grape Wine Res. 2016, 22, 384–390. [Google Scholar] [CrossRef]
- Boulton, R.B.; Singleton, V.L.; Bisson, L.F.; Kunkee, R.E. Microbiological Spoilage of Wine and its Control. In Principles and Practices of Winemaking; Chapman & Hall: London, UK, 1996; pp. 352–381. [Google Scholar]
- Ciani, M.; Maccarelli, F. Oenological properties of non-Saccharomyces yeasts associated with wine-making. World J. Microbiol. Biotechnol. 1998, 14, 199–203. [Google Scholar] [CrossRef]
- Domizio, P.; Romani, C.; Comitini, F.; Gobbi, M.; Lencioni, L.; Mannazzu, I.; Ciani, M. Potential spoilage non-Saccharomyces yeasts in mixed cultures with Saccharomyces cerevisiae. Ann. Microbiol. 2011, 61, 137–144. [Google Scholar] [CrossRef]
- Fernández, M.; Ubeda, J.; Briones, A. Typing of non-Saccharomyces yeasts with enzymatic activities of interest in wine-making. Int. J. Food Microbiol. 2000, 59, 29–36. [Google Scholar] [CrossRef]
- Jolly, N.P.; Varela, C.; Pretorius, I.S. Not your ordinary yeast: Non-Saccharomyces yeasts in wine production uncovered. FEMS Yeast Res. 2014, 14, 215–237. [Google Scholar] [CrossRef]
- Maturano, Y.P.; Assaf, L.A.R.; Toro, M.E.; Nally, M.C.; Vallejo, M.; De Figueroa, L.I.C.; Combina, M.; Vazquez, F. Multi-enzyme production by pure and mixed cultures of Saccharomyces and non-Saccharomyces yeasts during wine fermentation. Int. J. Food Microbiol. 2012, 155, 43–50. [Google Scholar] [CrossRef]
- Mendes-Ferreira, A.; Clímaco, M.C.; Mendes-Faia, A. The role of non-Saccharomyces species in releasing glycosidic bound fraction of grape aroma components Ð a preliminary study. J. Appl. Microbiol. 2001, 91, 67–71. [Google Scholar] [CrossRef]
- Rosi, I.; Vinella, M.; Domizio, P. Characterization of Beta-Glucosidase Activity in Yeasts of Enological Origin. J. Appl. Bacteriol. 1994, 77, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Strauss, M.; Jolly, N.; Lambrechts, M.; Van Rensburg, P. Screening for the production of extracellular hydrolytic enzymes by non-Saccharomyces wine yeasts. J. Appl. Microbiol. 2001, 91, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Viana, F.; Gil, J.; Genoves, S.; Valles, S.; Manzanares, P. Rational selection of non-Saccharomyces wine yeasts for mixed starters based on ester formation and enological traits. Food Microbiol. 2008, 25, 778–785. [Google Scholar] [CrossRef] [PubMed]
- Comitini, F.; Gobbi, M.; Domizio, P.; Romani, C.; Lencioni, L.; Mannazzu, I.; Ciani, M. Selected non-Saccharomyces wine yeasts in controlled multistarter fermentations with Saccharomyces cerevisiae. Food Microbiol. 2011, 28, 873–882. [Google Scholar] [CrossRef]
- De Benedictis, M.; Bleve, G.; Tristezza, M.; Tufariello, M.; Grieco, F. An optimized procedure for the enological selection of non-Saccharomyces starter cultures. Antonie Leeuwenhoek Int. J. Gen. Mol. Microbiol. 2011, 99, 189–200. [Google Scholar] [CrossRef]
- Domizio, P.; Romani, C.; Lencioni, L.; Comitini, F.; Gobbi, M.; Mannazzu, I.; Ciani, M. Outlining a future for non-Saccharomyces yeasts: Selection of putative spoilage wine strains to be used in association with Saccharomyces cerevisiae for grape juice fermentation. Int. J. Food Microbiol. 2011, 147, 170–180. [Google Scholar] [CrossRef]
- Belda, I.; Ruiz, J.; Alastruey-Izquierdo, A.; Navascués, E.; Marquina, D.; Santos, A. Unraveling the enzymatic basis of wine “Flavorome”: A phylo-functional study of wine related yeast species. Front. Microbiol. 2016, 7, 1–13. [Google Scholar] [CrossRef]
- Berbegal, C.; Spano, G.; Tristezza, M.; Grieco, F.; Capozzi, V. Microbial Resources and Innovation in the Wine Production Sector. S. Afr. J. Enol. Vitic. 2017, 38, 156–166. [Google Scholar] [CrossRef]
- Barbosa, C.; Lage, P.; Esteves, M.; Chambel, L.; Mendes-Faia, A.; Mendes-Ferreira, A. Molecular and Phenotypic Characterization of Metschnikowia pulcherrima Strains from Douro Wine Region. Fermentation 2018, 4, 8. [Google Scholar] [CrossRef]
- Nisiotou, A.; Sgouros, G.; Mallouchos, A.; Nisiotis, C.-S.; Michaelidis, C.; Tassou, C.; Banilas, G. The use of indigenous Saccharomyces cerevisiae and Starmerella bacillaris strains as a tool to create chemical complexity in local wines. Food Res. Int. 2018, 111, 498–508. [Google Scholar] [CrossRef]
- Binati, R.L.; Innocente, G.; Gatto, V.; Celebrin, A.; Polo, M.; Felis, G.E.; Torriani, S. Exploring the diversity of a collection of native non-Saccharomyces yeasts to develop co-starter cultures for winemaking. Food Res. Int. 2019, 122, 432–442. [Google Scholar] [CrossRef] [PubMed]
- Zironi, R.; Romano, P.; Suzzi, G.; Battistutta, F.; Comi, G. Volatile metabolites produced in wine by mixed and sequential cultures of Hanseniaspora guilliermondii or Kloeckera apiculata and Saccharomyces cerevisiae. Biotechnol. Lett. 1993, 15, 235–238. [Google Scholar] [CrossRef]
- Ciani, M.; Fatichenti, F. Selective sugar consumption by apiculate yeasts. Lett. Appl. Microbiol. 1999, 28, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Moreira, N.; Mendes, F.; De Pinho, P.G.; Hogg, T.; Vasconcelos, I. Heavy sulphur compounds, higher alcohols and esters production profile of Hanseniaspora uvarum and Hanseniaspora guilliermondii grown as pure and mixed cultures in grape must. Int. J. Food Microbiol. 2008, 124, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Andorrà, I.; Berradre, M.; Mas, A.; Esteve-Zarzoso, B.; Guillamón, J.M. Effect of mixed culture fermentations on yeast populations and aroma profile. LWT Food Sci. Technol. 2012, 49, 8–13. [Google Scholar] [CrossRef]
- Hu, K.; Jin, G.-J.; Mei, W.-C.; Li, T.; Tao, Y.-S. Increase of medium-chain fatty acid ethyl ester content in mixed H. uvarum/S. cerevisiae fermentation leads to wine fruity aroma enhancement. Food Chem. 2018, 239, 495–501. [Google Scholar] [CrossRef]
- Nevado, F.P.; Albergaria, H.; Hogg, T.; Gírio, F. Cellular death of two non-Saccharomyces wine-related yeasts during mixed fermentations with Saccharomyces cerevisiae. Int. J. Food Microbiol. 2006, 108, 336–345. [Google Scholar]
- Lage, P.; Barbosa, C.; Mateus, B.; Vasconcelos, I.; Mendes-Faia, A.; Mendes-Ferreira, A. H. guilliermondii impacts growth kinetics and metabolic activity of S. cerevisiae: The role of initial nitrogen concentration. Int. J. Food Microbiol. 2014, 172, 62–69. [Google Scholar] [CrossRef]
- Viana, F.; Belloch, C.; Vallés, S.; Manzanares, P. International Journal of Food Microbiology Monitoring a mixed starter of Hanseniaspora vineae—Saccharomyces cerevisiae in natural must: Impact on 2-phenylethyl acetate production. Int. J. Food Microbiol. 2011, 151, 235–240. [Google Scholar] [CrossRef]
- Medina, K.; Boido, E.; Dellacassa, E.; Carrau, F. Growth of non-Saccharomyces yeasts affects nutrient availability for Saccharomyces cerevisiae during wine fermentation. Int. J. Food Microbiol. 2012, 157, 245–250. [Google Scholar] [CrossRef]
- Medina, K.; Boido, E.; Farina, L.; Gioia, O.; Gómez, M.; Barquet, M.; Gaggero, C.; Dellacassa, E.; Carrau, F. Increased flavour diversity of Chardonnay wines by spontaneous fermentation and co-fermentation with Hanseniaspora vineae. Food Chem. 2013, 141, 2513–2521. [Google Scholar] [CrossRef] [PubMed]
- Lleixà, J.; Martín, V.; Portillo, M.D.C.; Carrau, F.; Beltran, G.; Mas, A. Comparison of Fermentation and Wines Produced by Inoculation of Hanseniaspora vineae and Saccharomyces cerevisiae. Front. Microbiol. 2016, 7, 338. [Google Scholar] [CrossRef] [PubMed]
- Cocolin, L.; Bisson, L.; Mills, D. Direct profiling of the yeast dynamics in wine fermentations. FEMS Microbiol. Lett. 2000, 189, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Contreras, A.; Hidalgo, C.; Schmidt, S.; Henschke, P.; Curtin, C.; Varela, C. The application of non-Saccharomyces yeast in fermentations with limited aeration as a strategy for the production of wine with reduced alcohol content. Int. J. Food Microbiol. 2015, 205, 7–15. [Google Scholar] [CrossRef]
- Ruiz, J.; Belda, I.; Beisert, B.; Navascués, E.; Marquina, D.; Calderón, F.; Benito, S. Analytical impact of Metschnikowia pulcherrima in the volatile profile of Verdejo white wines. Appl. Microbiol. Biotechnol. 2018, 102, 8501–8509. [Google Scholar] [CrossRef]
- Bely, M.; Stoeckle, P.; Masneuf-Pomarede, I.; Dubourdieu, D. Impact of mixed Torulaspora delbrueckii–Saccharomyces cerevisiae culture on high-sugar fermentation. Int. J. Food Microbiol. 2008, 122, 312–320. [Google Scholar] [CrossRef]
- Renault, P.; Coulon, J.; De Revel, G.; Barbe, J.-C.; Bely, M. Increase of fruity aroma during mixed T. delbrueckii/S. cerevisiae wine fermentation is linked to specific esters enhancement. Int. J. Food Microbiol. 2015, 207, 40–48. [Google Scholar] [CrossRef]
- Azzolini, M.; Fedrizzi, B.; Tosi, E.; Finato, F.; Vagnoli, P.; Scrinzi, C.; Zapparoli, G. Effects of Torulaspora delbrueckii and Saccharomyces cerevisiae mixed cultures on fermentation and aroma of Amarone wine. Eur. Food Res. Technol. 2012, 235, 303–313. [Google Scholar] [CrossRef]
- Taillandier, P.; Lai, Q.P.; Julien-Ortiz, A.; Brandam, C. Interactions between Torulaspora delbrueckii and Saccharomyces cerevisiae in wine fermentation: Influence of inoculation and nitrogen content. World J. Microbiol. Biotechnol. 2014, 30, 1959–1967. [Google Scholar] [CrossRef]
- Tondini, F.; Lang, T.; Chen, L.; Herderich, M.; Jiranek, V. Linking gene expression and oenological traits: Comparison between Torulaspora delbrueckii and Saccharomyces cerevisiae strains. Int. J. Food Microbiol. 2019, 294, 42–49. [Google Scholar] [CrossRef]
- Canonico, L.; Comitini, F.; Ciani, M. Influence of vintage and selected starter on Torulaspora delbrueckii/Saccharomyces cerevisiae sequential fermentation. Eur. Food Res. Technol. 2015, 241, 827–833. [Google Scholar] [CrossRef]
- Mora, J.; Barbas, J.I.; Mulet, A. Growth of Yeast Species during the Fermentation of Musts Inoculated with Kluyveromyces thermotolerans and Saccharomyces cerevisiae. Am. J. Enol. Vitic. 1990, 41, 156–159. [Google Scholar]
- Kapsopoulou, K.; Mourtzini, A.; Anthoulas, M.; Nerantzis, E. Biological acidification during grape must fermentation using mixed cultures of Kluyveromyces thermotolerans and Saccharomyces cerevisiae. World J. Microbiol. Biotechnol. 2007, 23, 735–739. [Google Scholar] [CrossRef]
- Gobbi, M.; Comitini, F.; Domizio, P.; Romani, C.; Lencioni, L.; Mannazzu, I.; Ciani, M. Lachancea thermotolerans and Saccharomyces cerevisiae in simultaneous and sequential co-fermentation: A strategy to enhance acidity and improve the overall quality of wine. Food Microbiol. 2013, 33, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Benito, Á.; Calderón, F.; Palomero, F.; Benito, S. Quality and Composition of Airén Wines Fermented by Sequential Inoculation of Lachancea thermotolerans and Saccharomyces cerevisiae. Food Technol. Biotechnol. 2016, 54, 135–144. [Google Scholar] [CrossRef]
- Morales, M.; Fierro-Risco, J.; Ríos-Reina, R.; Ubeda, C.; Paneque, P. Influence of Saccharomyces cerevisiae and Lachancea thermotolerans co-inoculation on volatile profile in fermentations of a must with a high sugar content. Food Chem. 2019, 276, 427–435. [Google Scholar] [CrossRef]
- Ciani, M.; Ferraro, F.R. Combined use of immobilized Candida stellata cells and Saccharomyces cerevisiae to improve the quality of wines. J. Appl. Microbiol. 1998, 85, 247–254. [Google Scholar] [CrossRef]
- Soden, A.; Francis, I.; Oakey, H.; Henschke, P. Effects of co-fermentation with Candida stellata and Saccharomyces cerevisiae on the aroma and composition of Chardonnay wine. Aust. J. Grape Wine Res. 2000, 6, 21–30. [Google Scholar] [CrossRef]
- Ciani, M.; Comitini, F.; Mannazzu, I.; Domizio, P. Controlled mixed culture fermentation: A new perspective on the use of non-Saccharomyces yeasts in winemaking. FEMS Yeast Res. 2010, 10, 123–133. [Google Scholar] [CrossRef]
- Domizio, P.; Liu, Y.; Bisson, L.; Barile, D. Use of non-Saccharomyces wine yeasts as novel sources of mannoproteins in wine. Food Microbiol. 2014, 43, 5–15. [Google Scholar] [CrossRef]
- Englezos, V.; Torchio, F.; Cravero, F.; Marengo, F.; Giacosa, S.; Gerbi, V.; Cocolin, L. Aroma profile and composition of Barbera wines obtained by mixed fermentations of Starmerella bacillaris (synonym Candida zemplinina) and Saccharomyces cerevisiae. LWT Food Sci. Technol. 2016, 73, 567–575. [Google Scholar] [CrossRef]
- Englezos, V.; Rantsiou, K.; Cravero, F.; Torchio, F.; Pollon, M.; Fracassetti, D.; Ortiz-Julien, A.; Gerbi, V.; Rolle, L.; Cocolin, L.; et al. Volatile profile of white wines fermented with sequential inoculation of Starmerella bacillaris and Saccharomyces cerevisiae. Food Chem. 2018, 257, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Benito, Á.; Calderón, F.; Benito, S. Mixed alcoholic fermentation of Schizosaccharomyces pombe and Lachancea thermotolerans and its influence on mannose-containing polysaccharides wine Composition. AMB Express 2019, 9, 17. [Google Scholar] [CrossRef] [PubMed]
- Belda, I.; Navascués, E.; Marquina, D.; Santos, A.; Calderon, F.; Benito, S. Dynamic analysis of physiological properties of Torulaspora delbrueckii in wine fermentations and its incidence on wine quality. Appl. Microbiol. Biotechnol. 2014, 99, 1911–1922. [Google Scholar] [CrossRef]
- Englezos, V.; Rantsiou, K.; Cravero, F.; Torchio, F.; Giacosa, S.; Ortiz-Julien, A.; Gerbi, V.; Rolle, L.; Cocolin, L. Volatile profiles and chromatic characteristics of red wines produced with Starmerella bacillaris and Saccharomyces cerevisiae. Food Res. Int. 2018, 109, 298–309. [Google Scholar] [CrossRef]
- Sadoudi, M.; Tourdot-Maréchal, R.; Rousseaux, S.; Steyer, D.; Gallardo-Chacón, J.-J.; Ballester, J.; Vichi, S.; Guérin-Schneider, R.; Caixach, J.; Alexandre, H. Yeast–yeast interactions revealed by aromatic profile analysis of Sauvignon Blanc wine fermented by single or co-culture of non-Saccharomyces and Saccharomyces yeasts. Food Microbiol. 2012, 32, 243–253. [Google Scholar] [CrossRef]
- Petruzzi, L.; Capozzi, V.; Berbegal, C.; Corbo, M.R.; Bevilacqua, A.; Spano, G.; Sinigaglia, M. Microbial Resources and Enological Significance: Opportunities and Benefits. Front. Microbiol. 2017, 8, 1–13. [Google Scholar] [CrossRef]
- Phaff, H.J.; Miller, M.W.; Em, M. The Life of Yeasts, 2nd ed.; Harvard University Press: Cambridge, UK, 1978. [Google Scholar]
- Romano, P.; Marchese, R.; Laurita, C.; Saleano, G.; Turbanti, L. Biotechnological suitability of Saccharomycodes ludwigii for fermented beverages. World J. Microbiol. Biotechnol. 1999, 15, 451–454. [Google Scholar] [CrossRef]
- Thomas, D.S. Yeasts as Spoilage Organisms in Beverages. In The Yeasts, 2nd ed.; Rose, J.S., Harrison, A.H., Eds.; Academic Press: New York, NY, USA, 1993; pp. 517–561. [Google Scholar]
- Stratford, M.; Morgan, P.; Rose, A.H. Sulphur Dioxide Resistance in Saccharomyces cerevisiae and Saccharomycodes ludwigii. Microbiology 1987, 133, 2173–2179. [Google Scholar] [CrossRef]
- Estela-Escalante, W.; Hatta-Sakoda, B.; Ludeña-Cervantes, Z.; Melzoch, K.; Rychtera, M.; Sarmiento-Casavilca, V.; Chaquilla-Quilca, G. Actividad Fermentativa de Saccharomycodes ludwigii y Evaluación de la Síntesis de Compuestos de Importancia Sensorial durante la Fermentacion de Jugo de Manzana. Rev. Espec. Cienc. Quim. Biol. 2011, 14, 12–23. [Google Scholar]
- De Francesco, G.; Turchetti, B.; Sileoni, V.; Marconi, O.; Perretti, G. Screening of new strains of Saccharomycodes ludwigii and Zygosaccharomyces rouxii to produce low-alcohol beer. J. Inst. Brew. 2015, 121, 113–121. [Google Scholar] [CrossRef]
- Liu, Y.; Li, H.; Du, J.-H. Non-alcoholic Beer Production by Saccharomycodes ludwigii. Food Sci. 2011, 15, 186–190. [Google Scholar]
- Tavares, M.J.; Güldener, U.; Esteves, M.; Mendes-Faia, A.; Mendes-Ferreira, A.; Mira, N.P. Genome Sequence of the Wine Yeast Saccharomycodes ludwigii UTAD17. Microbiol. Resour. Announc. 2018, 7, e01195-18. [Google Scholar] [CrossRef]
- Castellucci, F. Guidelines for the Characterization of Wine Yeasts of the Genus Saccharomyces Isolated from Vitivinicultural Environments; International Organisation of Vine and Wine: Paris, France, 2012. [Google Scholar]
- Henschke, P.A.; Jiranek, V. Wine Microbiology and Biotechnology (Fleet GH.); Harwood Academic: Chur, Switzerland, 1993. [Google Scholar]
- Mendes-Ferreira, A.; Barbosa, C.; Falco, V.; Leão, C.; Mendes-Faia, A. The production of hydrogen sulphide and other aroma compounds by wine strains of Saccharomyces cerevisiae in synthetic media with different nitrogen concentrations. J. Ind. Microbiol. Biotechnol. 2009, 36, 571–583. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y. Detection of Wild Yeasts in the Brewery. J. Inst. Brew. 1971, 77, 513–516. [Google Scholar]
- OIV. Compendium of International Methods of Analysis of Musts and Wines; International Organisation of Vine and Wine: Paris, France, 2016. [Google Scholar]
- Moreira, N.; De Pinho, P.G.; Santos, C.; Vasconcelos, I. Relationship between nitrogen content in grapes and volatiles, namely heavy sulphur compounds, in wines. Food Chem. 2011, 126, 1599–1607. [Google Scholar] [CrossRef]
- Alexandre, H.; Charpentier, C. Biochemical aspects of stuck and sluggish fermentation in grape must. J. Ind. Microbiol. Biotechnol. 1998, 20, 20–27. [Google Scholar] [CrossRef]
- Albertin, W.; Marullo, P.; Aigle, M.; Dillmann, C.; De Vienne, D.; Bely, M.; Sicard, D. Population size drives industrial Saccharomyces cerevisiae alcoholic fermentation and is under genetic control. Appl. Environ. Microbiol. 2011, 77, 2772–2784. [Google Scholar] [CrossRef]
- Heard, G.; Fleet, G. The effects of temperature and pH on the growth of yeast species during the fermentation of grape juice. J. Appl. Bacteriol. 1988, 65, 23–28. [Google Scholar] [CrossRef]
- Seixas, I.; Barbosa, C.; Mendes-Faia, A.; Güldener, U.; Tenreiro, R.; Mendes-Ferreira, A.; Mira, N.P. Genome sequence of the non-conventional wine yeast Hanseniaspora guilliermondii UTAD222 unveils relevant traits of this species and of the Hanseniaspora genus in the context of wine fermentation. Curr. Neuropharmacol. 2019, 26, 67–83. [Google Scholar] [CrossRef]
- Bataillon, M.; Rico, A.; Sablayrolles, J.-M.; Salmon, J.-M.; Barré, P. Early thiamin assimilation by yeasts under enological conditions: Impact on alcoholic fermentation kinetics. J. Ferment. Bioeng. 1996, 82, 145–150. [Google Scholar] [CrossRef]
- Brion, C.; Ambroset, C.; Delobel, P.; Sanchez, I.; Blondin, B. Deciphering regulatory variation of THI genes in alcoholic fermentation indicate an impact of Thi3p on PDC1 expression. BMC Genom. 2014, 15, 1085. [Google Scholar] [CrossRef] [PubMed]
- Langenberg, A.; Bink, F.J.; Wolff, L.; Walter, S.; Grossmann, M.; Schmitz, H. Glycolytic Functions Are Conserved in the Genome of the Wine Yeast. Appl. Environ. Microbiol. 2017, 83, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, C.; Lage, P.; Vilela, A.; Mendes-Faia, A.; Mendes-Ferreira, A. Phenotypic and metabolic traits of commercial Saccharomyces cerevisiae yeasts. AMB Express 2014, 4, 39. [Google Scholar] [CrossRef] [PubMed]
- Brice, C.; Cubillos, F.A.; Dequin, S.; Camarasa, C.; Martinez, C. Adaptability of the Saccharomyces cerevisiae yeasts to wine fermentation conditions relies on their strong ability to consume nitrogen. PLoS ONE 2018, 13, e0192383. [Google Scholar] [CrossRef] [PubMed]
- Nissen, P.; Nielsen, D.S.; Arneborg, N. Viable Saccharomyces cerevisiae cells at high concentrations cause early growth arrest of non-Saccharomyces yeasts in mixed cultures by a cell-cell contact-mediated mechanism. Yeast 2003, 20, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Goddard, M.R. Quantifying the complexities of Saccharomyces cerevisiae’s ecosystem engineering via fermentation. Ecology 2008, 89, 2077–2082. [Google Scholar] [CrossRef]
- Werner, M.; Rauhut, D.; Cottereau, P. Yeast and Natural Production of Sulphites. Int. J. Enol. Vitic. 2009, 12, 1–5. [Google Scholar]
- Remize, F.; Andrieu, E.; Dequin, S. Engineering of the pyruvate dehydrogenase bypass in Saccharomyces cerevisiae: Role of the cytosolic Mg2+and mitochondrial K+acetaldehyde dehydrogenases Ald6p and Ald4p in acetate formation during alcoholic fermentation. Appl. Environ. Microbiol. 2000, 66, 3151–3159. [Google Scholar] [CrossRef]
- Andorrà, I.; Berradre, M.; Rozès, N.; Mas, A.; Guillamón, J.M.; Esteve-Zarzoso, B. Effect of pure and mixed cultures of the main wine yeast species on grape must fermentations. Eur. Food Res. Technol. 2010, 231, 215–224. [Google Scholar] [CrossRef]
- Hazelwood, L.A.; Daran, J.-M.; Van Maris, A.J.A.; Pronk, J.T.; Dickinson, J.R. The Ehrlich Pathway for Fusel Alcohol Production: A Century of Research on Saccharomyces cerevisiae Metabolism. Appl. Environ. Microbiol. 2008, 74, 3920. [Google Scholar] [CrossRef]
- Bloem, A.; Sanchez, I.; Dequin, S.; Camarasa, C. Metabolic Impact of Redox Cofactor Perturbations on the Formation of Aroma Compounds in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2016, 82, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Saerens, S.M.G.; Delvaux, F.; Verstrepen, K.J.; Van Dijck, P.; Thevelein, J.M.; Delvaux, F.R. Parameters Affecting Ethyl Ester Production by Saccharomyces cerevisiae during Fermentation. Appl. Environ. Microbiol. 2008, 74, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Lilly, M.; Bauer, F.F.; Lambrechts, M.G.; Swiegers, J.H.; Cozzolino, D.; Pretorius, I.S. The effect of increased yeast alcohol acetyltransferase and esterase activity on the flavour profiles of wine and distillates. Yeast 2006, 23, 641–659. [Google Scholar] [CrossRef]
- Miyake, T.; Shibamoto, T. Quantitative analysis of acetaldehyde in foods and beverages. J. Agric. Food Chem. 1993, 41, 1968–1970. [Google Scholar] [CrossRef]
- Guth, H. Identification of Character Impact Odorants of Different White Wine Varieties. J. Agric. Food Chem. 1997, 45, 3022–3026. [Google Scholar] [CrossRef]
- Ferreira, V.; Ortín, N.; Escudero, A.; López, R.; Cacho, J. Chemical Characterization of the Aroma of Grenache Rosé Wines: Aroma Extract Dilution Analysis, Quantitative Determination, and Sensory Reconstitution Studies. J. Agric. Food Chem. 2002, 50, 4048–4054. [Google Scholar] [CrossRef]
- Lopez, R.; Ortín, N.; Pérez-Trujillo, J.P.; Cacho, J.; Ferreira, V. Impact Odorants of Different Young White Wines from the Canary Islands. J. Agric. Food Chem. 2003, 51, 3419–3425. [Google Scholar] [CrossRef]
- Peinado, R.A.; Moreno, J.; Bueno, J.E.; Moreno, J.A.; Mauricio, J.C.G. Comparative study of aromatic compounds in two young white wines subjected to pre-fermentative cryomaceration. Food Chem. 2004, 84, 585–590. [Google Scholar] [CrossRef]
- Moreno, J.A.; Zea, L.; Moyano, L.; Medina, M. Aroma compounds as markers of the changes in sherry wines subjected to biological ageing. Food Control 2005, 16, 333–338. [Google Scholar] [CrossRef]
- Peng, C.-T.; Wen, Y.; Tao, Y.-S.; Lan, Y.-Y. Modulating the Formation of Meili Wine Aroma by Prefermentative Freezing Process. J. Agric. Food Chem. 2013, 61, 1542–1553. [Google Scholar] [CrossRef] [PubMed]
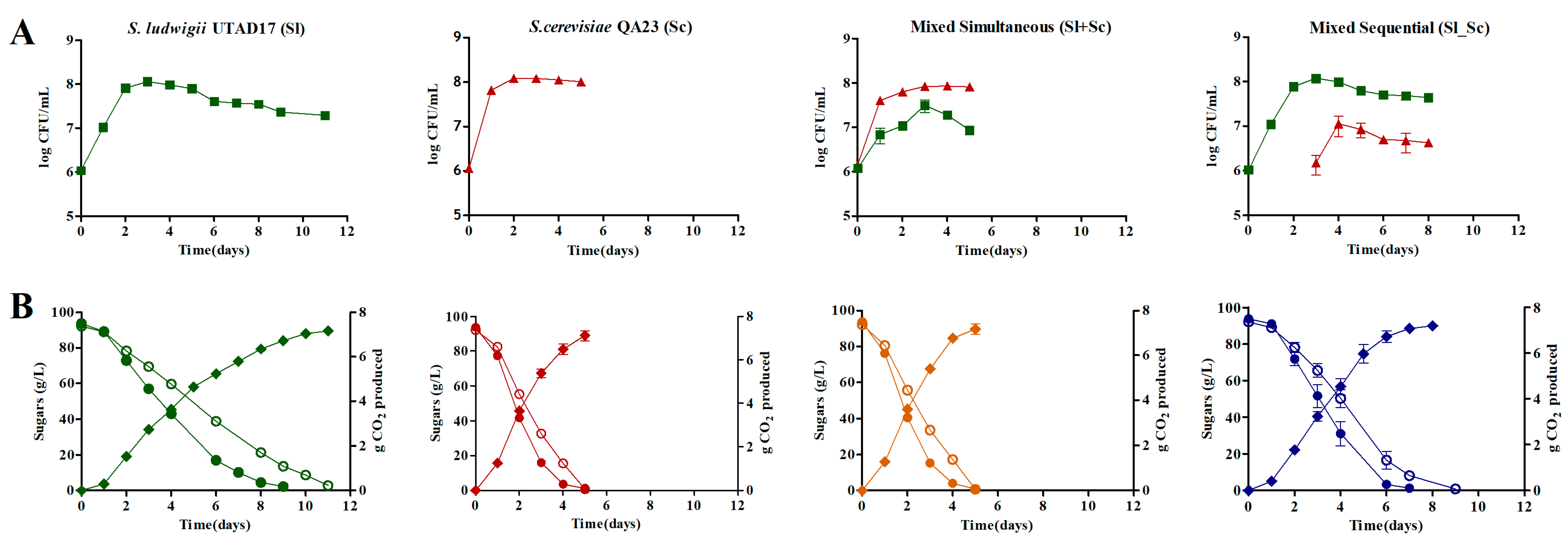
| Compound | Grape-Must | Sl | Sc | Sl+Sc | Sl_Sc |
|---|---|---|---|---|---|
| Sugars (g L−1) | 182.140 ± 3.62 | 2.273 ± 0.733 a | 0.328 ± 0.284 b | 0.190 ± 0.242 b | 0.018 ± 0.009 b |
| Ethanol (% v:v−1) | - | 10.195 ± 0.194 a | 10.391 ± 0.025 a | 10.201 ± 0.077 a | 10.355 ± 0.271 a |
| Glycerol (g L−1) | - | 6.279 ± 0.024 c | 7.671 ± 0.060 ab | 7.659 ± 0.302 a | 6.800 ± 0.658 bc |
| Acetic Acid (g L−1) | - | 0.138 ± 0.004 bc | 0.170 ± 0.018 a | 0.149 ± 0.004 ab | 0.120 ± 0.011 c |
| Titratable Acidity (g L−1) | 8.010 ± 0.350 | 8.100 ± 0.120 ab | 7.980 ± 0.000 a | 8.390 ± 0.010 a | 7.690 ± 0.130 b |
| Total SO2 (mg L−1) | - | 14.830 ± 0.430 a | 15.300 ± 0.820 a | 15.360 ± 0.000 a | 16.380 ± 3.020 a |
| pH | 2.990 ± 0.011 | 2.957 ± 0.003 a | 2.926 ± 0.031 b | 2.961 ± 0.000 a | 2.956 ± 0.004 a |
| YAN (mg L−1) | 196.869 ± 2.339 | 61.214 ± 5.029 a | 5.250 ± 0.354 c | 4.000 ± 0.000 c | 22.594 ± 6.731 b |
| YAN(72 h) (mg L−1) | - | 79.584 ± 1.996 a | 12.250 ± 3.889 b | 13.168 ± 0.289 b | 68.418 ± 19.340 a |
| PAN (mg L−1) | 92.000 ± 9.899 | 20.500 ± 2.121 a | 5.250 ± 0.354 b | 4.000 ± 0.000 b | 19.167 ± 4.537 a |
| PAN(72 h) (mg L−1) | - | 29.000 ± 1.414 a | 12.250 ± 3.889 b | 13.168 ± 0.289 b | 26.333 ± 4.646 a |
| NH4 (mg L−1) | 127.500 ± 9.192 | 49.500 ± 3.536 a | nd c | nd c | 4.170 ± 2.843 b |
| NH4(72 h) (mg L−1) | - | 61.500 ± 0.707 a | nd b | nd b | 51.167 ± 17.905 a |
| Sugars 72 h (g L−1) | - | 109.252 ± 1.555 a | 41.174 ± 0.246 b | 42.883 ± 2.115 b | 101.200 ± 11.232 a |
| MaxFR (g of CO2 V h−1). | - | 0.051 ± 0.004 b | 0.100 ± 0.005 a | 0.098 ± 0.002 a | 0.058 ± 0.006 b |
| FP | - | 0.014 ± 0.001 a,b | 0.017 ± 0.002 a | 0.015 ± 0.001 a | 0.011 ± 0.002 b |
| Compound (mg L−1) | Sl | Sc | Sl+Sc | Sl_Sc | OT (mg/L) | OD |
|---|---|---|---|---|---|---|
| Alcohols | ||||||
| 1-propanol | 14.315 ± 0.402 c | 44.383 ± 1.298 a | 43.985 ± 0.516 a | 17.934 ± 1.557 b | 306.000 | Alcohol, ripe fruit |
| 1-butanol | 35.428 ± 1.045 a | 15.040 ± 0.220 c | 15.335 ± 1.428 c | 30.792 ± 0.316 b | 150.000 | Medicinal |
| 2-Methyl-1-butanol | 17.882 ± 1.033 ab | 19.040 ± 0.721 a | 18.966 ± 1.087 a | 15.123 ± 0.436 b | 30.000 | Alcohol, nail polish |
| 3-Methyl-1-butanol | 74.963 ± 6.858 b | 102.984 ± 3.465 a | 101.176 ± 1.794 a | 69.184 ± 1.217 b | 30.000 | Whiskey, nail polish |
| 2-Phenylethanol | 18.945 ± 0.728 b | 27.040 ± 1.881 a | 26.997 ± 1.398 a | 16.869 ± 1.015 b | 14.000 | Rose, honey |
| ⅀ | 161.534 ± 8.609 b | 208.486 ± 3.824 a | 206.459 ± 6.122 a | 149.962 ± 4.092 b | ||
| Acetate Esters | ||||||
| Phenylethyl Acetate | ndb | 0.181 ± 0.011 a | 0.177 ± 0.024 a | ndb | 0.250 | Flowery |
| Isoamyl Acetate | ndc | 0.807 ± 0.106 a | 0.725 ± 0.155 a | 0.171 ± 0.120 b | 0.030 | Banana |
| ⅀ | ndc | 0.988 ± 0.117 a | 0.902 ± 0.163 a | 0.171 ± 0.120 b | ||
| Ethyl Esters | ||||||
| Ethyl Acetate | 32.679 ± 6.895 b | 40.995 ± 0.393 ab | 47.392 ± 7.071 a | 28.387 ± 5.742 b | 7.500 | Fruity, vinegar, nail polish, acetic |
| Ethyl Butanoate | ndb | 0.604 ± 0.182 a | 0.630 ± 0.066 a | 0.669 ± 0.118 a | 0.020 | Apple, strawberry, fruity |
| Ethyl Hexanoate | ndd | 0.301 ± 0.012 a | 0.231 ± 0.024 b | 0.082 ± 0.020 c | 0.005 | Green apple, fruity |
| Ethyl Octanoate | ndb | 0.312 ± 0.046 a | 0.410 ± 0.080 a | ndb | 0.002 | Pear, fruity |
| ⅀ (except ethyl acetate) | ndc | 1.216 ± 0.149 a | 1.271 ± 0.160 a | 0.752 ± 0.132 b | ||
| Fatty Acids | ||||||
| Isobutyric Acid | 2.430 ± 0.072 a | 1.077 ± 0.194 b | 1.242 ± 0.205 b | 2.494 ± 0.172 a | 2.300 | Fatty |
| Butyric Acid | 1.892 ± 0.074 a | 0.631 ± 0.089 b | 0.726 ± 0.056 b | 1.880 ± 0.074 a | 10.000 | Fatty, rancid |
| Isovaleric Acid | ndb | 0.231 ± 0.040 a | 0.251 ± 0.045 a | ndb | 0.033 | Fatty, rancid |
| Hexanoic Acid | 0.591 ± 0.023 c | 1.435 ± 0.064 a | 1.350 ± 0.027 b | 0.558 ± 0.034 c | 0.420 | Cheese, fatty |
| Octanoic Acid | 0.727 ± 0.175 c | 2.383 ± 0.060 a | 1.559 ± 0.127 b | 0.710 ± 0.145 c | 0.500 | Fatty, unpleasant |
| Decanoic Acid | 0.222 ± 0.005 a | 0.224 ± 0.031 a | nd b | nd b | 1.000 | Fat, rancid |
| ⅀ | 5.861 ± 0.293 a | 5.980 ±0.475 a | 5.127 ± 0.145 b | 5.642 ± 0.329 ab | ||
| Acetaldehyde | 20.279 ± 0.472 b | 25.328 ± 1.400 a | 25.661 ± 1.500 a | 25.944 ± 2.672 a | 10.000 | Sherry, nutty, bruised apple |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esteves, M.; Barbosa, C.; Vasconcelos, I.; Tavares, M.J.; Mendes-Faia, A.; Pereira Mira, N.; Mendes-Ferreira, A. Characterizing the Potential of the Non-Conventional Yeast Saccharomycodes ludwigii UTAD17 in Winemaking. Microorganisms 2019, 7, 478. https://doi.org/10.3390/microorganisms7110478
Esteves M, Barbosa C, Vasconcelos I, Tavares MJ, Mendes-Faia A, Pereira Mira N, Mendes-Ferreira A. Characterizing the Potential of the Non-Conventional Yeast Saccharomycodes ludwigii UTAD17 in Winemaking. Microorganisms. 2019; 7(11):478. https://doi.org/10.3390/microorganisms7110478
Chicago/Turabian StyleEsteves, Marcos, Catarina Barbosa, Isabel Vasconcelos, Maria João Tavares, Arlete Mendes-Faia, Nuno Pereira Mira, and Ana Mendes-Ferreira. 2019. "Characterizing the Potential of the Non-Conventional Yeast Saccharomycodes ludwigii UTAD17 in Winemaking" Microorganisms 7, no. 11: 478. https://doi.org/10.3390/microorganisms7110478
APA StyleEsteves, M., Barbosa, C., Vasconcelos, I., Tavares, M. J., Mendes-Faia, A., Pereira Mira, N., & Mendes-Ferreira, A. (2019). Characterizing the Potential of the Non-Conventional Yeast Saccharomycodes ludwigii UTAD17 in Winemaking. Microorganisms, 7(11), 478. https://doi.org/10.3390/microorganisms7110478





