Transposition of Insertion Sequences was Triggered by Oxidative Stress in Radiation-Resistant Bacterium Deinococcus geothermalis
Abstract
1. Introduction
2. Materials and Methods
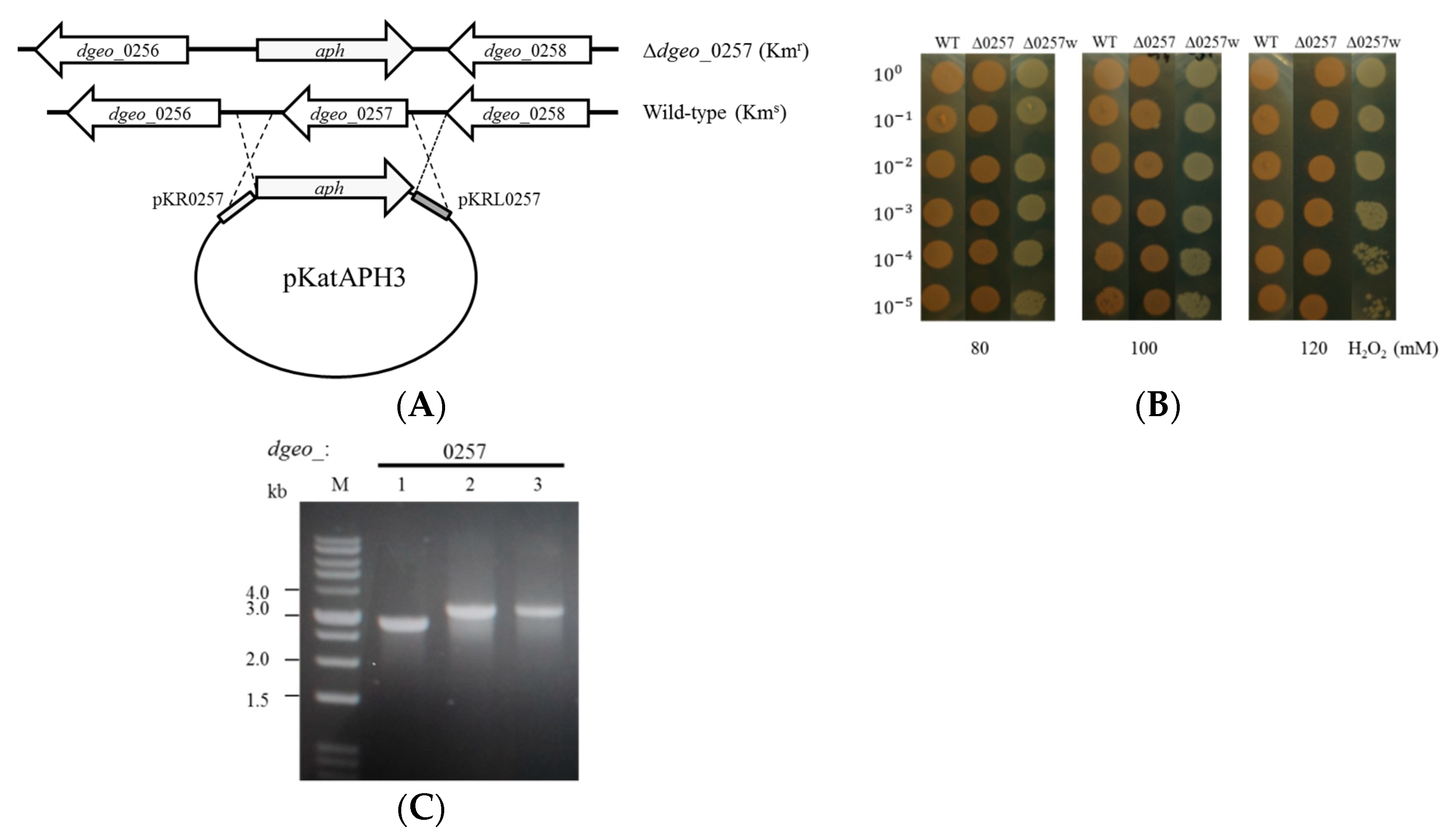
2.1. Bacterial Strains, Culture Conditions, and Construction of the Mutant
2.2. Viability Test Studies in D. Geothermalis
2.3. PCR Detection of Transposition and Sequence Analysis
2.4. RNA-Seq Analysis
2.5. Quantitative Reverse Transcriptase (qRT) PCR
3. Results
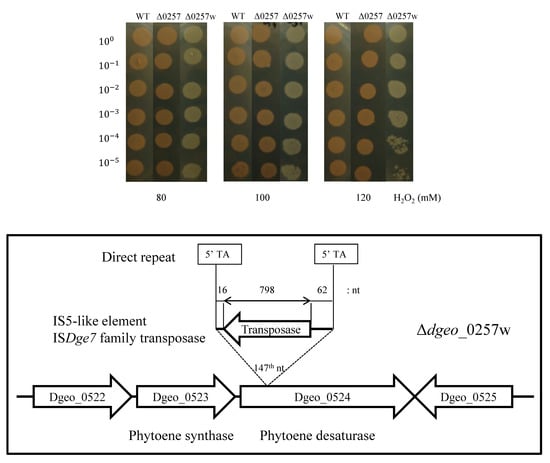
3.1. Construction of the D. Geothermalis Δdgeo_0257 Mutant Strain
3.2. The Effect of H2O2 on the Viability of the Δdgeo_0257 Mutant Strain
3.3. Detection of Transposition in Non-Pigmented Strain
3.4. RNA-Seq Analysis of Δdgeo_0257 and Δdgeo_0257w and Identification of New Transpositions
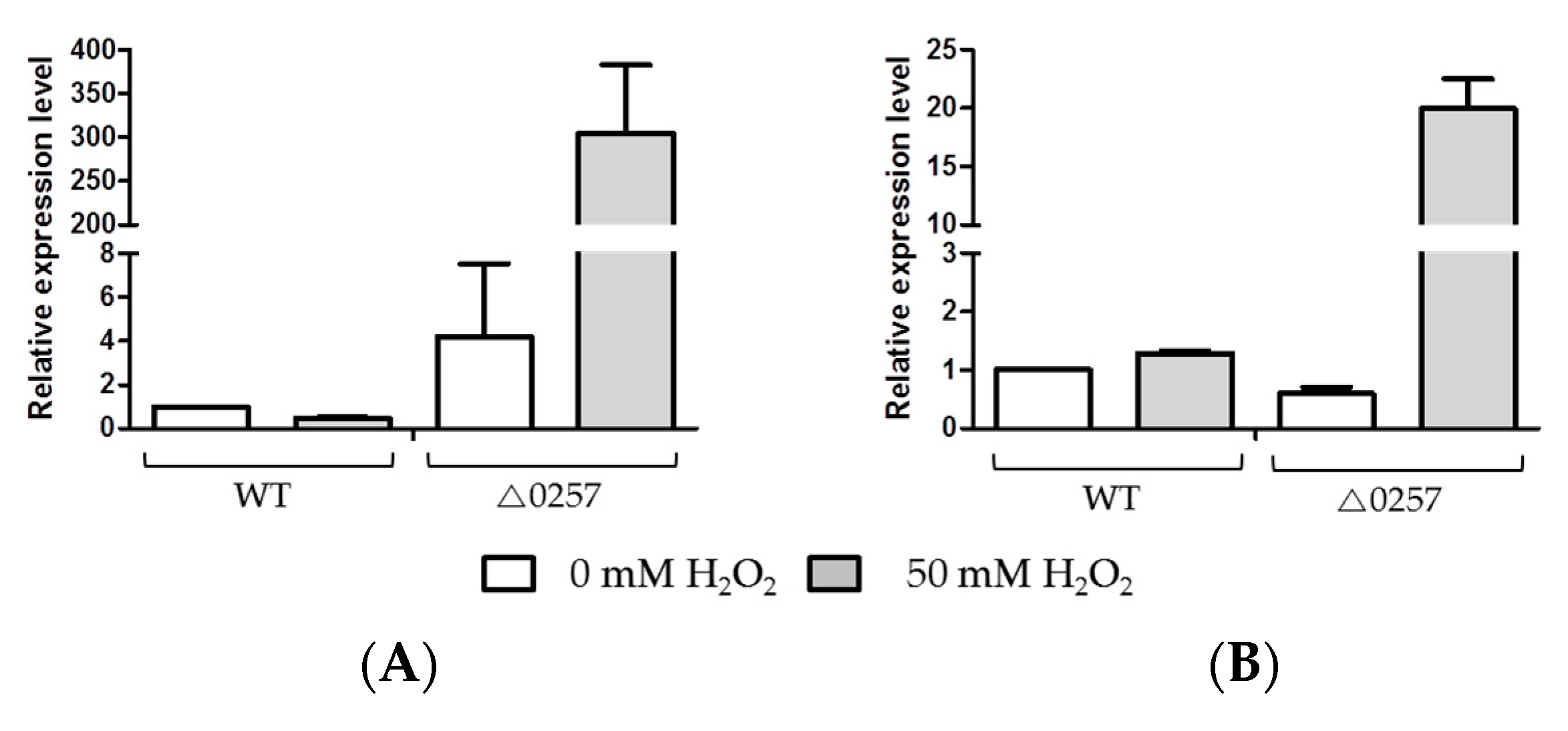
3.5. Detection of Expression Levels of Transposases on Oxidative Stress Condition
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cox, M.M.; Battista, J.R. Deinococcus radiodurans—The consummate survivor. Nature Rev. Microbiol. 2005, 3, 882–892. [Google Scholar] [CrossRef] [PubMed]
- Slade, D.; Radman, M. Oxidative stress resistance in Deinococcus radiodurans. Microbiol. Mol. Biol. Rev. 2011, 75, 133–191. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.P.; Mitchell, E.P.; Franquelim, H.G.; Castanho, M.A.R.B.; Abreu, I.A.; Romao, C.V. Dps from Deinococcus radiodurans: Oligomeric forms of Dps1 with distinct cellular functions and Dps2 involved in metal storage. FEBS J. 2015, 282, 4307–4327. [Google Scholar] [CrossRef] [PubMed]
- Battista, J.R.; Earl, A.M.; Park, M.J. Why is Deinococcus radiodurans so resistant to ionizing radiation? Trends Microbiol. 1999, 7, 362–365. [Google Scholar] [CrossRef]
- Agapov, A.A.; Kulbachinskiy, A.V. Mechanisms of stress resistance and gene regulation in the radioresistant bacterium Deinococcus radiodurans. Biochem. (Mosc). 2015, 80, 1201–1216. [Google Scholar] [CrossRef]
- Makarova, K.S.; Omelchenko, M.V.; Gaidamakova, E.K.; Matrosova, V.Y.; Vasilenko, A.; Zhai, M.; Lapidus, A.; Copeland, A.; Kim, E.; Land, M.; et al. Deinococcus geothermalis: The pool of extreme radiation resistance genes shrinks. PLoS ONE 2007, 2, e955. [Google Scholar]
- Lim, S.; Jung, J.; Blanchard, L.; de Groot, A. Conservation and diversity of radiation and oxidative stress resistance mechanisms in Deinococcus species. FEMS Microbiol. Rev. 2019, 43, 19–52. [Google Scholar] [CrossRef]
- Almiron, M.; Link, A.J.; Furlong, D.; Kolter, R. A novel DNA binding protein with regulatory and protective roles in starved Escherichia coli. Genes Dev. 1992, 6, 2646–2654. [Google Scholar] [CrossRef]
- Altuvia, S.; Almiron, M.; Huisman, G.; Kolter, R.; Storz, G. The dps promoter is activated by OxyR during growth and by IHF and in stationary phase. Mol. Microbiol. 1994, 13, 265–272. [Google Scholar] [CrossRef]
- Ceci, P.; Cellai, S.; Falvo, E.; Rivetti, C.; Rossi, G.L.; Chiancone, E. DNA condensation and self-aggregation of Escherichia coli Dps are coupled phenomena related to the properties of the N-terminus. Nucleic Acid Res. 2004, 32, 5935–5944. [Google Scholar] [CrossRef]
- Calhoun, L.N.; Kwon, Y.M. Structure, function and regulation of the DNA-binding protein Dps and its role in acid and oxidative stress resistance in Escherichia coli: A review. J. Appl. Microbiol. 2011, 110, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.H.; Smith, L.T.; Xiao, L.; Bhattacharyya, G.; Grove, A. On the stoichiometry of Deinococcus radiodurans Dps-1 binding to duplex DNA. Proteins 2012, 80, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.C.; Nobre, M.F.; Rainey, F.A.; Silva, M.T.; Wait, R.; Burghardt, J.; Chung, A.P.; da Costa, M.S. Deinococcus geothermalis sp. nov. and Deinococcus murrayi sp. nov., two extremely radiation-resistant and slightly thermophilic species from hot springs. Int. J. Syst. Bacteriol. 1997, 47, 939–947. [Google Scholar] [PubMed]
- Mahillon, J.; Chandler, M. Insertion sequences. Microbiol. Mol. Biol. Rev. 1998, 62, 725–774. [Google Scholar] [PubMed]
- Siguier, P.; Perochon, J.; Lestrade, L.; Mahillon, J.; Chandler, M. ISfinder: The reference centre for bacterial insertion sequences. Nucleic Acids Res. 2006, 34, D32–D36. [Google Scholar] [CrossRef] [PubMed]
- Blesa, A.; Sanchez, M.; Sacristan-Horcajada, E.; Fuente, S.G.; Peiro, R.; Berenguer, J. Into the Thermus mobilome: presence, diversity and recent activities of insertion sequences across Thermus spp. Microorganisms 2019, 7, 25. [Google Scholar] [CrossRef] [PubMed]
- Pasternak, C.; Ton-Hoang, B.; Coste, G.; Bailone, A.; Chandler, M.; Sommer, S. Irradiation-induced Deinococcus radiodurans genome fragmentation triggers transposition of a single resident insertion sequence. PLoS Genet. 2010, 6, e1000799. [Google Scholar] [CrossRef]
- Ohba, H.; Satoh, K.; Yanagisawa, T.; Narumi, I. The radiation responsive promoter of the Deinococcus radiodurans pprA gene. Gene 2005, 363, 133–141. [Google Scholar] [CrossRef]
- Brim, H.; Venkateswaran, A.; Kostandarithes, H.M.; Fredrickson, J.K.; Daly, M.J. Engineering Deinococcus geothermalis for bioremediation of high-temperature radioactive waste environments. Appl. Environ. Microbiol. 2003, 69, 4575–4582. [Google Scholar] [CrossRef]
- Kim, M.; Jeong, S.; Lim, S.; Sim, J.; Rhie, H.G.; Lee, S.J. Oxidative stress response of Deinococcus geothermalis via a cystine importer. J. Microbiol. 2017, 55, 137–146. [Google Scholar] [CrossRef]
- Tian, B.; Hua, Y. Carotenoid biosynthesis in extremeophilic Deinococcus-Thermus bacteria. Trends Microbiol. 2010, 18, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Sawyer, S.A.; Dykhuizen, D.E.; DuBose, R.F.; Green, L.; Mutangadura-Mhlanga, T.; Wolczyk, D.F.; Hartl, D.L. Distribution and abundance of insertion sequences among natural isolates of Escherichia coli. Genetics 1987, 115, 51–63. [Google Scholar] [PubMed]
- Siguier, P.; Gourbeyre, E.; Chandler, M. Bacterial insertion sequences: Their genomic impact and diversity. FEMS Rev. 2014, 38, 865–891. [Google Scholar] [CrossRef] [PubMed]
- Siguier, P.; Gourbeyre, E.; Varani, A.; Ton-Hoang, B.; Chandler, M. Everyman’s guide to bacterial insertion sequences. Microbiol. Spectrum. 2015, 3, MDNA3-0030-2014. [Google Scholar]
- Palmenaer, D.D.; Siguier, P.; Mahillon, J. IS4 family goes genomic. BMC Evol. Biol. 2008, 8, 18. [Google Scholar] [CrossRef] [PubMed]
- Guerillot, R.; Siguier, P.; Gourbeyre, E.; Chandler, M.; Glaser, P. The diversity of prokaryotic DDE transposases of the mutator superfamily, insertion specificity, and association with conjugation machineries. Genome Biol. Evol. 2014, 6, 260–272. [Google Scholar] [CrossRef] [PubMed]
- Vandecraen, J.; Chandler, M.; Aertsen, A.; Van Houdt, R. The impact of insertion sequences on bacterial genome plasticity and adaptability. Crit. Rev. Microbiol. 2017, 43, 709–730. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-López, M.; García-Pérez, J.L. DNA transposons: nature and applications in genomics. Curr. Genom. 2010, 11, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Sousa, A.; Bourgard, C.; Wahl, L.M.; Gordo, I. Rates of transposition in Escherichia coli. Biol. Lett. 2018, 9, 20130838. [Google Scholar] [CrossRef] [PubMed]
- Narumi, I.; Cherdchu, K.; Kitayama, S.; Watanabe, H. The Deinococcus radiodurans uvrA gene: identification of mutation sites in two mitomycin-sensitive strains and the first discovery of insertion sequence element from deinobacteria. Gene 1997, 198, 115–126. [Google Scholar] [CrossRef]
- Hua, Y.; Narumi, I.; Gao, G.; Tian, B.; Satoh, K.; Kitayama, S.; Shen, S. PprI: A general switch responsible for extreme radioresistance of Deinococcus radiodurans. Biochem. Biophys. Res. Commu. 2003, 306, 354–360. [Google Scholar] [CrossRef]
- Mennecier, S.; Servant, P.; Coste, G.; Bailone, A.; Sommer, S. Mutagenesis via IS transposition in Deinococcus radiodurans. Mol. Microbiol. 2006, 59, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Ohtsubo, Y.; Genka, H.; Komatsu, H.; Nagata, Y.; Tsuda, M. High-temperature-induced transposition of insertion elements in Burkholderia multivorans ATCC17616. Appl. Environ. Microbiol. 2005, 71, 1822–1828. [Google Scholar] [CrossRef] [PubMed][Green Version]

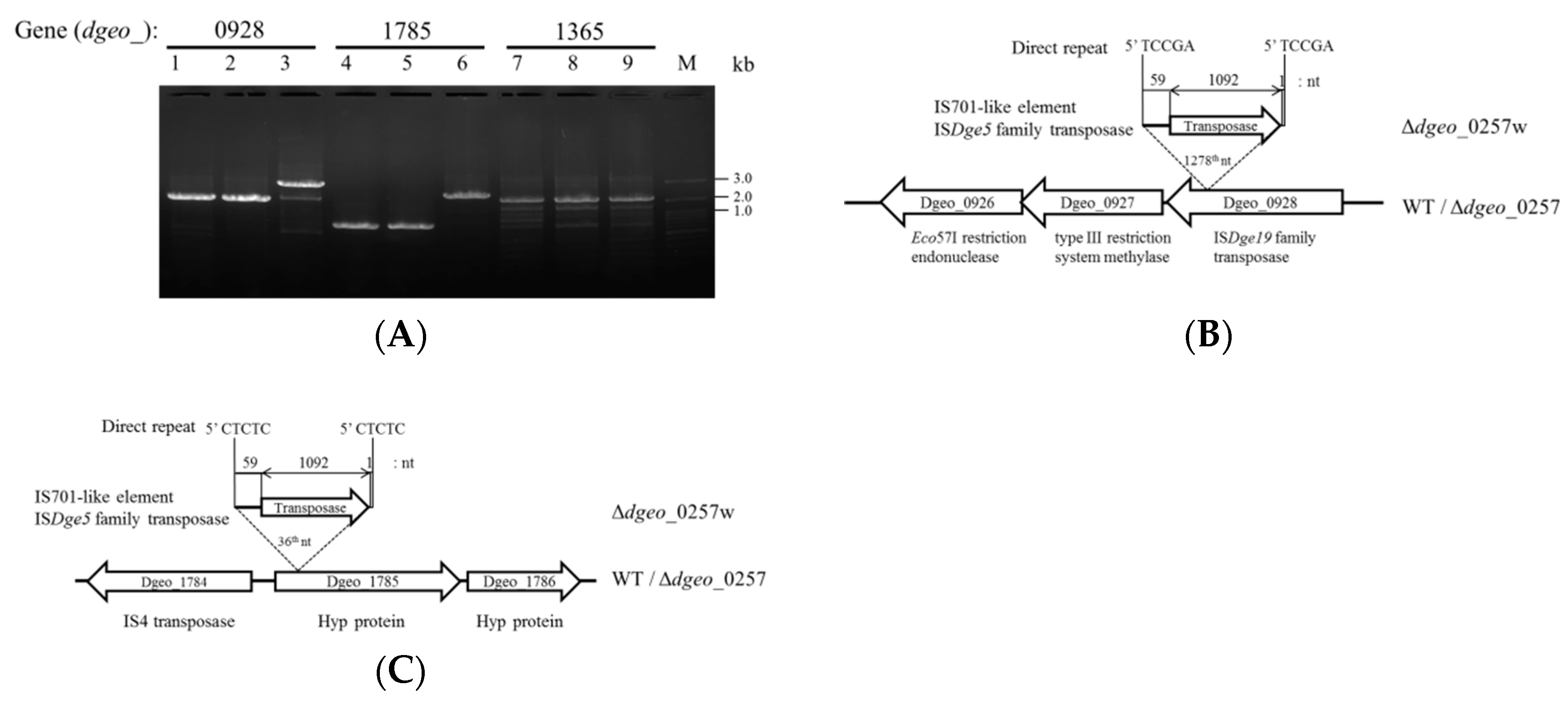
| Gene | Δ0257/WT | Δ0257w/WT | Δ0257w/Δ0257 |
|---|---|---|---|
| dgeo_0927 | 2.35 | 0.21 | 0.09 |
| dgeo_0926 | 2.10 | 0.28 | 0.13 |
| dgeo_1785 | 0.61 | 0.09 | 0.15 |
| dgeo_1365 | 1.58 | 0.45 | 0.28 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, C.; Choi, N.; Bae, M.K.; Choo, K.; Lee, S.-J. Transposition of Insertion Sequences was Triggered by Oxidative Stress in Radiation-Resistant Bacterium Deinococcus geothermalis. Microorganisms 2019, 7, 446. https://doi.org/10.3390/microorganisms7100446
Lee C, Choi N, Bae MK, Choo K, Lee S-J. Transposition of Insertion Sequences was Triggered by Oxidative Stress in Radiation-Resistant Bacterium Deinococcus geothermalis. Microorganisms. 2019; 7(10):446. https://doi.org/10.3390/microorganisms7100446
Chicago/Turabian StyleLee, Chanjae, Nakjun Choi, Min K. Bae, Kyungsil Choo, and Sung-Jae Lee. 2019. "Transposition of Insertion Sequences was Triggered by Oxidative Stress in Radiation-Resistant Bacterium Deinococcus geothermalis" Microorganisms 7, no. 10: 446. https://doi.org/10.3390/microorganisms7100446
APA StyleLee, C., Choi, N., Bae, M. K., Choo, K., & Lee, S.-J. (2019). Transposition of Insertion Sequences was Triggered by Oxidative Stress in Radiation-Resistant Bacterium Deinococcus geothermalis. Microorganisms, 7(10), 446. https://doi.org/10.3390/microorganisms7100446