Taking Advantage of Bacterial Adaptation in Order to Optimize Industrial Production of Dry Propionibacterium freudenreichii
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Pre-Culture
2.2. Optimization of P. freudenreichii Resistance to Heat and Oxidative Challenges
2.2.1. Growth and Adaptation Conditions in the Experimental Design
2.2.2. Stress Challenges
2.3. Identification and Quantification of Compatible Solutes Accumulated by P. freudenreichii CIRM−BIA 129
2.3.1. Extraction of Accumulated Compatible Solutes
2.3.2. NMR (Nuclear Magnetic Resonance) Analyses
2.4. Spray Drying and Powder Storage
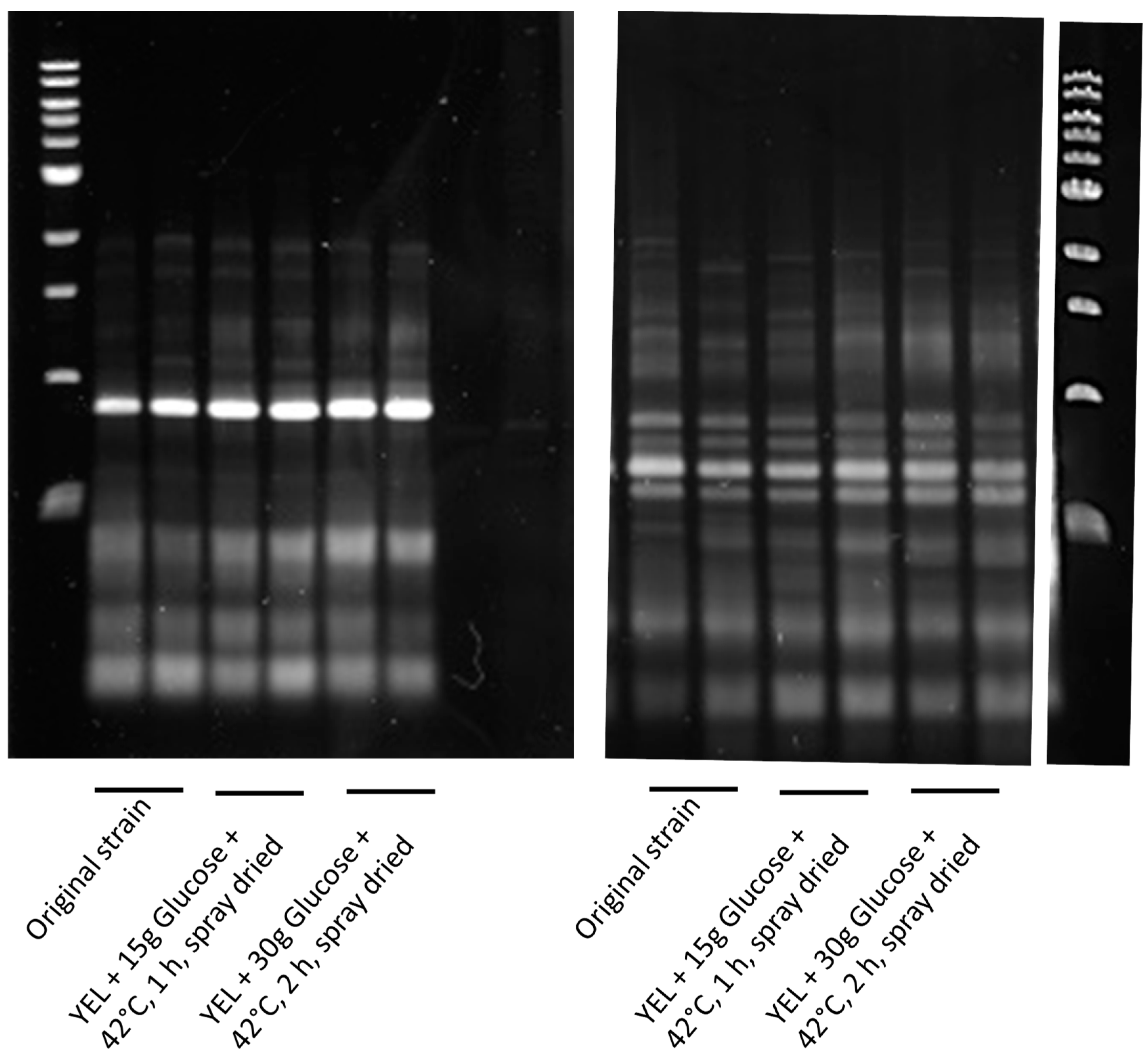
2.5. Random Amplified Polymorphic DNA (RAPD) Analysis
2.6. Revivification of dried P. freudenreichii in a cheese-like dairy medium
2.6.1. Culture with Dried P. freudenreichii in a Cheese-Like Dairy Medium
2.6.2. Organic Acids Quantification
2.7. Statistical Analysis
3. Results
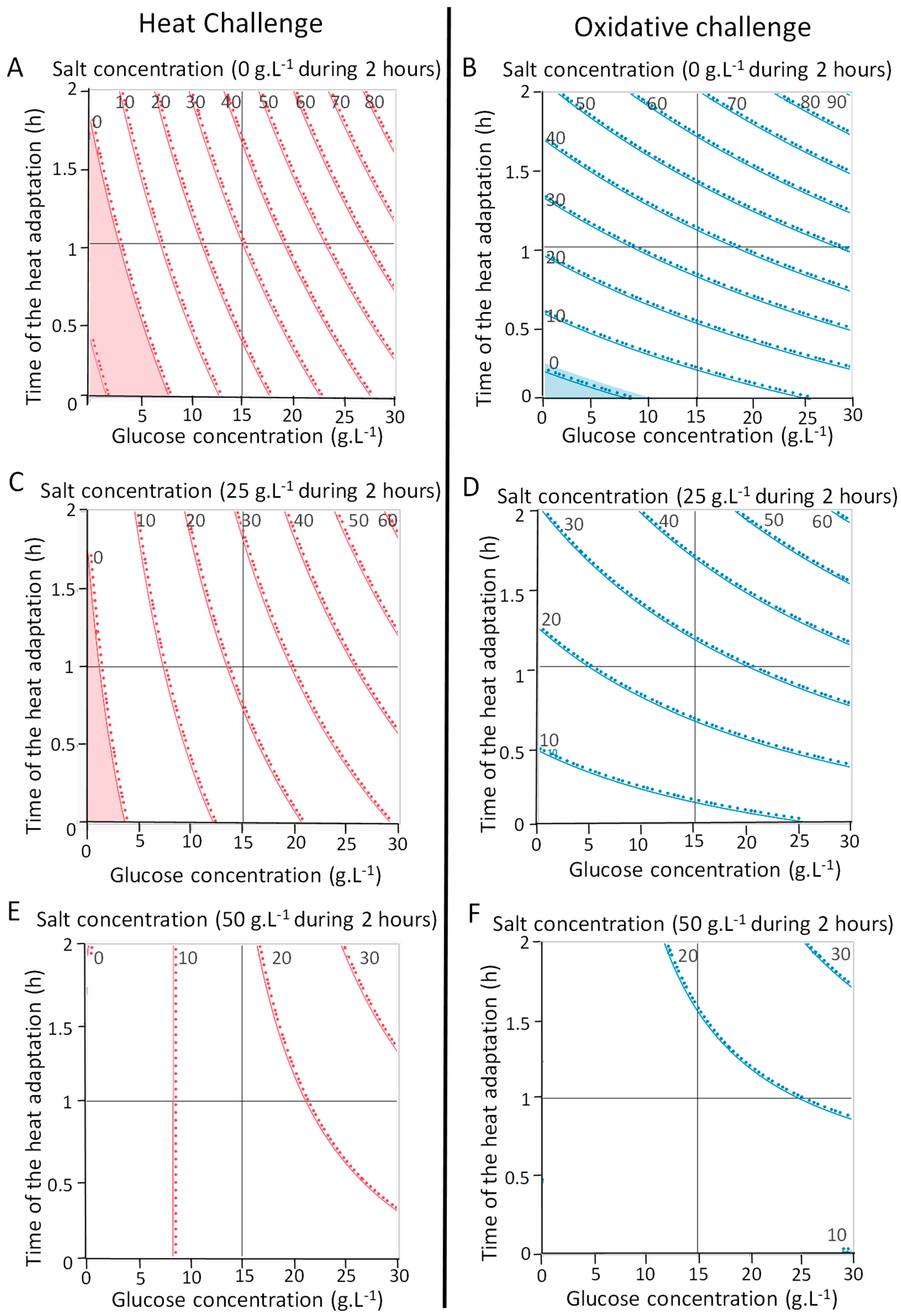
3.1. Optimization of P. freudenreichii Stress Tolerance with an Experimental Design
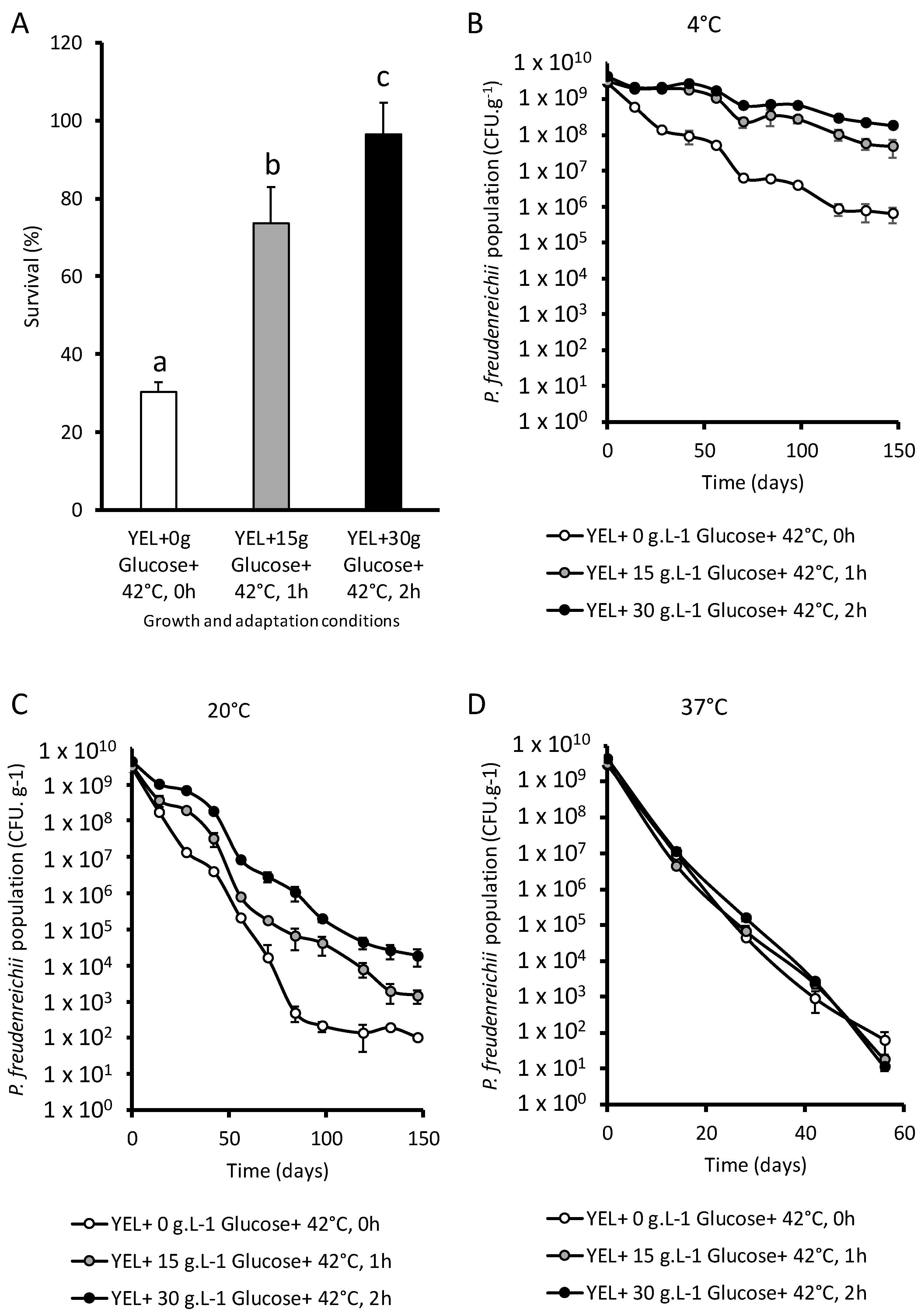
3.2. P. freudenreichii Viability after Spray Drying and Storage
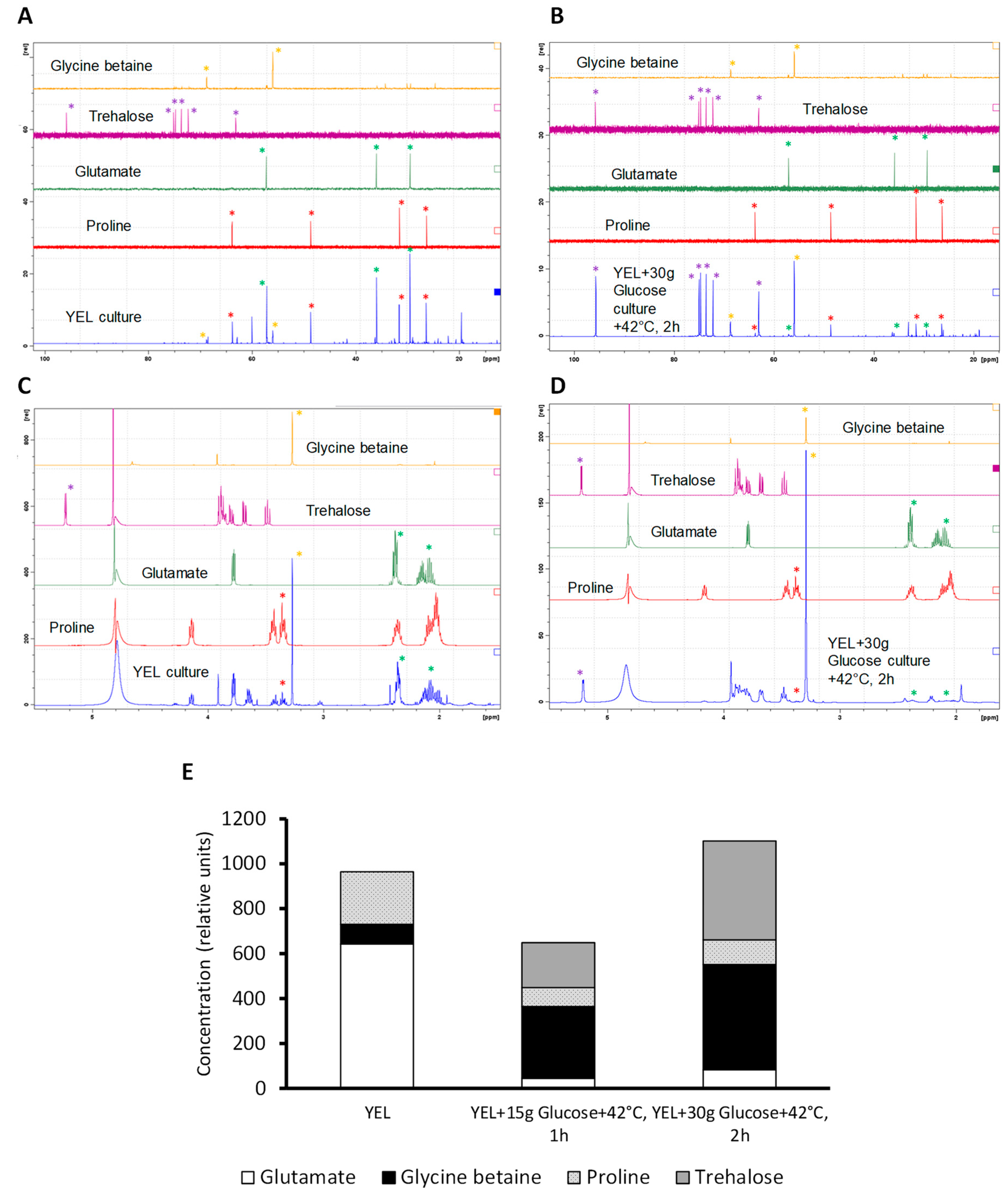
3.3. Compatible Solutes Accumulation Depends on P. freudenreichii Growth Medium and Adaptation Conditions
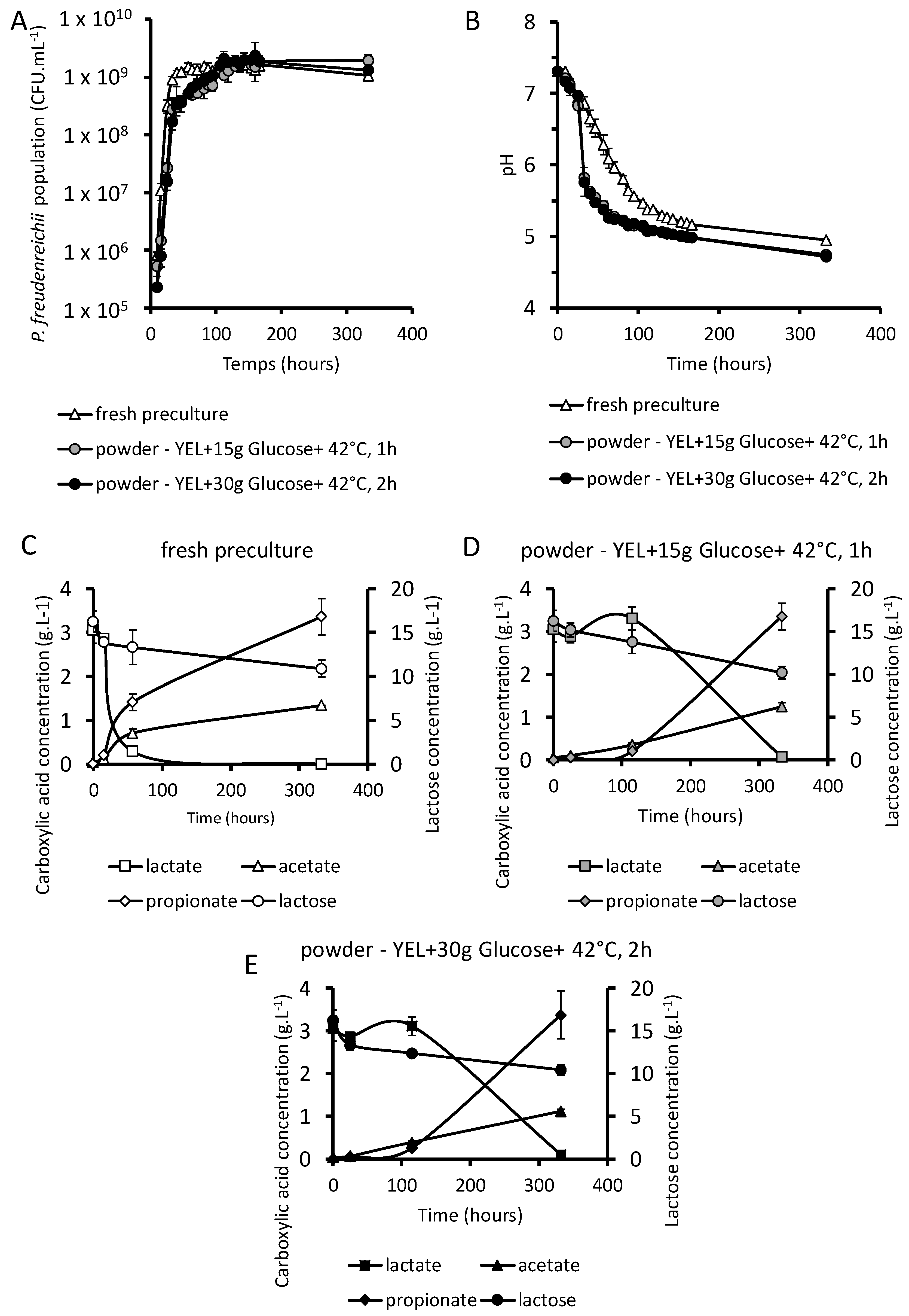
3.4. P. freudenreichii Revivification
3.4.1. Growth of Dried P. freudenreichii in a Cheese-Like Medium
3.4.2. Fate of Saccharides and Organic Acids
4. Discussion
4.1. Addition of Glucose and Heat-Adaptation Both Induce Cross-Protections to P. freudenreichii
4.2. Combining Glucose Aaddition and Thermo-Adaptation Increases P. freudenreichii Viability during Spray Drying and Storage
4.3. Combining Glucose Addition and Thermo-Adaptation Leads to Compatible Solutes Accumulation and Increases P. freudenreichii Viability to Spray Drying
4.4. Combining Glucose Addition and Heat Adaptation Leads to Production of Efficient Propionic Starter
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Moco, S.; Candela, M.; Chuang, E.; Draper, C.; Cominetti, O.; Montoliu, I.; Barron, D.; Kussmann, M.; Brigidi, P.; Gionchetti, P.; et al. Systems biology approaches for inflammatory bowel disease: Emphasis on gut microbial metabolism. Inflamm. Bowel Dis. 2014, 20, 2104–2114. [Google Scholar] [CrossRef] [PubMed]
- Soularue, E.; Lepage, P.; Colombel, J.F.; Coutzac, C.; Faleck, D.; Marthey, L.; Collins, M.; Chaput, N.; Robert, C.; Carbonnel, F. Enterocolitis due to immune checkpoint inhibitors: A systematic review. Gut 2018, 67, 2056–2067. [Google Scholar] [CrossRef] [PubMed]
- Skonieczna-Żydecka, K.; Marlicz, W.; Misera, A.; Koulaouzidis, A.; Łoniewski, I. Microbiome—the missing link in the Ggt-brain axis: Focus on its role in gastrointestinal and mental health. JCM 2018, 7, 521. [Google Scholar] [CrossRef] [PubMed]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Plé, C.; Breton, J.; Richoux, R.; Nurdin, M.; Deutsch, S.-M.; Falentin, H.; Hervé, C.; Chuat, V.; Lemée, R.; Maguin, E.; et al. Combining selected immunomodulatory Propionibacterium freudenreichii and Lactobacillus delbrueckii strains: Reverse engineering development of an anti-inflammatory cheese. Mol. Nutr. Food Res. 2016, 60, 935–948. [Google Scholar] [CrossRef] [PubMed]
- Ebner, S.; Smug, L.N.; Kneifel, W.; Salminen, S.J.; Sanders, M.E. Probiotics in dietary guidelines and clinical recommendations outside the European Union. WJG 2014, 20, 16095–16100. [Google Scholar] [CrossRef] [PubMed]
- FAO/WHO Evaluation of Health and Nutritional Properties of Powder Milk and Live Lactic Acid Bacteria. Food and Agriculture Organization of the United Nations and World Health Organization Expert Consultation Report 2001. Available online: http://www.fao.org/3/a-a0512e.pdf (accessed on 22 October 2019).
- Fung, W.-Y.; Lye, H.-S.; Lim, T.-J.; Kuan, C.-Y.; Liong, M.-T. Roles of probiotic on gut health. In Probiotics; Liong, M.-T., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; Volume 21, pp. 139–165. ISBN 978-3-642-20837-9. [Google Scholar]
- Ganji-Arjenaki, M.; Rafieian-Kopaei, M. Probiotics are a good choice in remission of inflammatory bowel diseases: A meta analysis and systematic review. J. Cell. Physiol. 2018, 233, 2091–2103. [Google Scholar] [CrossRef]
- Butel, M.-J.; Waligora-Dupriet, A.-J.; Wydau-Dematteis, S. The developing gut microbiota and its consequences for health. J. Dev. Orig. Health Dis. 2018, 9, 590–597. [Google Scholar] [CrossRef]
- Fujimura, K.E.; Sitarik, A.R.; Havstad, S.; Lin, D.L.; Levan, S.; Fadrosh, D.; Panzer, A.R.; LaMere, B.; Rackaityte, E.; Lukacs, N.W.; et al. Neonatal gut microbiota associates with childhood multisensitized atopy and T cell differentiation. Nat. Med. 2016, 22, 1187–1191. [Google Scholar] [CrossRef]
- Colliou, N.; Ge, Y.; Sahay, B.; Gong, M.; Zadeh, M.; Owen, J.L.; Neu, J.; Farmerie, W.G.; Alonzo, F.; Liu, K.; et al. Commensal Propionibacterium strain UF1 mitigates intestinal inflammation via Th17 cell regulation. J. Clin. Investig. 2017, 127, 3970–3986. [Google Scholar] [CrossRef]
- Chang, H.-Y.; Chen, J.-H.; Chang, J.-H.; Lin, H.-C.; Lin, C.-Y.; Peng, C.-C. Multiple strains probiotics appear to be the most effective probiotics in the prevention of necrotizing enterocolitis and mortality: An updated meta-analysis. PLoS ONE 2017, 12, e0171579. [Google Scholar] [CrossRef] [PubMed]
- Milani, C.; Duranti, S.; Bottacini, F.; Casey, E.; Turroni, F.; Mahony, J.; Belzer, C.; Delgado Palacio, S.; Arboleya Montes, S.; Mancabelli, L.; et al. The first microbial colonizers of the human gut: Composition, activities, and health implications of the infant gut microbiota. Microbiol. Mol. Biol. Rev. 2017, 81, e00036-17. [Google Scholar] [CrossRef]
- Repa, A.; Thanhaeuser, M.; Endress, D.; Weber, M.; Kreissl, A.; Binder, C.; Berger, A.; Haiden, N. Probiotics (Lactobacillus acidophilus and Bifidobacterium bifidum) prevent NEC in VLBW infants fed breast milk but not in formula. Pediatr. Res. 2015, 77, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Bouglé, N.D.; Roland, F. Lebeurrier Effect of Propionibacteria supplementation on fecal Bifidobacteria and segmental colonic transit time in healthy human subjects. Scand. J. Gastroenterol. 1999, 34, 144–148. [Google Scholar]
- Mitsuyama, K.; Masuda, J.; Yamasaki, H.; Kitazaki, S.; Koga, H.; Uchida, M.; Sata, M. Treatment of Ulcerative Colitis with Milk Whey Culture with Propionibacterium freudenreichii 2007. J. Intest. Microbiol. 2007, 21, 143–147. [Google Scholar]
- Hojo, K.; Yoda, N.; Tsuchita, H.; Ohtsu, T.; Seki, K.; Taketomo, N.; Murayama, T.; Iino, H. Effect of ingested culture of Propionibacterium freudenreichii ET-3 on fecal microflora and stool frequency in healthy females. Biosci. Microflora 2002, 21, 115–120. [Google Scholar] [CrossRef]
- Seki, K.; Nakao, H.; Umino, H.; Isshiki, H.; Yoda, N.; Tachihara, R.; Ohuchi, T.; Saruta, H.; Suzuki, K.; Mitsuoka, T. Effects of Fermented Milk Whey Containing Novel Bifidogenic Growth Stimulator Produced by Propionibacterium on Fecal Bacteria, Putrefactive Metabolite, Defecation Frequency and Fecal Properties in Senile Volunteers Needed Serious Nursing-Care Taking Enteral Nutrition by Tube Feeding 2004. J. Intest. Microbiol. 2004, 18, 107–115. [Google Scholar]
- Rabah, H.; Ménard, O.; Gaucher, F.; do Carmo, F.L.R.; Dupont, D.; Jan, G. Cheese matrix protects the immunomodulatory surface protein SlpB of Propionibacterium freudenreichii during in vitro digestion. Food Res. Int. 2018, 106, 712–721. [Google Scholar] [CrossRef] [PubMed]
- Foligne, B.; Deutsch, S.-M.; Breton, J.; Cousin, F.J.; Dewulf, J.; Samson, M.; Pot, B.; Jan, G. Promising immunomodulatory effects of selected strains of dairy propionibacteria as evidenced in vitro and in vivo. Appl. Environ. Microbiol. 2010, 76, 8259–8264. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). The Maintenance of the List of QPS Microorganisms Intentionally Added to Food or Feed-Scientific Opinion of the Panel on Biological Hazards 2008. Available online: https://www.efsa.europa.eu/fr/efsajournal/pub/923 (accessed on 22 October 2019).
- Rabah, H.; Rosa do Carmo, F.; Jan, G. Dairy propionibacteria: Versatile probiotics. Microorganisms 2017, 5, 24. [Google Scholar] [CrossRef]
- Huang, S.; Vignolles, M.-L.; Chen, X.D.; Le Loir, Y.; Jan, G.; Schuck, P.; Jeantet, R. Spray drying of probiotics and other food-grade bacteria: A review. Trends Food Sci. Technol. 2017, 63, 1–17. [Google Scholar] [CrossRef]
- Gaucher, F.; Bonnassie, S.; Rabah, H.; Leverrier, P.; Pottier, S.; Jardin, J.; Briard-Bion, V.; Marchand, P.; Jeantet, R.; Blanc, P.; et al. Benefits and drawbacks of osmotic adjustment in Propionibacterium freudenreichii. J. Proteom. 2019, 204, 103400. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Rabah, H.; Jardin, J.; Briard-Bion, V.; Parayre, S.; Maillard, M.-B.; Le Loir, Y.; Chen, X.D.; Schuck, P.; Jeantet, R.; et al. Hyperconcentrated sweet whey, a new culture medium that enhances Propionibacterium freudenreichii stress tolerance. Appl. Environ. Microbiol. 2016, 82, 4641–4651. [Google Scholar] [CrossRef] [PubMed]
- Jan, G.; Rouault, A.; Maubois, J.-L. Acid stress susceptibility and acid adaptation of Propionibacterium freudenreichii subsp. shermanii. Lait 2000, 80, 325–336. [Google Scholar] [CrossRef]
- Desmond, C.; Stanton, C.; Fitzgerald, G.F.; Collins, K.; Paul Ross, R. Environmental adaptation of probiotic lactobacilli towards improvement of performance during spray drying. Int. Dairy J. 2001, 11, 801–808. [Google Scholar] [CrossRef]
- Csonka, L.N.; Hanson, A.D. Prokaryotic Osmoregulation: Genetics and Physiology. Annu. Rev. Microbiol. 1991, 45, 569–606. [Google Scholar] [CrossRef] [PubMed]
- Boyaval, P.; Deborde, C.; Corre, C.; Blanco, C.; Bégué, É. Stress and osmoprotection in propionibacteria. Lait 1999, 79, 59–69. [Google Scholar] [CrossRef]
- Paéz, R.; Lavari, L.; Vinderola, G.; Audero, G.; Cuatrin, A.; Zaritzky, N.; Reinheimer, J. Effect of heat treatment and spray drying on lactobacilli viability and resistance to simulated gastrointestinal digestion. Food Res. Int. 2012, 48, 748–754. [Google Scholar] [CrossRef]
- Malik, A.C.; Reinbold, G.W.; Vedamuthu, E.R. An evaluation of the taxonomy of Propionibacterium. Can. J. Microbiol. 1968, 14, 1185–1191. [Google Scholar] [CrossRef]
- De Man, J.C.; Rogosa, M.; Sharpe, M.E. A medium for the cultivation of lactobacilli. J. Appl. Bacteriol. 1960, 23, 130–135. [Google Scholar] [CrossRef]
- Demirtas, M.U.; Kolhatkar, A.; Kilbane, J.J. Effect of aeration and agitation on growth rate of Thermus thermophilus in batch mode. J. Biosci. Bioeng. 2003, 95, 113–117. [Google Scholar] [CrossRef]
- Arnaud, J.-P.; Lacroix, C.; Choplin, L. Effect of agitation rate on cell release rate and metabolism during continuous fermentation with entrapped growing: Lactobacillus casei subsp. casei. Biotechnol. Tech. 1992, 6, 265–270. [Google Scholar] [CrossRef]
- Huang, S.; Cauty, C.; Dolivet, A.; Le Loir, Y.; Chen, X.D.; Schuck, P.; Jan, G.; Jeantet, R. Double use of highly concentrated sweet whey to improve the biomass production and viability of spray-dried probiotic bacteria. J. Funct. Foods 2016, 23, 453–463. [Google Scholar] [CrossRef]
- Cousin, F.J.; Louesdon, S.; Maillard, M.-B.; Parayre, S.; Falentin, H.; Deutsch, S.-M.; Boudry, G.; Jan, G. The first dairy product exclusively fermented by Propionibacterium freudenreichii: A new vector to study probiotic potentialities in vivo. Food Microbiol. 2012, 32, 135–146. [Google Scholar] [CrossRef]
- Gagnaire, V.; Jardin, J.; Rabah, H.; Briard-Bion, V.; Jan, G. Emmental cheese environment enhances Propionibacterium freudenreichii stress tolerance. PLoS ONE 2015, 10, e0135780. [Google Scholar] [CrossRef]
- Aburjaile, F.F.; Rohmer, M.; Parrinello, H.; Maillard, M.-B.; Beaucher, E.; Henry, G.; Nicolas, A.; Madec, M.-N.; Thierry, A.; Parayre, S.; et al. Adaptation of Propionibacterium freudenreichii to long-term survival under gradual nutritional shortage. BMC Genom. 2016, 17, 1007. [Google Scholar] [CrossRef]
- Kets, E.; Teunissen, P.; de Bont, J. Effect of compatible solutes on survival of lactic acid bacteria subjected to drying. Appl. Environ. Microbiol. 1996, 62, 259–261. [Google Scholar]
- Cardoso, F.S.; Castro, R.F.; Borges, N.; Santos, H. Biochemical and genetic characterization of the pathways for trehalose metabolism in Propionibacterium freudenreichii, and their role in stress response. Microbiology 2007, 153, 270–280. [Google Scholar] [CrossRef]
- Dalmasso, M.; Aubert, J.; Even, S.; Falentin, H.; Maillard, M.-B.; Parayre, S.; Loux, V.; Tanskanen, J.; Thierry, A. Accumulation of intracellular glycogen and trehalose by Propionibacterium freudenreichii under conditions mimicking cheese ripening in the cold. Appl. Environ. Microbiol. 2012, 78, 6357–6364. [Google Scholar] [CrossRef]
- Behrends, V.; Bundy, J.G.; Williams, H.D. Differences in strategies to combat osmotic stress in Burkholderia cenocepacia elucidated by NMR-based metabolic profiling: B. cenocepacia osmotic stress tolerance. Lett. Appl. Microbiol. 2011, 52, 619–625. [Google Scholar] [CrossRef]
- Pleitner, A.; Zhai, Y.; Winter, R.; Ruan, L.; McMullen, L.M.; Gänzle, M.G. Compatible solutes contribute to heat resistance and ribosome stability in Escherichia coli AW1.7. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2012, 1824, 1351–1357. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, S.; Dev, K.; Sourirajan, A. Distinct osmoadaptation strategies in the strict halophilic and halotolerant bacteria isolated from Lunsu salt water body of north west Himalayas. Curr. Microbiol. 2018, 75, 888–895. [Google Scholar] [CrossRef] [PubMed]
- Weinisch, L.; Kirchner, I.; Grimm, M.; Kühner, S.; Pierik, A.J.; Rosselló-Móra, R.; Filker, S. Glycine betaine and ectoine are the major compatible solutes used by four different halophilic heterotrophic ciliates. Microb. Ecol. 2019, 77, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Leverrier, P.; Vissers, J.P.C.; Rouault, A.; Boyaval, P.; Jan, G. Mass spectrometry proteomic analysis of stress adaptation reveals both common and distinct response pathways in Propionibacterium freudenreichii. Arch. Microbiol. 2004, 181, 215–230. [Google Scholar] [CrossRef] [PubMed]
- Flahaut, S.; Hartke, A.; Giard, J.; Benachour, A.; Boutibonnes, P.; Auffray, Y. Relationship between stress response towards bile salts, acid and heat treatment in Enterococcus faecalis. FEMS Microbiol. Lett. 1996, 138, 49–54. [Google Scholar] [CrossRef]
- Streeter, J.G.; Gomez, M.L. Three enzymes for trehalose synthesis in Bradyrhizobium cultured bacteria and in bacteroids from soybean nodules. Appl. Environ. Microbiol. 2006, 72, 4250–4255. [Google Scholar] [CrossRef]
- Teixido, N.; Canamas, T.P.; Usall, J.; Torres, R.; Magan, N.; Vinas, I. Accumulation of the compatible solutes, glycine-betaine and ectoine, in osmotic stress adaptation and heat shock cross-protection in the biocontrol agent Pantoea agglomerans CPA-2. Lett. Appl. Microbiol. 2005, 41, 248–252. [Google Scholar] [CrossRef]
- Cañamás, T.P.; Viñas, I.; Usall, J.; Magan, N.; Solsona, C.; Teixidó, N. Impact of mild heat treatments on induction of thermotolerance in the biocontrol yeast Candida sake CPA-1 and viability after spray-drying. J. Appl. Microbiol. 2008, 104, 767–775. [Google Scholar] [CrossRef]
- Ferreira, V.; Soares, V.; Santos, C.; Silva, J.; Gibbs, P.A.; Teixeira, P. Survival of Lactobacillus sakei during heating, drying and storage in the dried state when growth has occurred in the presence of sucrose or monosodium glutamate. Biotechnol. Lett. 2005, 27, 249–252. [Google Scholar] [CrossRef]
- Akashi, K.; Shibai, H.; Hirose, Y. Effect of oxygen supply on l -lysine, l -threonine and l -isoleucine fermentations. Agric. Biol. Chem. 1979, 43, 2087–2092. [Google Scholar] [CrossRef]
- Ghazi, A.; Demont-Caulet, N.; Richarme, G.; Caldas, T. Thermoprotection by glycine betaine and choline. Microbiology 1999, 145, 2543–2548. [Google Scholar]
- Holtmann, G.; Bremer, E. Thermoprotection of Bacillus subtilis by exogenously provided glycine betaine and structurally related compatible solutes: Involvement of Opu transporters. J. Bacteriol. 2004, 186, 1683–1693. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.A.; Lindquist, S. Thermotolerance in Saccharomyces cerevisiae: The Yin and Yang of trehalose. Trends Biotechnol. 1998, 16, 460–468. [Google Scholar] [CrossRef]
- Hottiger, T.; Schmutz, P.; Wiemken, A. Heat-induced accumulation and futile cycling of trehalose in Saccharomyces cerevisiae. J. Bacteriol. 1987, 169, 5518–5522. [Google Scholar] [CrossRef] [PubMed]
- Hazell, B.; Nevalainen, H.; Attfield, P. Evidence that the Saccharomyces cerevisiae CIF1 (GGS1/TPS1) gene modulates heat shock response positively. FEBS Lett. 1995, 377, 457–460. [Google Scholar] [PubMed]
- Fitz, A. Ueber Spaltpilzgährungen. Ber. Dtsch. Chem. Ges. 1878, 11, 1890–1899. [Google Scholar] [CrossRef]
| Design Matrice | Working Matrice | |||||
|---|---|---|---|---|---|---|
| Samples | NaCl Concentration | Glucose Concentration | Time of Heat Adaptation | NaCl Concentration (g·L−1) | Glucose Concentration (g·L−1) | Time of Heat Adaptation (h) |
| 1 | −1 | −1 | −1 | 0 | 0 | 0 |
| 2 | −1 | −1 | 0 | 0 | 0 | 1 |
| 3 | −1 | −1 | 1 | 0 | 0 | 2 |
| 4 | −1 | 0 | −1 | 0 | 15 | 0 |
| 5 | −1 | 0 | 0 | 0 | 15 | 1 |
| 6 | −1 | 0 | 1 | 0 | 15 | 2 |
| 7 | −1 | 1 | −1 | 0 | 30 | 0 |
| 8 | −1 | 1 | 0 | 0 | 30 | 1 |
| 9 | −1 | 1 | 1 | 0 | 30 | 2 |
| 10 | 0 | −1 | −1 | 25 | 0 | 0 |
| 11 | 0 | −1 | 0 | 25 | 0 | 1 |
| 12 | 0 | −1 | 1 | 25 | 0 | 2 |
| 13 | 0 | 0 | −1 | 25 | 15 | 0 |
| 14 | 0 | 0 | 0 | 25 | 15 | 1 |
| 15 | 0 | 0 | 0 | 25 | 15 | 1 |
| 16 | 0 | 0 | 0 | 25 | 15 | 1 |
| 17 | 0 | 0 | 1 | 25 | 15 | 2 |
| 18 | 0 | 1 | −1 | 25 | 30 | 0 |
| 19 | 0 | 1 | 0 | 25 | 30 | 1 |
| 20 | 0 | 1 | 1 | 25 | 30 | 2 |
| 21 | 1 | −1 | −1 | 50 | 0 | 0 |
| 22 | 1 | −1 | 0 | 50 | 0 | 1 |
| 23 | 1 | −1 | 1 | 50 | 0 | 2 |
| 24 | 1 | 0 | −1 | 50 | 15 | 0 |
| 25 | 1 | 0 | 0 | 50 | 15 | 1 |
| 26 | 1 | 0 | 1 | 50 | 15 | 2 |
| 27 | 1 | 1 | −1 | 50 | 30 | 0 |
| 28 | 1 | 1 | 0 | 50 | 30 | 1 |
| 29 | 1 | 1 | 1 | 50 | 30 | 2 |
| Estimation | Prob. >|t| | |
|---|---|---|
| Constant | 23 | <0.0001 |
| NaCl concentration(0.50) | −7 | 0.0077 |
| Glucose concentration(0.30) | 24 | <0.0001 |
| Time of heat adaptation(0.2) | 9 | 0.0011 |
| NaCl concentration × Glucose concentration | −13 | 0.0003 |
| NaCl concentration × Time of heat adaptation | NS | 0.0638 |
| Glucose concentration × Time of heat adaptation | 7 | 0.0429 |
| Estimation | Prob. >|t| | |
|---|---|---|
| Constant | 29 | <0.0001 |
| NaCl concentration(0.50) | −10 | 0.0067 |
| Glucose concentration(0.30) | 10 | 0.0068 |
| Time of heat adaptation(0.2) | 19 | <0.0001 |
| NaCl concentration × Glucose concentration | NA | 0.2329 |
| NaCl concentration × Time of heat adaptation | −14 | 0.0021 |
| Glucose concentration × Time of heat adaptation | NA | 0.1406 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaucher, F.; Gagnaire, V.; Rabah, H.; Maillard, M.-B.; Bonnassie, S.; Pottier, S.; Marchand, P.; Jan, G.; Blanc, P.; Jeantet, R. Taking Advantage of Bacterial Adaptation in Order to Optimize Industrial Production of Dry Propionibacterium freudenreichii. Microorganisms 2019, 7, 477. https://doi.org/10.3390/microorganisms7100477
Gaucher F, Gagnaire V, Rabah H, Maillard M-B, Bonnassie S, Pottier S, Marchand P, Jan G, Blanc P, Jeantet R. Taking Advantage of Bacterial Adaptation in Order to Optimize Industrial Production of Dry Propionibacterium freudenreichii. Microorganisms. 2019; 7(10):477. https://doi.org/10.3390/microorganisms7100477
Chicago/Turabian StyleGaucher, Floriane, Valérie Gagnaire, Houem Rabah, Marie-Bernadette Maillard, Sylvie Bonnassie, Sandrine Pottier, Pierre Marchand, Gwénaël Jan, Philippe Blanc, and Romain Jeantet. 2019. "Taking Advantage of Bacterial Adaptation in Order to Optimize Industrial Production of Dry Propionibacterium freudenreichii" Microorganisms 7, no. 10: 477. https://doi.org/10.3390/microorganisms7100477
APA StyleGaucher, F., Gagnaire, V., Rabah, H., Maillard, M.-B., Bonnassie, S., Pottier, S., Marchand, P., Jan, G., Blanc, P., & Jeantet, R. (2019). Taking Advantage of Bacterial Adaptation in Order to Optimize Industrial Production of Dry Propionibacterium freudenreichii. Microorganisms, 7(10), 477. https://doi.org/10.3390/microorganisms7100477





