Abstract
Human infections with Campylobacter are primarily associated with the consumption of contaminated poultry meat. In this study, we isolated Campylobacter jejuni from retail raw chicken and duck meat in Korea and compared their aerotolerance, antibiotic resistance, and virulence gene prevalence. Whereas C. jejuni isolates from chicken dominantly belonged to multilocus sequence typing (MLST) clonal complex (CC)-21, CC-45 is the common MLST sequence type in duck meat isolates. C. jejuni strains from both chicken and duck meat were highly tolerant to aerobic stress. The prevalence of virulence genes was higher in C. jejuni strains from chicken than those from duck meat. However, antibiotic resistance was higher in duck meat isolates than chicken isolates. Based on the prevalence of virulence genes and antibiotic resistance, fluoroquinolone-resistant C. jejuni strains harboring all tested virulence genes except virB11 were predominant on retail poultry. Fluoroquinolone-resistant C. jejuni strains carrying most virulence genes were more frequently isolated in summer than in winter. The comparative profiling analysis in this study successfully demonstrated that antibiotic-resistant and pathogenic strains of C. jejuni are highly prevalent on retail poultry and that retail duck meat is an important vehicle potentially transmitting C. jejuni to humans in Korea.
1. Introduction
Campylobacter is a leading etiological agent of gastroenteritis, accounting for approximately 166 million diarrheal cases globally per year []. Although estimates of the number of human campylobacterisosis cases in South Korea are not available, Campylobacter is considered as a major food safety concern in the country along with enterotoxigenic Escherichia coli, Salmonella, and Clostridium according to the Ministry of Food and Drug Safety (MFDS). (https://www.foodsafety korea.go.kr/portal/healthyfoodlife/foodPoisoningStat.do)
Campylobacter jejuni is the pathogenic species most frequently implicated in human infection and may result in diarrhea, fever and abdominal pains. In some cases, C. jejuni may lead to the onset of Guillain–Barré syndrome, an autoimmune disorder characterized by acute and progressive neuromuscular paralysis [].
The pathogenicity of C. jejuni is mediated by various virulence factors, such as flagellin, cytolethal distending toxin (CDT), adhesins (e.g., Campylobacter adhesion to Fibronectin (CadF), Peb1), Campylobacter invasion antigen (Cia) proteins, and phospholipase A (PldA). Flagellins are the building block of flagella that play an important role in motility, chicken colonization, autoagglutination, and biofilm formation in Campylobacter []. The CDT toxin consists of three subunits (CdtA, CdtB, and CdtC) [], and CdtB possesses a type I deoxyribonuclease activity and arrests the cell cycle in the G2/M transition phase []. Peb1 is an adhesin that is involved in C. jejuni interaction with INT 407 cells [] and affects host colonization []. CadF is an outer membrane protein that affects C. jejuni binding to epithelial cells [], and a mutation of cadF completely prevented C. jejuni from colonizing chicken intestines []. CiaB is associated with the internalization of C. jejuni into host cells []. PldA is an outer-membrane protein with a hemolytic activity []. PldA and CiaB also significantly affect the ability of C. jejuni to colonize chicken intestines [].
Human campylobacteriosis is usually self-limiting. However, severe and prolonged infections may require antimicrobial therapy [], and fluoroquinolone (FQ) and macrolide antibiotics are the drugs of choice to treat human campylobacteriosis [,]. For serious Campylobacter infections in humans, such as bacteremia, aminoglycosides are intravenously administered to patients []. However, increasing resistance in Campylobacter to clinically important antibiotics, particularly FQs, is a serious global public health problem, and the World Health Organization (WHO) recently classified FQ-resistant Campylobacter as a high-priority antibiotic-resistant pathogen for which new drugs need to be developed [].
C. jejuni is a microaerophilic bacterium and requires low oxygen concentrations for growth []. Thus, tolerance to aerobic stress is the forefront survival mechanism for C. jejuni in the food production and processing systems in a normal atmosphere where the oxygen level is high (ca. 21%). In contrast to our traditional knowledge about oxygen sensitivity in C. jejuni, recently, C. jejuni strains with increased aerotolerance have been isolated from retail raw chicken and humans [,]. In Canada, C. jejuni strains with increased aerotolerance are highly prevalent in retail raw chicken []. Additionally, aerotolerant C. jejuni survived on raw chicken under aerobic conditions for an extended period of time compared to oxygen-sensitive C. jejuni strains, potentially posing a serious threat to food safety [].
Campylobacter is isolated from a wide range of food-producing animals and their carcasses [], and poultry is considered as the primary reservoir for Campylobacter []. It has been estimated that 50% to 80% of human cases of campylobacteriosis are attributed to chickens, and 20% to 30% are directly associated with the handling, preparation, and consumption of broiler meat in the EU []. In addition, Campylobacter is isolated from duck meat and its parts in many countries [], and the consumption of duck meat and organs (e.g., liver pâté) has caused Campylobacter outbreaks [,]. While turkey is widely consumed in North America, duck is preferably consumed in most Asian countries including Korea. Since duck meat has been increasingly produced and consumed in Korea, human exposure to Campylobacter can be caused by eating contaminated duck meat. However, little has been investigated about the food safety risk of C. jejuni on duck meat.
To fill this knowledge gap, in this study, we isolated C. jejuni from retail chicken and duck meat in Korea and analyzed their multilocus sequence typing (MLST) clonal complexes (CCs), aerotolerance, virulence gene prevalence, and antibiotic resistance.
2. Materials and Methods
2.1. Isolation of C. jejuni from Retail Raw Chicken and Duck Meats
Eighty poultry whole carcasses (52 chickens and 28 duck meats) were collected from retail stores in seven different provinces in Korea in winter (from December 2016 to March 2017), and 114 poultry meats (81 chickens and 33 ducks) were purchased in summer (from April to June 2017). A total of 194 whole carcasses were subjected to enrichment with 1 L of Bolton broth supplemented with Bolton Campylobacter-selective supplement (Oxoid, UK) under microaerobic conditions (4% H2, 6% O2, 7% CO2, 83% N2) at 42 °C for 24 h. To increase isolation efficiency, an aliquot (20 mL) of the enriched broth was concentrated and resuspended in 1 mL of Bolton broth. The resuspension was serially diluted (100 to 10−5) and spread on six Preston agars supplemented with Preston Campylobacter-selective supplement (Thermo-Fisher Scientific, Waltham, MA, USA). The culture was incubated at 42 °C for 48 h under microaerobic conditions.
2.2. Identification of Campylobacter Species
Presumptive Campylobacter colonies were selected based on the typical colony morphology of Campylobacter, such as flat, shiny, and mucoid colonies. To avoid analyzing duplicate clones, only 1~2 isolates were selected from each sample depending on different colony morphologies for further confirmation using multiplex PCR and 16S rRNA sequencing. Since hipO has been used as a specific marker for C. jejuni in many studies, the isolates that were positive for hipO and cj0414 were considered as C. jejuni, and those that were positive for ask were determined as Campylobacter coli []. To prepared template DNA, the pure culture in Muller Hinton (MH, Oxoid) broth resuspended in MH broth to an optical density at 600 nm (OD600) of 0.1 was diluted 10-fold in distilled water and heated to 95 °C for 7 min to extract DNA. Cell debris was pelleted by centrifugation at 16,000 × g, 4 °C for 1 min, and the supernatant was used as template DNA. The isolates were further differentiated with 16S rRNA sequencing. The primer sequences were described in Table S3.
2.3. MLST Analysis
C. jejuni genotypes were characterized by MLST based on the method outlined in PubMLST (pubmlst.org) and a previous study []. Briefly, template DNA was prepared as described above. The seven housekeeping genes (aspA, glnA, gltA, glyA, pgm, tkt, and uncA) were amplified by PCR with the primer pairs described elsewhere []. The PCR amplicons were commercially sequenced by Macrogen (Seoul, Korea). Allele numbers and sequence types were assigned by using the Campylobacter MLST database [].
2.4. Aerotolerance Test
An aerotolerance test was performed as described previously []. Briefly, overnight cultures of C. jejuni on MH agar at 42 °C were resuspended in MH broth to an OD600 of 0.1. The bacterial suspension was incubated at 42 °C with shaking (200 rpm) under aerobic conditions. Samples were taken after 0, 12, and 24 h for serial dilution and bacterial counting. The strains that survived in aerobic shaking < 12 h were called oxygen-sensitive (OS), and those that survived 12~24 h and ≥ 24 h were considered aerotolerant (AT) and hyper-aerotolerant (HAT), respectively, according to the previous study [].
2.5. PCR Detection of Virulence Genes
The prevalence of nine virulence genes in C. jejuni was examined with single PCR. Template DNA was prepared as described above. The PCR reaction was performed with rTaq (Takara, Japan) under the following conditions: initial denaturation at 95 °C for 15 min followed by 35 cycles of denaturation at 95 °C for 30 s, variable annealing temperature (cadF, ciaB, iam, pldA, and flaA at 45 °C, docA, and peb1 at 50 °C, virB11 at 53 °C, and cdtB at 58 °C) for 1 min 30 s, extension at 72 °C for 2 min and the final extension at 72 °C for 7 min. The results were analyzed by electrophoresis with 1% agarose gels. The primer sequences are described in Table S3.
2.6. Antimicrobial Susceptibility Test
The minimum inhibitory concentrations (MICs) of the C. jejuni isolates were determined using a broth microdilution method with the Sensititre custom plate KRCAMP (TREK Diagnostics, Cleveland, OH, USA). The antimicrobials in the plate were two-fold diluted, including azithromycin, chloramphenicol, ciprofloxacin, erythromycin, gentamicin, nalidixic acid, streptomycin, tetracycline, and telithromycin. C. jejuni was suspended in cation-adjusted MH broth to a 0.5 McFarland standard. The microtiter plates were incubated at 42 °C for 24 h under microaerophilic conditions. Antimicrobial resistance was determined according to the interpretative criteria recommended by the Clinical and Laboratory Standards Institute [], and a report from the US Food and Drug Administration []. C. jejuni ATCC 33560 was included in the test as a quality control strain.
2.7. Data Analysis
The data on virulence genes and the MIC test were analyzed using BioNumerics 7.6.2 (Applied Maths, Belgium). Cluster analysis was performed, and a dendrogram was generated with BioNumerics using the similarity coefficient and unweighted-pair group method with average linkages (UPGMA) coefficient.
2.8. Statistical Analysis
Statistical analyses were performed using χ2 test with SPSS (IBM, USA).
3. Results
3.1. Frequencies of Campylobacter Isolation from Retail Raw Chicken and Duck Meat
Campylobacter was isolated from 54.1% (105/194) of the total poultry meat samples and more frequently from duck (62.3%) than chicken (50.4%) meat (Table 1). Compared to C. coli, C. jejuni was more prevalent on retail raw poultry in Korea, and both pathogenic species were simultaneously isolated from 9.8% of raw chicken and 18.0% of duck meat samples (Table 1). The isolation frequencies of Campylobacter from raw chicken were higher in summer than in winter; however, it was not statistically significant (p = 0.0648; Table 1). However, such a seasonal pattern was not observed in duck meat samples (Table 1). These results showed that Campylobacter contamination was highly prevalent on both raw chicken and duck meat and that retail raw duck meat is highly prone to Campylobacter contamination. The rest of the study was focused on the characterization of C. jejuni isolates.

Table 1.
Frequencies of Campylobacter isolation from retail raw chicken and duck meat.
3.2. MLST Analysis of C. jejuni Isolates from Raw Retail Chicken and Duck Meat
One hundred and thirty five strains of C. jejuni were isolated from 105 C. jejuni-positive samples (64 chicken and 41 duck meat samples); we selected only one or two isolates from each sample based on their morphological differences to avoid isolating duplicate clones. The results of the MLST analysis showed that C. jejuni strains belonging to CC-21 and CC-45 were dominant on both raw chicken and duck meats (Figure 1A). Whereas CC-21 was predominant in chicken isolates, CC-45 was highly prevalent in C. jejuni isolates from duck meat (p < 0.05; Figure 1A). The MLST CCs of 28.9% of duck meat isolates were not assigned (p < 0.05), and 17.8% of them were not typable (p < 0.05) with MLST; however, 14.4% of the chicken isolates were not assigned with MLST CC or non-typable (p < 0.05; Figure 1A). This suggests that the genetic background of certain C. jejuni isolates from duck meat may be different from that of chicken isolates.
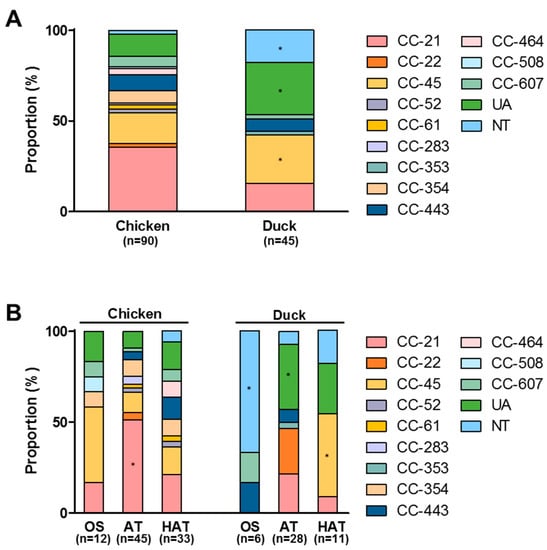
Figure 1.
Multilocus sequence typing (MLST) sequence types of C. jejuni strains from retail raw chicken and duck meat (A), and the distribution of MLST clonal complexes (CCs) in C. jejuni strains with different aerotolerance levels from retail raw chicken and duck meat (B) in Korea. UA: unassigned to any CC defined, NT: not typable. *: p < 0.05.
3.3. Aerotolerance in C. jejuni Isolates from Raw Chicken and Duck Meat
We investigated aerotolerance in C. jejuni isolates from raw chicken and duck meat. AT and HAT strains of C. jejuni were highly prevalent in both raw chicken and duck meat (Table 2). Although the prevalence of HAT C. jejuni was higher in raw chicken than duck meat, the combined prevalence of AT and HAT C. jejuni strains was similar between chicken isolates and duck meat isolates (86.7% in chicken isolates vs 86.6% in duck meat isolates; Table 2). MLST CC-21 was most common in AT and HAT strains of C. jejuni from retail raw chicken (Figure 1B). In addition, most (66.7%) OS strains from duck meat were non-typable (p < 0.05); however, CC-45 was predominant in AT and HAT strains (p < 0.05) of C. jejuni from duck meat (Figure 1B). The results suggest that the genetic background may differ in OS, AT and HAT strains of C. jejuni from retail poultry.

Table 2.
Aerotolerance in C. jejuni strains isolated from retail raw chicken and duck meat.
3.4. Antibiotic Resistance in C. jejuni Isolates from Retail Raw Chicken and Duck Meat
C. jejuni strains isolated from retail raw chicken and duck meat were highly resistant to ciprofloxacin and tetracycline (Table 3 and Figure S1). FQ resistance was higher in duck meat isolates (97.8%) than chicken isolates (p < 0.05; 85.6%) (Table 3). Similarly, higher rates of tetracycline resistance were observed in C. jejuni isolates from duck meat (57.8%) than chicken (p < 0.001; 27.8%) (Table 3). In contrast to the high levels of FQ and tetracycline resistance, only a single strain of C. jejuni was resistant to macrolides (e.g., erythromycin and azithromycin), the most important antibiotic class of clinical importance for treating human campylobacteriosis, and five strains were resistant to gentamicin (Table 3). The findings demonstrated that C. jejuni isolates from duck meat are highly resistant to antibiotics, particularly FQs and tetracycline.

Table 3.
Antibiotic resistance of C. jejuni strains from raw chicken and duck meat.
3.5. Virulence Gene Prevalence and Antibiotic Resistance in C. jejuni Isolates from Retail Raw Chicken and Duck Meat
To evaluate the virulence potential of C. jejuni isolates, the prevalence of virulence genes was determined with PCR. A total of 68.9% (93/135) of C. jejuni isolates harbored all tested virulence genes except virB11 (Figure S2), which is a virulence gene encoded on the plasmid pVir []. Approximately 74.4% (67/90) of C. jejuni strains from raw chicken harbored eight virulence genes (all except virB11), and 7.8% (7/90) of chicken isolates possessed all tested virulence genes (Figure S2), indicating that 82.2% (74/90) of chicken isolates carried at least eight virulence genes involved in colonization, adhesion, and invasion (Figure S2). Among 45 duck isolates, 57.8% (26/45) possessed eight virulence genes (all except virB11), and 2.2% (1/45) of duck strains harbored all tested virulence genes (Figure S2), meaning that 60.0% (27/45) of duck meat isolates carried more than eight virulence genes. The prevalence of virulence genes was higher in C. jejuni strains from chicken compared to duck meat strains (Table 4). The virulence genes were widely distributed in C. jejuni isolates from chicken regardless of the level of aerotolerance. Among duck meat isolates of C. jejuni, the prevalence of cadF, iam, pldA, docA, peb1, and flaA was higher in AT and HAT strains compared to OS strains (Table S1).

Table 4.
Prevalence of virulence genes in C. jejuni strains isolated from retail raw chicken and duck meat in Korea.
3.6. Integrative Comparison of Virulence Gene Prevalence and Antibiotic Resistance between C. jejuni Chicken Isolates and Duck Meat Isolates
Since both virulence factors and antibiotic resistance affect food safety associated with Campylobacter, 135 C. jejuni strains from retail raw chicken and duck meats were analyzed based on virulence gene prevalence and antibiotic resistance. In this study, we named it the virulence and antibiotic resistance (Viro and AMR) type. The analysis clustered the 135 C. jejuni isolates from chicken and duck meat into 30 different Viro and AMR types. Types 4 and 13 constituted 36.3% (49/135) and 20.0% (27/135) of the total strains, respectively (Figure 2A). C. jejuni strains in Type 4 harbored all tested virulence genes except virB11 and were resistant to FQs. Type 4 was predominant (42.2%; 38/90) with a statistical significance (p < 0.05) in chicken isolates, whereas Type 13 was highly prevalent (28.9%; 13/45) in duck meat isolates (Figure 2A). Type 13 was highly dominant and exhibited the same patterns of virulence gene prevalence and antibiotic resistance as Type 4 except for tetracycline resistance (Figure 2A). C. jejuni strains in Types 1 and 2, which harbored all tested virulence genes, were primarily isolated from chicken (Figure 2A).
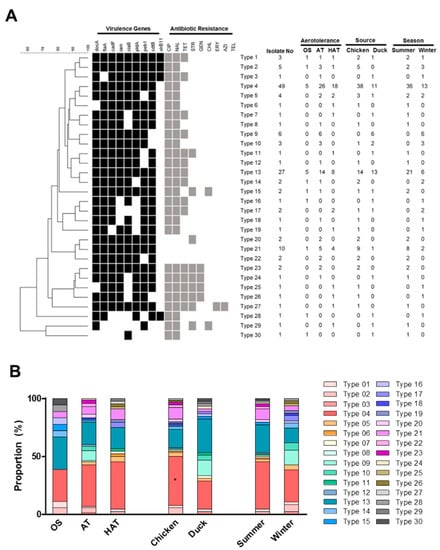
Figure 2.
The virulence gene prevalence and antibiotic resistance (Viro and AMR) types of 135 C. jejuni isolates from retail chicken and duck meats in Korea (A) and the distribution of different Viro and AMR types depending on aerotolerance (OS: oxygen-sensitive, AT: aerotolerant, HAT: hyper-aerotolerant), origin (chicken or duck meats), and the season (summer or winter) (B). *: p < 0.05.
C. jejuni strains belonging to Type 4 were prevalent in AT and HAT strains. The proportion of Type 4 was 27.8%, 35.6%, and 40.9% in OS, AT, and HAT strains, respectively (Figure 2B and Table S2). In contrast, Type 13 was less prevalent in HAT strains, constituting 27.8%, 19.2%, and 18.2% in OS, AT, and HAT strains, respectively (Figure 2B and Table S2). Whereas Type 4 was predominant in chicken isolates, Type 13 was predominant in duck meat isolates (Figure 2B). Furthermore, C. jejuni strains belonging to Types 4 and 13 were isolated more frequently in summer than in winter (Figure 2B).
C. jejuni strains in Types 4 and 13 exhibited different compositions of MLST sequence types. Whereas CC-21 was predominant in C. jejuni strains in Type 4 from both chicken and duck meats (p < 0.05; Figure 3), CC-45 was the major sequence type in Type 13 in both chicken and duck meat strains (p < 0.001; Figure 3). In addition, all nine C. jejuni strains (six from raw chicken and three from duck meats) belonging to CC-443 (Figure 1A) were clustered to Type 4 (Figure 3). The integrative analysis of virulence gene prevalence and antibiotic resistance suggest that antibiotic-resistant C. jejuni strains harboring virulence genes are highly prevalent on retail duck meat.
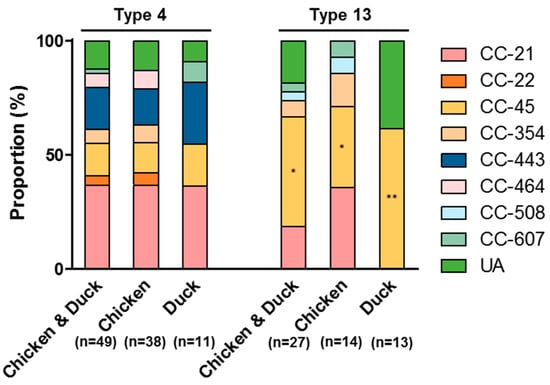
Figure 3.
MLST sequence types of the two primary Viro and AMR types, Type 4 and Type 13, in C. jejuni isolates from retail raw chicken and duck meat. *: p < 0.05, **: p <0.001.
4. Discussion
Aerotolerance in Campylobacter may directly affect food safety, as it increases the survival of this microaerophilic pathogen in the food supply chain. In this study, we first reported aerotolerance in C. jejuni strains from duck meat. Similar to a previous study in Canada [], the AT C. jejuni strains is highly prevalent on both retail raw chicken and duck meat in Korea. As a foodborne pathogen, C. jejuni encounters various stress conditions at different stages of food production, processing, preservation, distribution, and cooking, and aerobic stress would be a common stressor to this microaerophilic bacterium in the normal atmosphere. As it has been demonstrated that AT and HAT strains of C. jejuni survives on refrigerated raw chicken in air more effectively than OS strains [], the high prevalence of AT and HAT strains of C. jejuni on retail poultry may affect food safety [].
It has been well documented that contaminated chicken is frequently involved in human campylobacteriosis []. The findings in this study demonstrate that duck meat is also an important source that can transmit Campylobacter to humans since Campylobacter was more frequently isolated from retail duck meat (62.3%) than chicken (50.4%). Similarly, Wei et al. have reported that Campylobacter contamination is more frequent on duck meats than raw chicken in Korea []. According to the study of Little et al., 50.7% of raw duck meat in the UK is contaminated with Campylobacter, which is lower than Campylobacter contamination in raw chicken (60.9%) but greater than that in turkey (33.7%) []. The frequencies of Campylobacter isolation from raw chicken were higher in summer (56.8%) than in winter (40.4%). Similarly, the prevalence of Campylobacter-positive broiler flocks is significantly higher in summer (54~60%) than during the rest of the year (14~48%) in the UK []. However, such a seasonal pattern of Campylobacter isolation from chicken was not observed in duck meat, as the isolation frequency of Campylobacter from duck meat was similar between summer (63.6%) and winter (60.7%). Although the reason for the different seasonality patterns in Campylobacter isolation remains unexplained, the results clearly showed that duck meat is more likely to be contaminated with Campylobacter regardless of the season.
Duck meat isolates of C. jejuni harbored most of the tested virulence genes. However, the prevalence of the virulence genes was relatively lower in duck meat isolates compared to chicken isolates. Particularly, the genes involved in invasion (e.g., ciaB) and toxin production (i.g., cdtB) were less prevalent in C. jejuni strains from duck meat compared to chicken isolates. The prevalence of virB11 was low in both chicken and duck meat isolates. The prevalence of virulence genes was higher in C. jejuni strains from retail poultry in Korea compared to previous studies performed in other countries. The iam locus has been detected in 54.7% and 57.1% of C. jejuni isolates from chicken in Poland and Canada, respectively [,]. However, 97.8% and 88.9% of C. jejuni isolates from raw chicken and duck meats, respectively, were positive for iam in this study. Similarly, the pldA and ciaB genes have been detected from C. jejuni isolates from chicken carcasses at the frequencies of 63.6% and 67.3%, respectively, in Brazil [], 56% and 40% in the US [], and 84.3% and 68.6% in Canada []. In this study, 94.4% and 91.1% of C. jejuni isolates from raw chicken and duck meats, respectively, harbored pldA, and the ciaB gene was detected in 95.6% and 88.9% of C. jejuni strains from raw chicken and duck meats, respectively. In C. jejuni strains from raw chicken, the virulence genes were highly prevalent regardless of the aerotolerance level. Similar to a previous report [], however, some virulence genes, such as cadF, pldA, docA, and peb1, were more frequently detected in AT and HAT strains of C. jejuni from duck meats than OS strains. Since the virulence genes tested in this study play a critical role in the pathogenicity of Campylobacter, high prevalence of the virulence genes in C. jejuni isolates from duck meat suggest that these strains are potentially a food safety threat.
C. jejuni strains resistant to clinically important antibiotics, particularly FQs, were highly prevalent in retail raw poultry. High prevalence of FQ-resistant C. jejuni on retail chicken in Korea has been reported in multiple studies. Han et al. has reported that 92.2% of C. jejuni isolates from raw chicken in Korea are resistant to FQs []. Multidrug-resistant Campylobacter is highly prevalent in domestic and imported retail chickens in Korea, and FQ resistance is significantly high (95%) in Campylobacter strains from retail chicken []. In this study, we observed that FQ resistance was even higher in duck meat isolates (97.8%) than chicken isolates (85.6%). Wei et al. also reported that C. jejuni strains from duck meat (87.8%) were more resistant to FQs than the strains from chicken carcasses (83.3%) []. FQ resistance in C. jejuni from ducks and retail duck meats substantially vary depending on the country. In Malaysia, 76% of C. jejuni isolates from ducks and their farming and processing environments were resistant to FQs []. Among nine C. jejuni and 11 C. coli strains isolated from duck meats in the UK, FQ resistance was detected in 54.6% of C. coli, and none of the C. jejuni strains were resistant to FQs []. Since FQs are clinically important for treating gastroenteritis, the high prevalence of FQ-resistant C. jejuni in duck meat may be a serious public health concern. However, resistance to macrolides was low in C. jejuni strains from retail poultry.
MLST CC-21 and CC-45 were dominant in duck meat isolates. Compared to chicken isolates, the sequence type of 28.9% of duck meat isolates was not determined by MLST; this suggests that different C. jejuni populations may exist on duck meat. A study from Korea has reported that CC-21 and CC-45 are the primary MLST sequence types of C. jejuni isolates from duck farms []. In New Zealand, CC-45 and CC-1034 were dominant CCs in C. jejuni from mallard ducks inhabiting the public access sites of urban areas []. Although only a limited number of studies are available regarding the MLST sequence types of C. jejuni strains from ducks, CC-45 appears to be a common MLST sequence type in C. jejuni strains from duck and duck meat. Approximately 45.5% of HAT C. jejuni isolates from duck meat belonged to CC-45, whereas most (66.7%) of the OS strains of C. jejuni from duck meat were not typable with MLST.
By analyzing the prevalence of virulence genes and antibiotic resistance, we discovered that antibiotic-resistant and pathogenic C. jejuni populations, such as Types 4 and 13, are predominant on retail raw chicken and duck meats. Type 4 was more prevalent in HAT strains of C. jejuni compared to OS strains, whereas Type 13 was more dominant in OS strains than HAT strains. In addition, all nine strains belonging to MLST CC-443 were classified to Type 4, indicating that MLST CC-443 may be associated with antibiotic-resistant and pathogenic C. jejuni populations. C. jejuni strains belonging to Types 4 and 13 were predominant in summer (64.8%) compared to winter (40.5%). It has been reported that ambient temperatures are related to human infections with Campylobacter and human campylobacteriosis frequently occurs in summer []. Campylobacter is isolated from diarrheal patients in Korea more frequently in summer than in winter []. Along with the other factors affecting foodborne infections, such as cross-contamination and temperature abuse, the findings in our study suggest that C. jejuni strains with an increased public health risk of antibiotic resistance and virulence gene prevalence are more prevalent on retail raw chicken and duck meats in summer than in winter.
5. Conclusions
This study performed an extensive comparative profiling of C. jejuni strains isolated from retail poultry (raw chicken and duck meat). C. jejuni populations with antibiotic resistance and virulence potential are highly prevalent on both retail raw chicken and duck meat. Since poultry is the major reservoir for Campylobacter, human exposure to Campylobacter is mainly associated with poultry sources. Since dietary patterns may vary in different countries and ethnic groups, foodborne exposure to Campylobacter may occur through the consumption of different kinds of poultry meat. Although some countries frequently consume duck meat, little is known about C. jejuni from duck. The findings in this study successfully demonstrated that C. jejuni strains from retail duck meat are highly antibiotic-resistant, aerotolerant, and potentially pathogenic.
Supplementary Materials
Supplementary materials can be found at https://www.mdpi.com/2076-2607/7/10/433/s1. Table S1: Prevalence of virulence genes in oxygen-sensitive (OS), aerotolerant (AT), and hyper-aerotolerant (HAT) C. jejuni strains from retail raw chicken and duck meat; Table S2: Distribution of C. jejuni strains belonging to different virulence and antibiotic resistance (Viro and AMR) types depending on the aerotolerance, source (chicken and duck meat), and season (summer and winter); Table S3: Primers used in this study; Figure S1: Antibiotic resistance in C. jejuni strains from retail raw chicken and duck meat; Figure S2: Prevalence of nine virulence genes in 135 strains of C. jejuni from retail raw chicken and duck meats. The number of strains is indicated on the right.
Author Contributions
S.C., S.R., and B.J. designed the study; J.K. (Junhyung Kim)., H.P., J.K. (Junhyung Kim), J.H.K., and J.I.J. performed the experiments; J.K. (Jinshil Kim), S.C., S.R., and B.J. analyzed the data; J.K. (Jinshil Kim) and B.J. wrote the manuscript; J.K. (Jinshil Kim), S.C., S.R., and B.J. critically reviewed the manuscript.
Funding
This research was supported by a grant (16162MFDS029) from the Ministry of Food and Drug Safety in Korea. The antimicrobial susceptibility test was performed by J.H.K. and J.I.J. with a research grant (2017-NI41003) from the Korea Centers for Disease Control and Prevention. J.K. was supported by the BK21 Plus Program of the Department of Agricultural Biotechnology, Seoul National University, Seoul, Korea.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kirk, M.D.; Pires, S.M.; Black, R.E.; Caipo, M.; Crump, J.A.; Devleesschauwer, B.; Dopfer, D.; Fazil, A.; Fischer-Walker, C.L.; Hald, T.; et al. World health organization estimates of the global and regional disease burden of 22 foodborne bacterial, protozoal, and viral diseases, 2010: A data synthesis. PLoS Med. 2015, 12, e1001921. [Google Scholar]
- Humphrey, T.; O’Brien, S.; Madsen, M. Campylobacters as zoonotic pathogens: A food production perspective. Int. J. Food Microbiol. 2007, 117, 237–257. [Google Scholar] [CrossRef]
- Guerry, P. Campylobacter flagella: Not just for motility. Trends Microbiol. 2007, 15, 456–461. [Google Scholar] [CrossRef]
- Lara-Tejero, M.A.; Galán, J.E. CdtA, CdtB, and CdtC form a tripartite complex that is required for cytolethal distending toxin activity. Infect. Immun. 2001, 69, 4358–4365. [Google Scholar] [CrossRef]
- Lara-Tejero, M.; Galan, J.E. A bacterial toxin that controls cell cycle progression as a deoxyribonuclease i-like protein. Science 2000, 290, 354–357. [Google Scholar] [CrossRef]
- Pei, Z.; Blaser, M.J. Peb1, the major cell-binding factor of Campylobacter jejuni, is a homolog of the binding component in gram-negative nutrient transport systems. J. Biol. Chem. 1993, 268, 18717–18725. [Google Scholar]
- Hauser, M.; Arendt, R.; Kelsall, T.; Dwek, E.; Odegard, N.; Weiland, J.; Freudenreich, H.; Reach, W.; Silverberg, R.; Moseley, S. The COBE diffuse infrared background experiment search for the cosmic infrared background. i. limits and detections. Astrophys. J. 1998, 508, 25. [Google Scholar] [CrossRef]
- Konkel, M.E.; Garvis, S.G.; Tipton, S.L.; Anderson, J.; Donald, E.; Cieplak, W., Jr. Identification and molecular cloning of a gene encoding a fibronectin-binding protein (cadF) from Campylobacter jejuni. Mol. Microbiol. 1997, 24, 953–963. [Google Scholar] [CrossRef]
- Ziprin, R.L.; Young, C.R.; Stanker, L.H.; Hume, M.E.; Konkel, M.E. The absence of cecal colonization of chicks by a mutant of Campylobacter jejuni not expressing bacterial fibronectin-binding protein. Avian Dis. 1999, 43, 586–589. [Google Scholar] [CrossRef]
- Konkel, M.E.; Kim, B.J.; Rivera-Amill, V.; Garvis, S.G. Bacterial secreted proteins are required for the internaliztion of Campylobacter jejuni into cultured mammalian cells. Mol. Microbiol. 1999, 32, 691–701. [Google Scholar] [CrossRef]
- Grant, K.A.; Belandia, I.U.; Dekker, N.; Richardson, P.T.; Park, S.F. Molecular characterization of pldA, the structural gene for a phospholipase a from Campylobacter coli, and its contribution to cell-associated hemolysis. Infect. Immun. 1997, 65, 1172–1180. [Google Scholar] [PubMed]
- Ziprin, R.L.; Young, C.R.; Byrd, J.A.; Stanker, L.H.; Hume, M.E.; Gray, S.A.; Kim, B.J.; Konkel, M.E. Role of Campylobacter jejuni potential virulence genes in cecal colonization. Avian Dis. 2001, 45, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Luangtongkum, T.; Jeon, B.; Han, J.; Plummer, P.; Logue, C.M.; Zhang, Q. Antibiotic resistance in Campylobacter: Emergence, transmission and persistence. Future Microbiol. 2009, 4, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Allos, B.M. Campylobacter jejuni infections: Update on emerging issues and trends. Clin. Infect. Dis. 2001, 32, 1201–1206. [Google Scholar] [PubMed]
- Engberg, J.; Aarestrup, F.M.; Taylor, D.E.; Gerner-Smidt, P.; Nachamkin, I. Quinolone and macrolide resistance in Campylobacter jejuni and C. coli: Resistance mechanisms and trends in human isolates. Emerg. Infect. Dis. 2001, 7, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Aarestrup, F.M.; Engberg, J. Antimicrobial resistance of thermophilic Campylobacter. Vet. Res. 2001, 32, 311–321. [Google Scholar] [CrossRef] [PubMed]
- The World Health Organization. Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Bolton, F.J.; Coates, D. A study of the oxygen and carbon dioxide requirements of thermophilic campylobacters. J. Clin. Pathol. 1983, 36, 829–834. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Pocheron, A.L.; Hernould, M.; Haddad, N.; Tresse, O.; Cappelier, J.M. Description of Campylobacter jejuni Bf, an atypical aero-tolerant strain. Gut Pathog. 2015, 7, 30. [Google Scholar] [CrossRef]
- Oh, E.; McMullen, L.; Jeon, B. High prevalence of hyper-aerotolerant Campylobacter jejuni in retail poultry with potential implication in human infection. Front. Microbiol. 2015, 6, 1263. [Google Scholar] [CrossRef]
- Oh, E.; McMullen, L.M.; Chui, L.; Jeon, B. Differential survival of hyper-aerotolerant Campylobacter jejuni under different gas conditions. Front. Microbiol. 2017, 8, 954. [Google Scholar] [CrossRef]
- Huang, H.; Brooks, B.W.; Lowman, R.; Carrillo, C.D. Campylobacter species in animal, food, and environmental sources, and relevant testing programs in Canada. Can. J. Microbiol. 2015, 61, 701–721. [Google Scholar] [CrossRef] [PubMed]
- Skarp, C.P.; Hanninen, M.L.; Rautelin, H.I. Campylobacteriosis: The role of poultry meat. Clin. Microbiol. Infect. 2016, 22, 103–109. [Google Scholar] [CrossRef] [PubMed]
- EFSA. Scientific opinion on Campylobacter in broiler meat production: Control options and performance objectives and/or targets at different stages of the food chain. EFSA J. 2011, 9, 2105. [Google Scholar] [CrossRef]
- Adzitey, F.; Huda, N.; Ali, G.R. Prevalence and antibiotic resistance of Campylobacter, Salmonella, and L. monocytogenes in ducks: A review. Foodborne Pathog. Dis. 2012, 9, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Abid, M.; Wimalarathna, H.; Mills, J.; Saldana, L.; Pang, W.; Richardson, J.F.; Maiden, M.C.; McCarthy, N.D. Duck liver-associated outbreak of campylobacteriosis among humans, united kingdom, 2011. Emerg. Infect. Dis. 2013, 19, 1310–1313. [Google Scholar] [CrossRef]
- Young, N.J.; Day, J.; Montsho-Hammond, F.; Verlander, N.Q.; Irish, C.; Pankhania, B.; Oliver, I. Campylobacter infection associated with consumption of duck liver pate: A retrospective cohort study in the setting of near universal exposure. Epidemiol. Infect. 2014, 142, 1269–1276. [Google Scholar] [CrossRef]
- Yamazaki-Matsune, W.; Taguchi, M.; Seto, K.; Kawahara, R.; Kawatsu, K.; Kumeda, Y.; Kitazato, M.; Nukina, M.; Misawa, N.; Tsukamoto, T. Development of a multiplex pcr assay for identification of Campylobacter coli, Campylobacter fetus, Campylobacter hyointestinalis subsp. hyointestinalis, Campylobacter jejuni, Campylobacter lari and Campylobacter upsaliensis. J. Med. Microbiol. 2007, 56, 1467–1473. [Google Scholar] [CrossRef]
- Dingle, K.E.; Colles, F.M.; Wareing, D.R.; Ure, R.; Fox, A.J.; Bolton, F.E.; Bootsma, H.J.; Willems, R.J.; Urwin, R.; Maiden, M.C. Multilocus sequence typing system for Campylobacter jejuni. J. Clin. Microbiol. 2001, 39, 14–23. [Google Scholar] [CrossRef]
- PubMLST. Available online: http://pubmlst.org/campylobacter (accessed on 1 May 2018).
- The Clinical & Laboratory Standards Institute (CLSI). Methods for Antimicrobial Dilution and Disk Susceptibility Testing for Infrequently-Isolated or Fastidious Bacteria: Approved Guidelines; Publication no. M45-a2; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2010. [Google Scholar]
- FDA. National Antimicrobial Resistance Monitoring System—Enteric Bacteria (NARMS): 2011 Executive Report; Department of Health and Human Services, Food and Drug Administration: Rockville, MD, USA, 2013.
- Bacon, D.J.; Alm, R.A.; Hu, L.; Hickey, T.E.; Ewing, C.P.; Batchelor, R.A.; Trust, T.J.; Guerry, P. DNA sequence and mutational analyses of the pVir plasmid of Campylobacter jejuni 811–76. Infect. Immun. 2002, 70, 6242–6250. [Google Scholar] [CrossRef]
- Oh, E.; Andrews, K.J.; McMullen, L.M.; Jeon, B. Tolerance to stress conditions associated with food safety in Campylobacter jejuni strains isolated from retail raw chicken. Sci. Rep. 2019, 9, 11915. [Google Scholar] [CrossRef]
- Wei, B.; Cha, S.Y.; Yoon, R.H.; Kang, M.; Roh, J.H.; Seo, H.S.; Lee, J.A.; Jang, H.K. Prevalence and antimicrobial resistance of Campylobacter spp. isolated from retail chicken and duck meat in South Korea. Food Control 2016, 62, 63–68. [Google Scholar] [CrossRef]
- Little, C.L.; Richardson, J.F.; Owen, R.J.; de Pinna, E.; Threlfall, E.J. Prevalence, characterisation and antimicrobial resistance of Campylobacter and Salmonella in raw poultrymeat in the UK, 20032–005. Int. J. Environ. Health Res. 2008, 18, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, F.; Ellis-Iversen, J.; Rushton, S.; Bull, S.A.; Harris, S.A.; Bryan, S.J.; Gonzalez, A.; Humphrey, T.J. Influence of season and geography on Campylobacter jejuni and C. coli subtypes in housed broiler flocks reared in great britain. Appl. Environ. Microbiol. 2011, 77, 3741–3748. [Google Scholar] [CrossRef] [PubMed]
- Rozynek, E.; Dzierzanowska-Fangrat, K.; Jozwiak, P.; Popowski, J.; Korsak, D.; Dzierzanowska, D. Prevalence of potential virulence markers in polish Campylobacter jejuni and Campylobacter coli isolates obtained from hospitalized children and from chicken carcasses. J. Med. Microbiol. 2005, 54, 615–619. [Google Scholar] [CrossRef] [PubMed]
- Melo, R.T.; Nalevaiko, P.C.; Mendonca, E.P.; Borges, L.W.; Fonseca, B.B.; Beletti, M.E.; Rossi, D.A. Campylobacter jejuni strains isolated from chicken meat harbour several virulence factors and represent a potential risk to humans. Food Control 2013, 33, 227–231. [Google Scholar] [CrossRef]
- Hanning, I.; Biswas, D.; Herrera, P.; Roesler, M.; Ricke, S.C. Prevalence and characterization of Campylobacter jejuni isolated from pasture flock poultry. J. Food Sci. 2010, 75, M496–M502. [Google Scholar] [CrossRef]
- Han, K.; Jang, S.S.; Choo, E.; Heu, S.; Ryu, S. Prevalence, genetic diversity, and antibiotic resistance patterns of Campylobacter jejuni from retail raw chickens in Korea. Int. J. Food Microbiol. 2007, 114, 50–59. [Google Scholar] [CrossRef]
- Ku, B.K.; Kim, H.J.; Lee, Y.J.; Kim, Y.I.; Choi, J.S.; Park, M.Y.; Kwon, J.W.; Nam, H.M.; Kim, Y.H.; Jung, S.C.; et al. Genetic characterization and antimicrobial susceptibility of Campylobacter spp. isolated from domestic and imported chicken meats and humans in Korea. Foodborne Pathog. Dis. 2011, 8, 381–386. [Google Scholar] [CrossRef]
- Adzitey, F.; Rusul, G.; Huda, N.; Cogan, T.; Corry, J. Prevalence, antibiotic resistance and rapd typing of Campylobacter species isolated from ducks, their rearing and processing environments in penang, malaysia. Int. J. Food Microbiol. 2012, 154, 197–205. [Google Scholar] [CrossRef]
- Wei, B.; Cha, S.Y.; Kang, M.; Roh, J.H.; Seo, H.S.; Yoon, R.H.; Jang, H.K. Antimicrobial susceptibility profiles and molecular typing of Campylobacter jejuni and Campylobacter coli isolates from ducks in South Korea. Appl. Environ. Microbiol. 2014, 80, 7604–7610. [Google Scholar] [CrossRef]
- Mohan, V.; Stevenson, M.; Marshall, J.; Fearnhead, P.; Holland, B.R.; Hotter, G.; French, N.P. Campylobacter jejuni colonization and population structure in urban populations of ducks and starlings in new zealand. MicrobiologyOpen 2013, 2, 659–673. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.; Greiner, M.; Holler, C.; Messelhausser, U.; Rampp, A.; Klein, G. Association between the ambient temperature and the occurrence of human Salmonella and Campylobacter infections. Sci. Rep. 2016, 6, 28442. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.O.; Jung, S.M.; Na, H.Y.; Chung, G.T.; Yoo, C.K.; Seong, W.K.; Hong, S. Enteric bacteria isolated from diarrheal patients in Korea in 2014. Osong Public Health Res. Perspect. 2015, 6, 233–240. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).