Native Rhizobial Inoculation Improves Tomato Yield and Nutrient Uptake While Mitigating Heavy Metal Accumulation in a Conventional Farming System
Abstract
1. Introduction
2. Materials and Methods
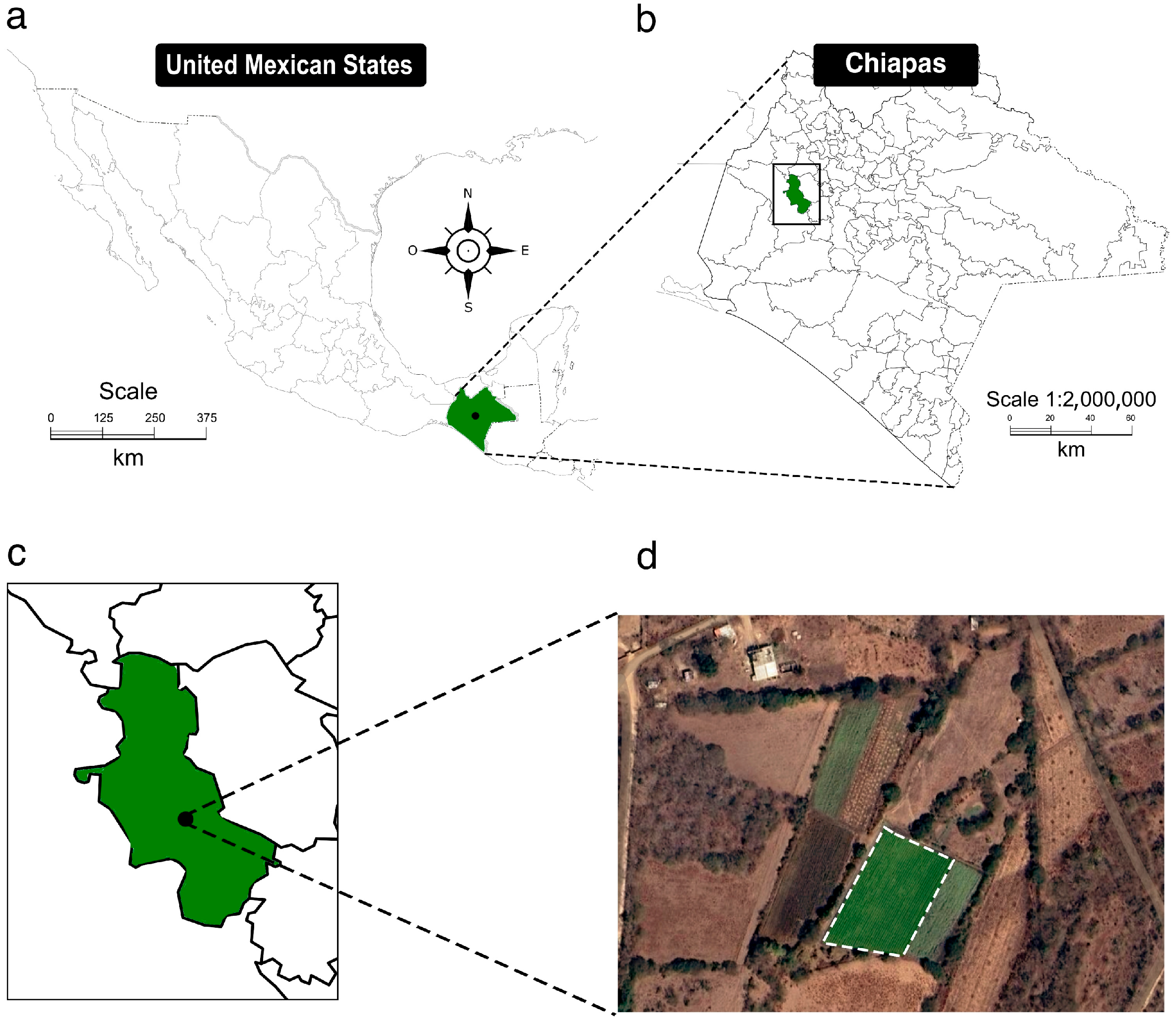
2.1. Field Site Description
2.2. Sampling Site and Field Experiment Establishment
2.3. Experimental Design
2.4. Bacterial Strains
2.5. Bacterial Inoculation Trial in Tomato Crop
2.6. Assessment of Morphological Traits, Fruit Yield, and Nutritional Composition in Plant Tissue
2.7. Molecular Analysis of Bacterial Communities
2.8. Statistical Analysis
3. Results
3.1. Bulk Soil Physicochemical Characteristics
3.2. Impact of Inoculation on Tomato Cultivation
3.3. Fruit Yield in Inoculated Plants
3.4. Nutritional Content in Plant Tissue
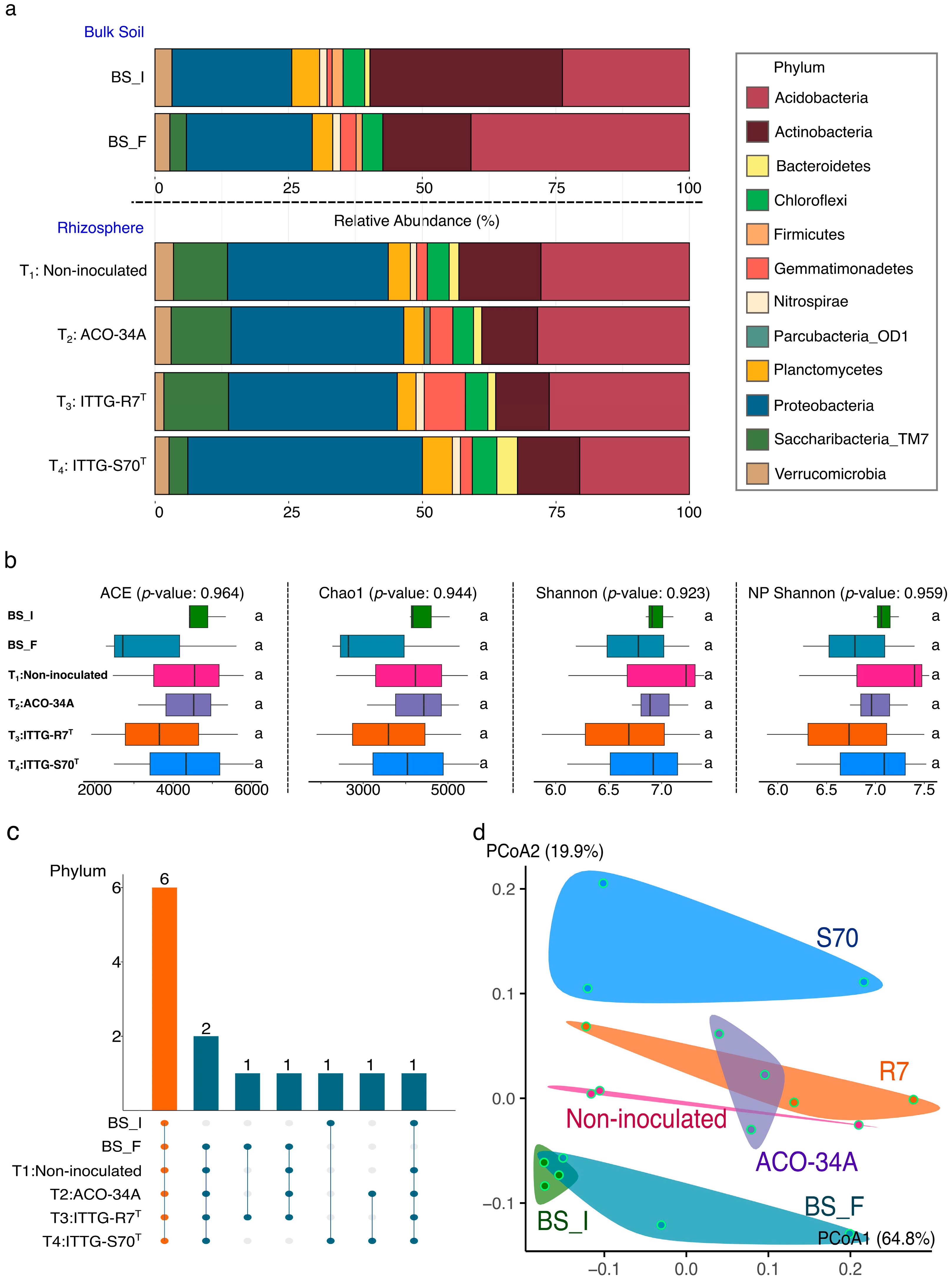
3.5. Bacterial Community Structure
3.6. Diversity and Species Richness of Bacterial Community
3.7. Core Soil Bacterial Community
3.8. Beta Diversity Analysis and Functional Biomarkers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arora, P.K.; Tripathi, S.; Omar, R.A.; Chauhan, P.; Sinhal, V.K.; Singh, A.; Srivastava, A.; Garg, S.K.; Singh, V.P. Next-generation fertilizers: The impact of bionanofertilizers on sustainable agriculture. Microb. Cell Factories 2024, 23, 254. [Google Scholar] [CrossRef]
- Giller, K.E.; Hijbeek, R.; Andersson, J.A.; Sumberg, J. Regenerative Agriculture: An agronomic perspective. Outlook Agric. 2021, 50, 13–25. [Google Scholar] [CrossRef]
- Govindasamy, P.; Muthusamy, S.K.; Bagavathiannan, M.; Mowrer, J.; Jagannadham, P.T.K.; Maity, A.; Halli, H.M.; K., S.G.; Vadivel, R.; K., D.T.; et al. Nitrogen use efficiency—A key to enhance crop productivity under a changing climate. Front. Plant Sci. 2023, 14, 1121073. [Google Scholar] [CrossRef]
- Harish, V.; Aslam, S.; Chouhan, S.; Pratap, Y.; Lalotra, S. Iron toxicity in plants: A Review. Int. J. Environ. Clim. Chang. 2023, 13, 1894. [Google Scholar] [CrossRef]
- Gen-Jiménez, A.; Flores-Félix, J.D.; Rincón-Molina, C.I.; Manzano-Gómez, L.A.; Villalobos-Maldonado, J.J.; Ruiz-Lau, N.; Roca-Couso, R.; Ruíz-Valdiviezo, V.M.; Rincón-Rosales, R. Native Rhizobium biofertilization enhances yield and quality in Solanum lycopersicum under field conditions. World J. Microbiol. Biotechnol. 2025, 41, 126. [Google Scholar] [CrossRef]
- Rincón-Molina, C.I.; Martínez-Romero, E.; Manzano-Gómez, L.A.; Rincón-Rosales, R. Growth Promotion of Guava “Pear” (Psidium guajava cv.) by Sinorhizobium mexicanum in Southern Mexican Agricultural Fields. Sustainability 2022, 14, 12391. [Google Scholar] [CrossRef]
- Tabassum, T.; Shahriar, S.; Araf, Y.; Ullah, M.A.; Islam, T. Potentials of Plant Probiotic Bacteria for Improving Growth and Health of Crop Plants. In Soil Bacteria: Biofertilization and Soil Health; Springer Nature: Singapore, 2024; pp. 333–358. [Google Scholar]
- Gupta, S.; Pandey, S. Plant growth promoting rhizobacteria to mitigate biotic and abiotic stress in plants. In Sustainable Agriculture Reviews; Springer: Cham, Switzerland, 2023; Volume 60, pp. 47–68. [Google Scholar]
- Mitter, E.K.; Tosi, M.; Obregón, D.; Dunfield, K.E.; Germida, J.J. Rethinking Crop Nutrition in Times of Modern Microbiology: Innovative Biofertilizer Technologies. Front. Sustain. Food Syst. 2021, 5, 606815. [Google Scholar] [CrossRef]
- Ivette, R.M.C.; Alberto, M.G.L.; Galdino, G.P.L.; Alexander, R.M.F.; Reiner, R.R. Advances and challenges in the production and use of native bacteria as plant probiotics in agronomic applications: A Mexican review. J. Agric. Food Res. 2025, 21, 101917. [Google Scholar] [CrossRef]
- Flores-Félix, J.D.; Menéndez, E.; Rivera, L.P.; Marcos-García, M.; Martínez-Hidalgo, P.; Mateos, P.F.; Martínez-Molina, E.; Velázquez Mde la, E.; García-Fraile, P.; Rivas, R. Use of Rhizobium leguminosarum as a potential biofertilizer for Lactuca sativa and Daucus carota crops. J. Plant Nutr. Soil Sci. 2013, 176, 876–882. [Google Scholar] [CrossRef]
- Flores-Félix, J.D.; Marcos-García, M.; Silva, L.R.; Menéndez, E.; Martínez-Molina, E.; Mateos, P.F.; Velázquez, E.; García-Fraile, P.; Andrade, P.; Rivas, R. Rhizobium as plant probiotic for strawberry production under microcosm conditions. Symbiosis 2015, 67, 25–32. [Google Scholar] [CrossRef]
- Jiménez-Gómez, A.; Flores-Félix, J.D.; García-Fraile, P.; Mateos, P.F.; Menéndez, E.; Velázquez, E.; Rivas, R. Probiotic activities of Rhizobium laguerreae on growth and quality of spinach. Sci. Rep. 2018, 8, 295. [Google Scholar] [CrossRef]
- De La Torre-Ruiz, N.; Ruiz-Valdiviezo, V.M.; Rincón-Molina, C.I.; Rodríguez-Mendiola, M.; Arias-Castro, C.; Gutiérrez-Miceli, F.A.; Palomeque-Dominguez, H.; Rincón-Rosales, R. Effect of plant growth-promoting bacteria on the growth and fructan production of Agave americana L. Braz. J. Microbiol. 2016, 47, 587–596. [Google Scholar] [CrossRef]
- García-Pérez, L.G.; Rincón-Molina, C.I.; Martínez-Romero, E.; Rogel, M.A.; Tapia-Torres, Y.; Manzano-Gómez, L.A.; Maldonado-Gómez, J.C.; Rincón-Molina, F.A.; Rincón-Rosales, R. Genomic Insights and Plant Growth-Promoting Potential of Rhizobial Strains from Agave americana. Horticulturae 2024, 10, 1370. [Google Scholar] [CrossRef]
- Maranto-Gómez, V.M.; Rincón-Molina, C.I.; Manzano-Gómez, L.A.; Gen-Jiménez, A.; Maldonado-Gómez, J.C.; Villalobos-Maldonado, J.J.; Ruiz-Valdiviezo, V.M.; Rincón-Rosales, R.; Rincón-Molina, F.A. Plant Probiotic Potential of Native Rhizobia to Enhance Growth and Sugar Content in Agave tequilana Weber var. Blue. Horticulturae 2025, 11, 137. [Google Scholar] [CrossRef]
- Gen-Jiménez, A.; Flores-Félix, J.D.; Rincón-Molina, C.I.; Manzano-Gomez, L.A.; Rogel, M.A.; Ruíz-Valdiviezo, V.M.; Rincón-Molina, F.A.; Rincón-Rosales, R. Enhance of tomato production and induction of changes on the organic profile mediated by Rhizobium biofortification. Front. Microbiol. 2023, 14, 1235930. [Google Scholar] [CrossRef]
- Le Campion, A.; Oury, F.-X.; Heumez, E.; Rolland, B. Conventional versus organic farming systems: Dissecting comparisons to improve cereal organic breeding strategies. Org. Agric. 2020, 10, 63–74. [Google Scholar] [CrossRef]
- Kumar, R.; Bhardwaj, A.; Singh, L.P.; Singh, G.; Kumar, A.; Pattnayak, K.C. Comparative life cycle assessment of environmental impacts and economic feasibility of tomato cultivation systems in northern plains of India. Sci. Rep. 2024, 14, 7084. [Google Scholar] [CrossRef]
- Gallegos-Cedillo, V.M.; Nájera, C.; Signore, A.; Ochoa, J.; Gallegos, J.; Egea-Gilabert, C.; Gruda, N.S.; Fernández, J.A. Analysis of global research on vegetable seedlings and transplants and their impacts on product quality. J. Sci. Food Agric. 2024, 104, 4950–4965. [Google Scholar] [CrossRef] [PubMed]
- Ebelhar, S.A.; Chesworth, W.; Paris, Q.; Spaargaren, O. Leptosols. In Encyclopedia of Soil Science; Chesworth, W., Ed.; Springer: Dordrecht, The Netherlands, 2008; pp. 437–438. [Google Scholar] [CrossRef]
- Manzano-Gómez, L.A.; Guzmán-Albores, J.M.; Rincón-Rosales, R.; Winkler, R.; Rincón-Molina, C.I.; Castañón-González, J.H.; Ruiz-Lau, N.; Gutiérrez-Miceli, F.A.; Rincón-Molina, F.A.; Ruíz-Valdiviezo, V.M. Evaluation of metabolomic profile and growth of moringa oleifera l. Cultivated with vermicompost under different soil types. Agronomy 2021, 11, 2061. [Google Scholar] [CrossRef]
- NOM-021-SEMARNAT-2000; Establece las Especificaciones de Fertilidad, Salinidad y Clasificación de Suelos. Estudios, Muestreo y Análisis. Secretaría de Medio Ambiente y Recursos Naturales: Ciudad de México, México, 2000. Available online: https://www.ordenjuridico.gob.mx/Documentos/Federal/wo69255.pdf (accessed on 13 August 2025).
- Orzolek, M. (Ed.) A Guide to the Manufacture, Performance, and Potential of Plastics in Agriculture; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–20. [Google Scholar] [CrossRef]
- Rincón-Molina, C.I.; Martínez-Romero, E.; Aguirre-Noyola, J.L.; Manzano-Gómez, L.A.; Zenteno-Rojas, A.; Rogel, M.A.; Rincón-Molina, F.A.; Ruíz-Valdiviezo, V.M.; Rincón-Rosales, R. Bacterial Community with Plant Growth-Promoting Potential Associated to Pioneer Plants from an Active Mexican Volcanic Complex. Microorganisms 2022, 10, 1568. [Google Scholar] [CrossRef] [PubMed]
- Rincón-Rosales, R.; Lloret, L.; Ponce, E.; Martínez-Romero, E. Rhizobia with different symbiotic efficiencies nodulate Acaciella angustissima in Mexico, including Sinorhizobium chiapanecum sp. nov. which has common symbiotic genes with Sinorhizobium mexicanum. FEMS Microbiol. Ecol. 2009, 67, 103–117. [Google Scholar] [CrossRef] [PubMed]
- Fan, G.; Guo, C.; Zhang, Q.; Liu, D.; Sun, Q.; Cui, Z.; Zhou, H.; Zhou, Y.; Guo, Z.; Ma, J.; et al. A secure visualization platform for pathogenic genome analysis with an accurate reference database. Biosaf. Health 2024, 6, 235–243. [Google Scholar] [CrossRef]
- Manzano-Gómez, L.A.; Rincón-Rosales, R.; Flores-Felix, J.D.; Gen-Jimenez, A.; Ruíz-Valdiviezo, V.M.; Ventura-Canseco, L.M.C.; Rincón-Molina, F.A.; Villalobos-Maldonado, J.J.; Rincón-Molina, C.I. Cost-Effective Cultivation of Native PGPB Sinorhizobium Strains in a Homemade Bioreactor for Enhanced Plant Growth. Bioengineering 2023, 10, 960. [Google Scholar] [CrossRef] [PubMed]
- Bashan, Y.; de-Bashan, L.E.; Prabhu, S.R.; Hernandez, J.P. Advances in plant growth-promoting bacterial inoculant technology: Formulations and practical perspectives (1998–2013). Plant Soil 2014, 378, 1–33. [Google Scholar] [CrossRef]
- Andrews, S. FASTQC: A Quality Control Tool for High Throughput Sequence Data (v 0.11.91). 2012. Available online: https://www.bibsonomy.org/bibtex/2b6052877491828ab53d3449be9b293b3/ozborn (accessed on 13 August 2025).
- McMurdie, P.J.; Holmes, S. Phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- RStudio Team. RStudio: Integrated Development Environment for R (1.4.1717). 2021. Available online: http://www.rstudio.com/ (accessed on 13 August 2025).
- Eddy, S.R. Accelerated Profile HMM Searches. PLoS Comput. Biol. 2011, 7, e1002195. [Google Scholar] [CrossRef]
- Lee, B.; Moon, T.; Yoon, S.; Weissman, T. DUDE-Seq: Fast, flexible, and robust denoising for targeted amplicon sequencing. PLoS ONE 2017, 12, e0181463. [Google Scholar] [CrossRef]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef]
- Yoon, S.-H.; Ha, S.-M.; Kwon, S.; Lim, J.; Kim, Y.; Seo, H.; Chun, J. Introducing EzBioCloud: A taxonomically united database of 16S rRNA gene sequences and whole-genome assemblies. Int. J. Syst. Evol. Microbiol. 2017, 67, 1613–1617. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; Available online: https://ggplot2.tidyverse.org (accessed on 13 August 2025).
- Chen, T.; Liu, Y.X.; Huang, L. ImageGP: An easy-to-use data visualization web server for scientific researchers. IMeta 2022, 1, e5. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Y.; Liu, H.; Li, X.; Zhao, J.; Dong, Z.; Li, J.; Kaka, N.A.; Nazar, M.; Shao, T. Using PICRUSt2 to explore the functional potential of bacterial community in alfalfa silage harvested at different growth stages. Chem. Biol. Technol. Agric. 2022, 9, 98. [Google Scholar] [CrossRef]
- De Mendiburu, F.; Yaseen, M. Agricolae: Statistical Procedures for Agricultural Research. 2020. Available online: https://cran.r-project.org/package=agricolae (accessed on 13 August 2025).
- KozaK, M.; BocianowsKi, J. Note on the use of coefficieNt of variatioN for data from agricultural factorial experiments. Bulg. J. Agric. Sci. 2013, 19, 644–646. [Google Scholar]
- Kassambara, A. factoextra: Extract and Visualize the Results of Multivariate Data Analyses. 2016. Available online: https://cran.r-project.org/web/packages/factoextra/readme/README.html (accessed on 13 August 2025).
- Akoglu, H. User’s guide to correlation coefficients. Turk. J. Emerg. Med. 2018, 18, 91–93. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Souza, T. Redundancy Analysis (RDA). In Advanced Statistical Analysis for Soil Scientists; Springer Nature: Cham, Switzerland, 2025; pp. 57–77. [Google Scholar]
- Lloret, L.; Ormeño-Orrillo, E.; Rincón, R.; Martínez-Romero, J.; Rogel-Hernández, M.A.; Martínez-Romero, E. Ensifer mexicanus sp. nov. a new species nodulating Acacia angustissima (Mill.) Kuntze in Mexico. Syst. Appl. Microbiol. 2007, 30, 280–290. [Google Scholar] [CrossRef]
- Feng, Y.; Tian, B.; Xiong, J.; Lin, G.; Cheng, L.; Zhang, T.; Lin, B.; Ke, Z.; Li, X. Exploring IAA biosynthesis and plant growth promotion mechanism for tomato root endophytes with incomplete IAA synthesis pathways. Chem. Biol. Technol. Agric. 2024, 11, 187. [Google Scholar] [CrossRef]
- Ruíz-Valdiviezo, V.M.; Rogel-Hernandez, M.A.; Guerrero, G.; Rincón-Molina, C.I.; García-Perez, L.G.; Gutiérrez-Miceli, F.A.; Villalobos-Maldonado, J.J.; López-López, A.; Martinez-Romero, E.; Rincón-Rosales, R. Complete genome sequence of a novel nonnodulating Rhizobium species isolated from Agave americana L. rhizosphere. Genome Announc. 2017, 5, e01280-17. [Google Scholar] [CrossRef]
- Kassam, A.; Kassam, L. Paradigms of agriculture. In Rethinking Food and Agriculture; Kassam, A., Kassam, L., Eds.; Woodhead Publishing: Cambridge, UK, 2021; pp. 181–218. [Google Scholar] [CrossRef]
- Sumberg, J.; Giller, K.E. What is ‘conventional’ agriculture? Glob. Food Secur. 2022, 32, 100617. [Google Scholar] [CrossRef]
- Bissadu, K.D.; Sonko, S.; Hossain, G. Society 5.0 enabled agriculture: Drivers, enabling technologies, architectures, opportunities, and challenges. Inf. Process. Agric. 2025, 12, 112–124. [Google Scholar] [CrossRef]
- Plank, C.O. Plant Analysis Handbook for Georgia. Cooperative Extension Service, University of Georgia College of Agriculture. 1989. Available online: https://books.google.com.mx/books?id=L2ScGwAACAAJ (accessed on 13 August 2025).
- Arifur Rahman, M.; Harker, T.; Lewis, W.; Islam, K.R. Chelated- and nano iron fertilization affects nutrient uptake and translocation in fresh market tomatoes. J. Plant Nutr. 2024, 47, 1152–1174. [Google Scholar] [CrossRef]
- Alves, A.; Ribeiro, R.; Azenha, M.; Cunha, M.; Teixeira, J. Effects of Exogenously Applied Copper in Tomato Plants’ Oxidative and Nitrogen Metabolisms under Organic Farming Conditions. Horticulturae 2023, 9, 323. [Google Scholar] [CrossRef]
- Garber, A.I.; Nealson, K.H.; Okamoto, A.; McAllister, S.M.; Chan, C.S.; Barco, R.A.; Merino, N. FeGenie: A Comprehensive Tool for the Identification of Iron Genes and Iron Gene Neighborhoods in Genome and Metagenome Assemblies. Front. Microbiol. 2020, 11, 37. [Google Scholar] [CrossRef]
- Krewulak, K.D.; Vogel, H.J. Structural biology of bacterial iron uptake. Biochim. Biophys. Acta—Biomembr. 2008, 1778, 1781–1804. [Google Scholar] [CrossRef]
- Gudipaty, S.A.; McEvoy, M.M. The histidine kinase CusS senses silver ions through direct binding by its sensor domain. Biochim. Biophys. Acta—Proteins Proteom. 2014, 1844, 1656–1661. [Google Scholar] [CrossRef]
- Outten, F.W.; Huffman, D.L.; Hale, J.A.; O’Halloran, T.V. The Independent cue and cus Systems Confer Copper Tolerance during Aerobic and Anaerobic Growth in Escherichia coli. J. Biol. Chem. 2001, 276, 30670–30677. [Google Scholar] [CrossRef]
- Rowland, J.L.; Niederweis, M. A multicopper oxidase is required for copper resistance in mycobacterium tuberculosis. J. Bacteriol. 2013, 195, 3724–3733. [Google Scholar] [CrossRef]
- Wekesa, C.; Kiprotich, K.; Okoth, P.; Asudi, G.O.; Muoma, J.O.; Furch, A.C.; Oelmüller, R. Molecular characterization of indigenous rhizobia from Kenyan soils nodulating with common beans. Int. J. Mol. Sci. 2023, 24, 9509. [Google Scholar] [CrossRef]
- Pajuelo, E.; Carrasco, J.A.; Flores-Duarte, N.J.; Rodríguez-Llorente, I.D.; Mesa-Marín, J.; Mateos-Naranjo, E.; Redondo-Gómez, S.; Navarro-Torre, S. Designing Tailored Bioinoculants for Sustainable Agrobiology in Multi-Stressed Environments. Sustain. Agrobiol. Des. Dev. Microb. Consortia 2023, 43, 359–397. [Google Scholar]
- Zuluaga, M.Y.A.; Milani, K.M.L.; Miras-Moreno, B.; Lucini, L.; Valentinuzzi, F.; Mimmo, T.; Pii, Y.; Cesco, S.; Rodrigues, E.P.; Oliveira, A.L.M.d.e. Inoculation with plant growth-promoting bacteria alters the rhizosphere functioning of tomato plants. Appl. Soil Ecol. 2021, 158, 103784. [Google Scholar] [CrossRef]
- Adedayo, A.; Fadiji, A.; Babalola, O. The Effects of Plant Health Status on the Community Structure and Metabolic Pathways of Rhizosphere Microbial Communities Associated with Solanum lycopersicum. Horticulturae 2022, 8, 404. [Google Scholar] [CrossRef]
- Gu, Y.; Dong, K.; Geisen, S.; Yang, W.; Yan, Y.; Gu, D.; Liu, N.; Borisjuk, N.; Luo, Y.; Friman, V.P. The effect of microbial inoculant origin on the rhizosphere bacterial community composition and plant growth-promotion. Plant Soil. 2020, 452, 105–117. [Google Scholar] [CrossRef]
- Xing, Y.; Wang, X.; Mustafa, A. Exploring the link between soil health and crop productivity. Ecotoxicol. Environ. Saf. 2025, 289, 117703. [Google Scholar] [CrossRef]
- Naz, M.; Dai, Z.; Hussain, S.; Tariq, M.; Danish, S.; Khan, I.U.; Qi, S.; Du, D. The soil pH and heavy metals revealed their impact on soil microbial community. J. Environ. Manag. 2022, 321, 115770. [Google Scholar] [CrossRef]
- Naylor, D.; McClure, R.; Jansson, J. Trends in microbial community composition and function by soil depth. Microorganisms 2022, 10, 540. [Google Scholar] [CrossRef]
- Wan, W.; Tan, J.; Wang, Y.; Qin, Y.; He, H.; Wu, H.; Zuo, W.; He, D. Responses of the rhizosphere bacterial community in acidic crop soil to pH: Changes in diversity, composition, interaction, and function. Sci. Total Environ. 2020, 700, 134418. [Google Scholar] [CrossRef]
- Trivedi, P.; Leach, J.E.; Tringe, S.G.; Sa, T.; Singh, B.K. Plant–microbiome interactions: From community assembly to plant health. Nat. Rev. Microbiol. 2020, 18, 607–621. [Google Scholar] [CrossRef]
- Liu, Z.; Cao, S.; Sun, Z.; Wang, H.; Qu, S.; Lei, N.; He, J.; Dong, Q. Tillage effects on soil properties and crop yield after land reclamation. Sci. Rep. 2021, 11, 4611. [Google Scholar] [CrossRef]
- Spohn, M.; Braun, S.; Sierra, C.A. Continuous decrease in soil organic matter despite increased plant productivity in an 80-years-old phosphorus-addition experiment. Commun. Earth Environ. 2023, 4, 251. [Google Scholar] [CrossRef]



| Physical characteristics | ||||||
| Sample | Texture | SP (%) | WHC (%) | PWP (%) | HC (cm h−1) | BD (g cm−3) |
| BS_I | Clay | 78.47 ± (3.90) A Ψ | 42.17 ± (2.16) A | 25.07 ± (1.29) A | 0.10 ± (0.01) B | 1.21 ± (0.05) A |
| BS_F | Clay | 63.36 ± (4.83) A | 33.84 ± (2.44) A | 20.47 ± (1.74) A | 0.69 ± (0.03) A | 1.13 ± (0.03) A |
| p < 0.05 | 0.0631 | 0.0602 | 0.0732 | 0.0005 | 0.1863 | |
| Chemical characteristics | ||||||
| Sample | pH | TC (%) | EC (dS m−1) | CEC (meq 100 g−1) | SOM (%) | |
| BS_I | 7.96 ± (0.23) A Ψ | 26.30 ± (9.01) A | 1.18 ± (0.27) A | 48.47 ± (1.15) A | 5.39 ± (0.16) A | |
| BS_F | 8.67 ± (0.34) A | 39.82 ± (1.90) A | 1.50 ± (0.04) A | 44.0 ± (1.55) A | 5.01 ± (0.15) A | |
| p < 0.05 | 0.1151 | 0.1419 | 0.2119 | 0.0585 | 0.0768 | |
| Soil macronutrients (ppm) | ||||||
| Sample | NO3 | P | K | Ca | S | Mg |
| BS_I α | 31.93 ± (1.99) B Ψ | 26.10 ± (15.86) A | 215.00 ± (9.64) B | 8424.33 ± (201.40) A | 9.95 ± (1.82) B | 708.67 ± (13.58) A |
| BS_F Ω | 38.40 ± (0.94) A | 20.24 ± (0.88) A | 447.89 ± (35.73) A | 7666.33 ± (240.73) A | 26.97± (0.98) A | 782.67 ± (34.15) A |
| p < 0.05 | 0.0453 | 0.5889 | 0.0055 | 0.0952 | 0.0046 | 0.1134 |
| Soil micronutrients (ppm) | ||||||
| Sample | Na | Fe | Zn | Mn | Cu | B |
| BS_I | 24.17 ± (0.21) B Ψ | 28.97 ± (0.78) A | 1.04 ± (0.01) B | 2.85 ± (0.15) A | 3.47 ± (0.17) A | 1.15 ± (0.04) B |
| BS_F | 27.65 ± (1.01) A | 8.51 ± (0.36) B | 1.80 ± (0.08) A | 3.58 ± (0.18) A | 2.17 ± (0.12) B | 2.15 ± (0.13) A |
| p < 0.05 | 0.0377 | 0.0006 | 0.0036 | 0.0588 | 0.0045 | 0.0028 |
| Treatment | Plant Height (cm) | Plant Stem Width (mm) | Plant Dry Weight (g) | Chlorophyll (SPAD Index) |
|---|---|---|---|---|
| T1: non-inoculated | 117.33 ± (3.79) A Ψ | 12.45 ± (1.91) C | 246.67 ± (68.25) AB | 37.09 ± (3.63) D |
| T2: ACO-34 A | 85.33 ± (8.39) B | 13.89 ± (2.55) B | 170.00 ± (52.92) B | 39.41 ± (2.90) C |
| T3: ITTG-R7 T | 119.33 ± (3.06) A | 16.12 ± (3.10) A | 306.67 ± (15.28) A | 41.26 ± (2.92) B |
| T4: ITTG-S70 T | 108.00 ± (15.62) AB | 15.41 ± (3.51) A | 135.00 ± (13.23) B | 43.19 ± (2.39) A |
| p-value | 0.0069 | 0.0000 | 0.0059 | 0.0000 |
| HSD £ (p < 0.05) | 24.0354 | 0.9425 | 115.9548 | 0.9961 |
| CV * (%) | 8.51 | 19.53 | 20.63 | 7.44 |
| Treatment | Fruits per Plant | Fruit Height (cm) | Fruit Width (mm) | Fruit Weight (g) | EFV (cm3) |
|---|---|---|---|---|---|
| T1: non-inoculated | 15.61 ± (7.24) B Ψ | 72.37 ± (5.14) B | 65.46 ± (6.99) C | 155.50 ± (33.67) D | 164.19 ± (37.67) C |
| T2: ACO-34A | 22.23 ± (5.84) A | 77.68 ± (7.40) A | 83.75 ± (8.44) A | 281.56 ± (86.29) A | 291.25 ± (75.63) A |
| T3: ITTG-R7 T | 21.91 ± (5.92) A | 77.47 ± (5.35) A | 79.29 ± (7.50) B | 246.92 ± (53.57) B | 257.05 ± (53.83) B |
| T4: ITTG-S70 T | 21.18 ± (3.81) A | 73.25 ± (5.30) B | 79.05 ± (6.06) B | 218.85 ± (46.40) C | 241.62 ± (46.05) B |
| p-value | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
| HSD £ (p < 0.05) | 1.9405 | 2.7588 | 3.2307 | 27.4065 | 25.8819 |
| CV * (%) | 28.86 | 7.81 | 9.49 | 25.81 | 23.17 |
| Macronutrients Ω (g plant−1) | ||||||||
| Treatment | N | P | K | Ca | Mg | S | ||
| T1: non-inoculated | 4.80 ± (1.28) AB Ψ | 0.49 ± (0.14) AB | 3.66 ± (0.94) AB | 14.56 ± (3.86) A | 1.71 ± (0.44) AB | 2.75 ± (0.80) A | ||
| T2: ACO-34A | 3.45 ± (1.06) AB | 0.34 ± (0.09) B | 3.26 ± (0.99) AB | 7.49 ± (2.29) B | 2.10 ± (0.62) AB | 1.02 ± (0.30) B | ||
| T3: ITTG-R7 T | 5.67 ± (0.25) A | 0.71 ± (0.06) A | 4.96 ± (0.29) A | 19.12 ± (0.75) A | 2.52 ± (0.14) A | 3.19 ± (0.15) A | ||
| T4: ITTG-S70 T | 3.27 ± (0.30) B | 0.31 ± (0.04) B | 2.92 ± (0.27) B | 7.79 ± (0.80) B | 1.24 ± (0.14) B | 1.28 ± (0.11) B | ||
| p-value | 0.0253 | 0.0022 | 0.0343 | 0.0007 | 0.0215 | 0.0007 | ||
| HSD £ (p < 0.05) | 2.2323 | 0.2324 | 1.8542 | 6.043 | 1.0318 | 1.1449 | ||
| Micronutrients Ω (mg plant−1) | ||||||||
| Treatment | Na | Fe | Zn | Mn | Cu | B | Ni | Mo |
| T1: non-inoculated | 133.01 ± (37.08) B Ψ | 341.82 ± (93.24) A | 45.17 ± (12.19) B | 104.04 ± (29.95) A | 40.46 ± (11.21) A | 15.55 ± (4.23) A | 3.73 ± (1.00) A | 0.25 ± (0.07) A |
| T2: ACO-34A | 135.43 ± (42.76) B | 44.06 ± (13.86) B | 25.57 ± (7.70) C | 21.73 ± (6.62) B | 8.30 ± (2.53) C | 6.67 ± (2.04) B | 0.79 ± (0.25) B | 0.13 ± (0.04) B |
| T3: ITTG-R7 T | 236.13 ± (11.57) A | 40.18 ± (2.13) B | 68.82 ± (3.59) A | 81.49 ± (4.78) A | 24.34 ± (1.27) B | 14.37 ± (0.95) A | 1.22 ± (0.06) B | 0.16 ± (0.01) AB |
| T4: ITTG-S70 T | 109.70 ± (10.54) B | 41.61 ± (3.91) B | 15.71 ± (1.66) C | 28.77 ± (3.01) B | 8.16 ± (0.75) C | 6.51 ± (0.62) B | 0.82 ± (0.08) B | 0.16 ± (0.02) AB |
| p-value | 0.0033 | 0.0001 | 0.000 | 0.0005 | 0.0004 | 0.0024 | 0.0003 | 0.0382 |
| HSD £ (p < 0.05) | 76.7702 | 123.3776 | 19.542 | 40.7789 | 15.1435 | 6.3124 | 1.3515 | 0.1118 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manzano-Gómez, L.A.; Rincón-Molina, C.I.; Martínez-Romero, E.; Stopol-Martínez, S.S.; Santos-Santiago, A.; Villalobos-Maldonado, J.J.; Ruíz-Valdiviezo, V.M.; Rincón-Rosales, R. Native Rhizobial Inoculation Improves Tomato Yield and Nutrient Uptake While Mitigating Heavy Metal Accumulation in a Conventional Farming System. Microorganisms 2025, 13, 1904. https://doi.org/10.3390/microorganisms13081904
Manzano-Gómez LA, Rincón-Molina CI, Martínez-Romero E, Stopol-Martínez SS, Santos-Santiago A, Villalobos-Maldonado JJ, Ruíz-Valdiviezo VM, Rincón-Rosales R. Native Rhizobial Inoculation Improves Tomato Yield and Nutrient Uptake While Mitigating Heavy Metal Accumulation in a Conventional Farming System. Microorganisms. 2025; 13(8):1904. https://doi.org/10.3390/microorganisms13081904
Chicago/Turabian StyleManzano-Gómez, Luis Alberto, Clara Ivette Rincón-Molina, Esperanza Martínez-Romero, Simón Samuel Stopol-Martínez, Amado Santos-Santiago, Juan José Villalobos-Maldonado, Víctor Manuel Ruíz-Valdiviezo, and Reiner Rincón-Rosales. 2025. "Native Rhizobial Inoculation Improves Tomato Yield and Nutrient Uptake While Mitigating Heavy Metal Accumulation in a Conventional Farming System" Microorganisms 13, no. 8: 1904. https://doi.org/10.3390/microorganisms13081904
APA StyleManzano-Gómez, L. A., Rincón-Molina, C. I., Martínez-Romero, E., Stopol-Martínez, S. S., Santos-Santiago, A., Villalobos-Maldonado, J. J., Ruíz-Valdiviezo, V. M., & Rincón-Rosales, R. (2025). Native Rhizobial Inoculation Improves Tomato Yield and Nutrient Uptake While Mitigating Heavy Metal Accumulation in a Conventional Farming System. Microorganisms, 13(8), 1904. https://doi.org/10.3390/microorganisms13081904









