Isolation and Screening of the Novel Multi-Trait Strains for Future Implications in Phytotechnology
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Collection
2.2. Isolation of Microbial Strains
2.3. Screening Microbial Isolates for Growth-Promoting Properties
2.3.1. Indole-3-Acetic Acid (IAA) Synthesis Assay
2.3.2. Phosphate Solubilisation Assay
2.3.3. Determination of N2 Fixation Activity
2.3.4. Determination of Antifungal Activity
2.3.5. Zinc (Zn) Solubilization Assay
2.4. Biochemical Properties of Microbial Isolates
2.4.1. Determination of Citrate Assimilation
2.4.2. Determination of Carbohydrate Fermentation
2.4.3. Catalase Assay
2.4.4. Determination of Amylolytic Activity
2.4.5. Determination of Protease Activity
2.4.6. Determination of Lipase Activity
2.4.7. Determination of Cellulolytic Activity
2.5. 16S rRNA Gene Sequencing
Phylogenetic Identification of Microbial Isolates
2.6. Seed Germination Assay
2.7. Statistical Analysis
3. Results
3.1. Isolation and Morphological Characterisation of Microbial Strains
3.2. Screening Microbial Isolates for Plant Growth-Promoting Properties
3.2.1. Indole-3-Acetic Acid Production
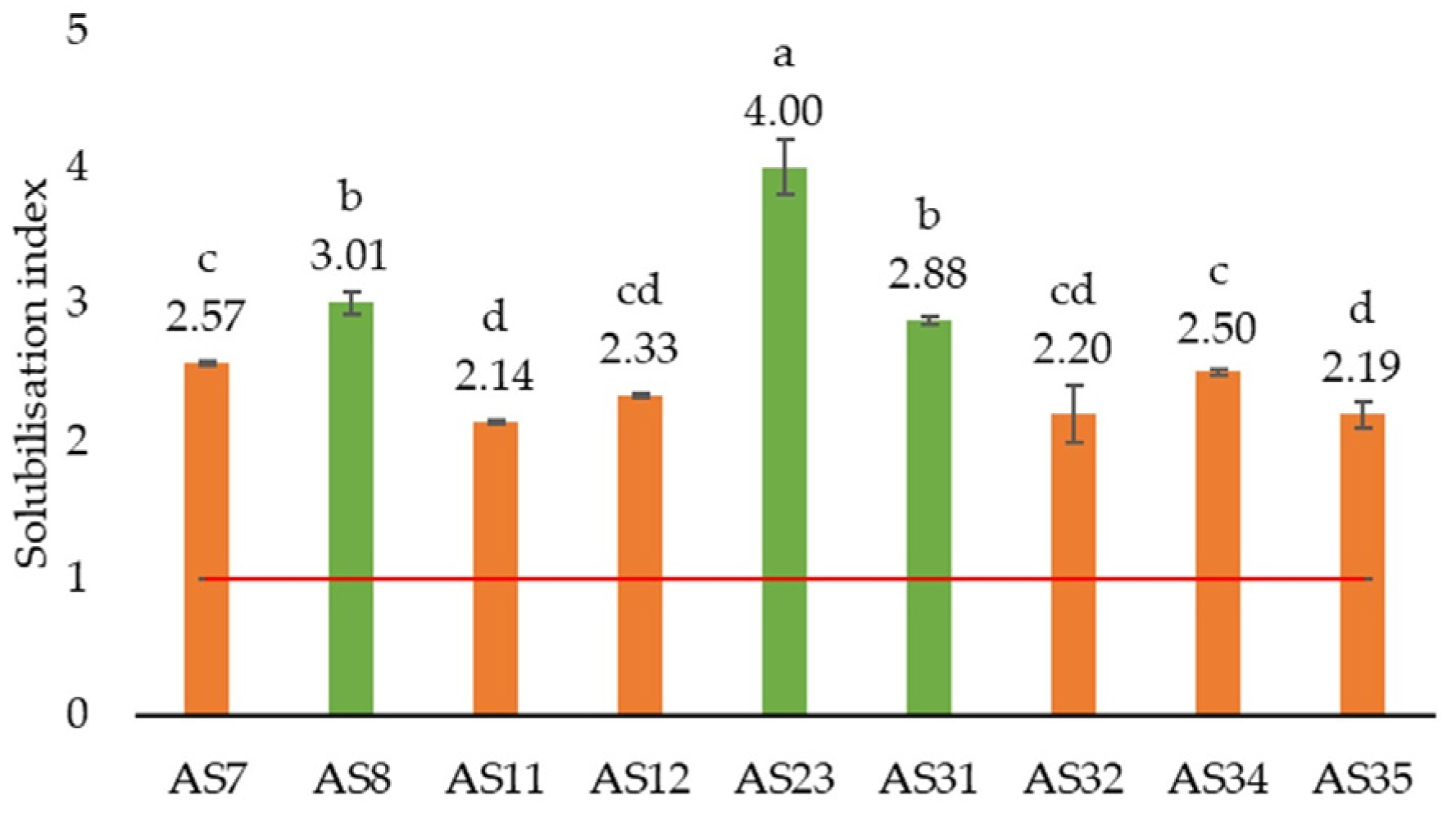
3.2.2. Phosphate Solubilisation
3.2.3. Nitrogen Fixation
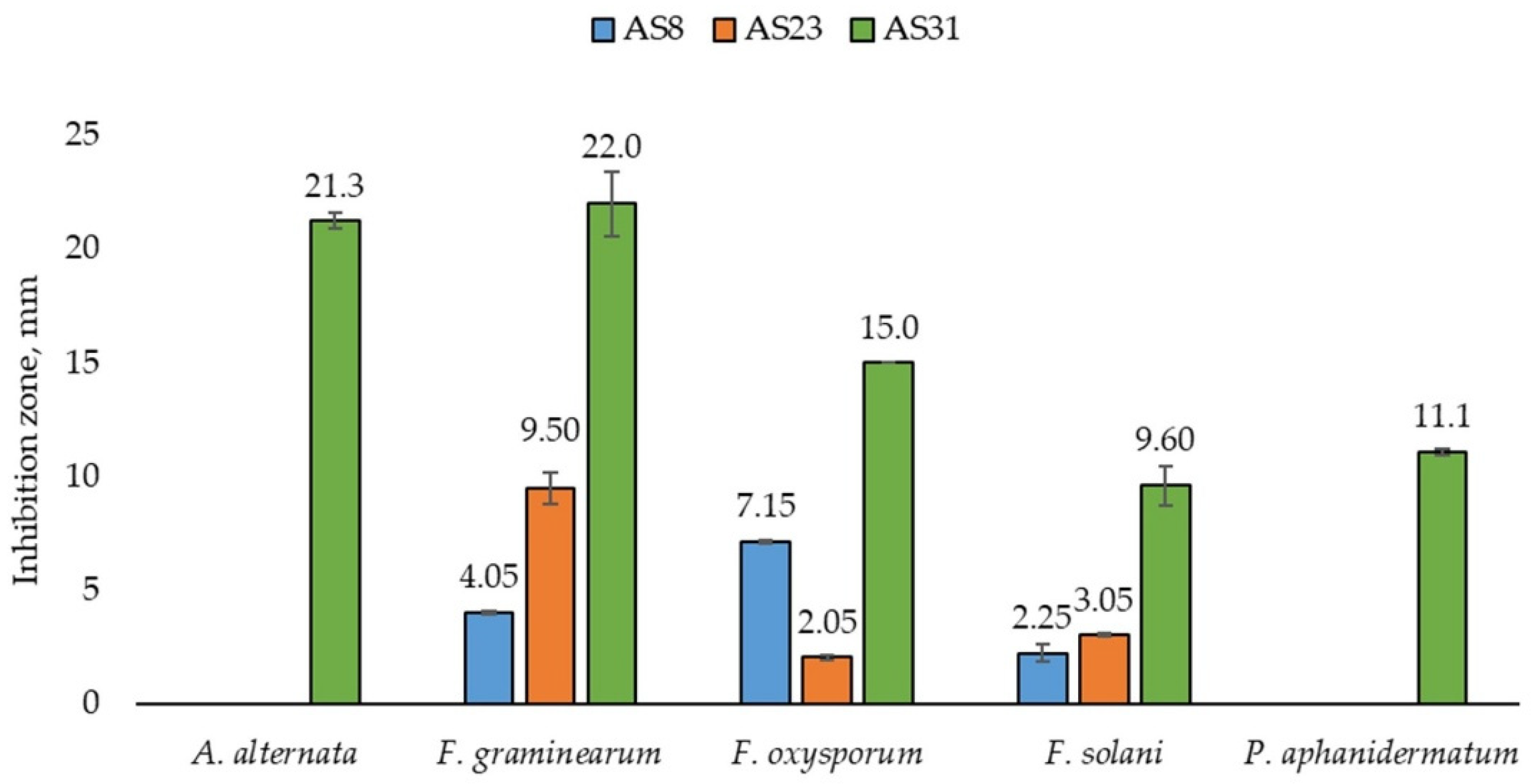
3.2.4. Antifungal Activity
3.2.5. Zinc Solubilization
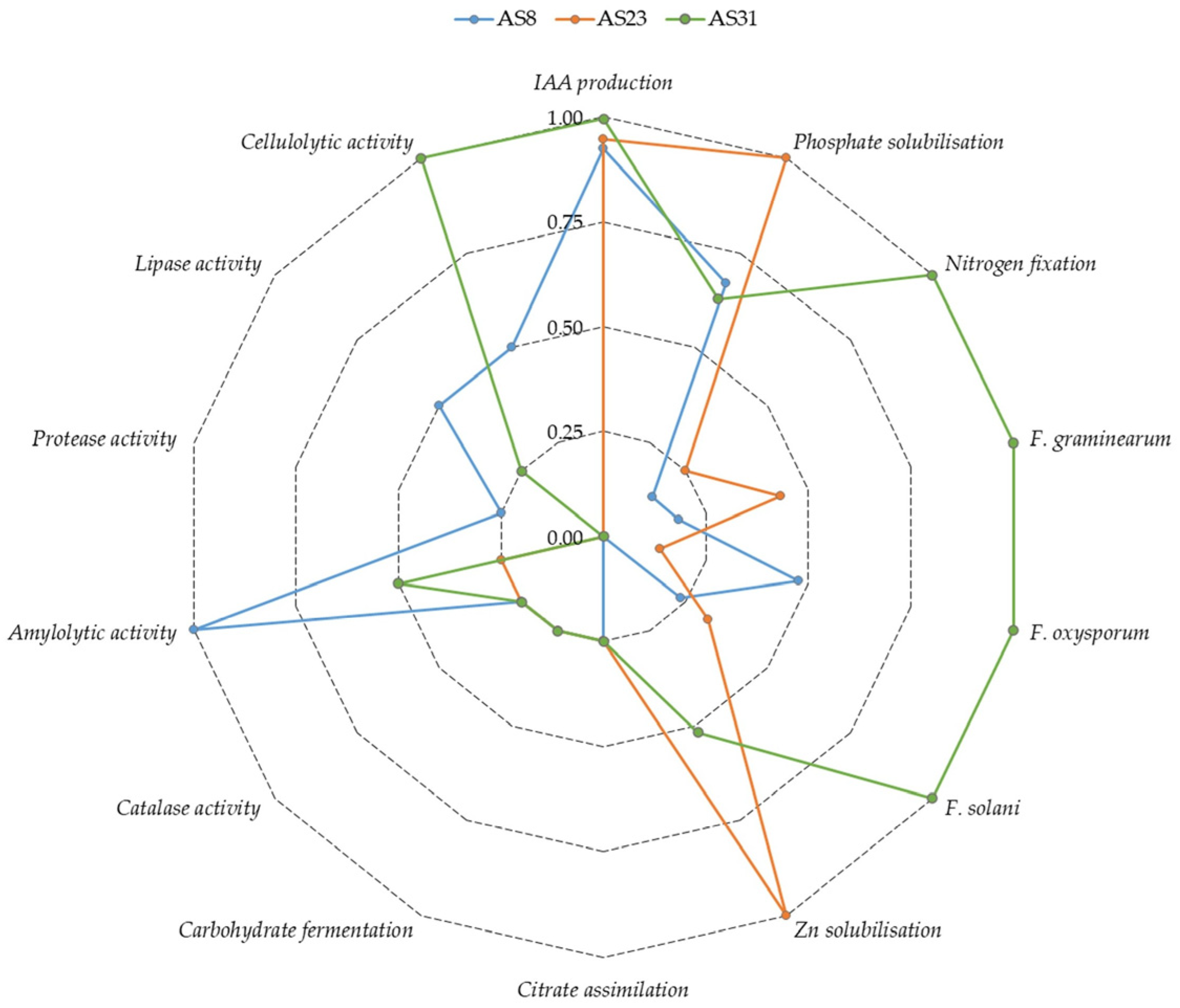
3.3. Biochemical Characterisation of Promising Strains
3.4. Genetic Identification of Promising Strains
3.5. Influence of PGPRs on Seed Germination
4. Discussion
4.1. Plant Growth-Promoting Properties of Isolates
4.1.1. Indole-3-Acetic Acid Production
4.1.2. Nutrient Solubilization
4.2. Synergy of Plant Growth-Promoting Traits
4.3. Biocontrol Potential of Isolates
4.3.1. Hydrolytic Enzymes Activity
4.3.2. Antifungal Activity
4.4. Comparative Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PGPR | Plant growth-promoting rhizobacteria |
| PGP | Plant growth-promoting |
| IAA | Indole-3-acetic acid |
References
- Tripathi, A.D.; Mishra, R.; Maurya, K.K.; Singh, R.B.; Wilson, D.W. Chapter 1—Estimates for World Population and Global Food Availability for Global Health. In The Role of Functional Food Security in Global Health; Singh, R.B., Watson, R.R., Takahashi, T., Eds.; Academic Press: New York, NY, USA, 2019; pp. 3–24. ISBN 978-0-12-813148-0. [Google Scholar]
- FAO; IFAD; UNICEF; WFP; WHO. The State of Food Security and Nutrition in the World 2024—Financing to End Hunger. In Food Insecurity and Malnutrition in All Its Forms; FAO: Rome, Italy, 2024. [Google Scholar]
- Akanmu, A.O.; Olowe, O.M.; Phiri, A.T.; Nirere, D.; Odebode, A.J.; Karemera Umuhoza, N.J.; Asemoloye, M.D.; Babalola, O.O. Bioresources in Organic Farming: Implications for Sustainable Agricultural Systems. Horticulturae 2023, 9, 659. [Google Scholar] [CrossRef]
- Nurzhanova, A.; Pidlisnyuk, V.; Nurmagambetova, A.; Zhumasheva, Z.; Naizabayeva, L.; Mamirova, A. Biochar as a Tool to Optimise Miscanthus sinensis Resilience and Phytoremediation Efficiency: Case Study of Contamination by Mixture of Ni and 4.4′-DDE. Environ. Chem. Ecotoxicol. 2025, 7, 802–818. [Google Scholar] [CrossRef]
- Mishra, S.; Keswani, C.; Abhilash, P.C.; Fraceto, L.F.; Singh, H.B. Integrated Approach of Agri-Nanotechnology: Challenges and Future Trends. Front. Plant Sci. 2017, 8, 471. [Google Scholar] [CrossRef]
- Smirnova, I.; Sadanov, A.; Baimakhanova, G.; Faizulina, E.; Tatarkina, L. Metabolic Interaction at the Level of Extracellular Amino Acids between Plant Growth-Promoting Rhizobacteria and Plants of Alfalfa (Medicago sativa L.). Rhizosphere 2022, 21, 100477. [Google Scholar] [CrossRef]
- Zharlygassov, Z.; Kalimov, N.; Ansabayeva, A.; Zharlygassov, Z.; Moskvicheva, E.; İslamzade, R.; Ay, A.; Akça, İ.; Kızılkaya, R. Sustainable Nutrient Management and Agricultural Productivity in Chernozem Soils of the Kostanay Region, Kazakhstan. Eur. J. Sport Sci. 2025, 14, 98–106. [Google Scholar] [CrossRef]
- Batykova, Z.K.; Kistaubayeva, A.S.; Savitskaya, I.S.; Pidlisnyuk, V. Isolation and Study of Plant Growth Promoting Rhizobacteria from Triticosecale Wittmack Growing in Almaty Region. Int. J. Biol. Chem. 2024, 17, 53–59. [Google Scholar] [CrossRef]
- European Commission. Organic Action Plan. Available online: https://agriculture.ec.europa.eu/farming/organic-farming/organic-action-plan_en (accessed on 4 August 2024).
- Ajijah, N.; Fiodor, A.; Pandey, A.K.; Rana, A.; Pranaw, K. Plant Growth-Promoting Bacteria (PGPB) with Biofilm-Forming Ability: A Multifaceted Agent for Sustainable Agriculture. Diversity 2023, 15, 112. [Google Scholar] [CrossRef]
- Nosheen, S.; Ajmal, I.; Song, Y. Microbes as Biofertilizers, a Potential Approach for Sustainable Crop Production. Sustainability 2021, 13, 1868. [Google Scholar] [CrossRef]
- Umesha, S.; Manukumar, H.M.G.; Chandrasekhar, B. Chapter 3—Sustainable Agriculture and Food Security. In Biotechnology for Sustainable Agriculture; Singh, R.L., Mondal, S., Eds.; Woodhead Publishing: Cambridge, UK, 2018; pp. 67–92. ISBN 978-0-12-812160-3. [Google Scholar]
- Wang, T.; Xu, J.; Chen, J.; Liu, P.; Hou, X.; Yang, L.; Zhang, L. Progress in Microbial Fertilizer Regulation of Crop Growth and Soil Remediation Research. Plants 2024, 13, 346. [Google Scholar] [CrossRef]
- Pidlisnyuk, V.; Mamirova, A.; Pranaw, K.; Shapoval, P.Y.; Trögl, J.; Nurzhanova, A. Potential Role of Plant Growth-Promoting Bacteria in Miscanthus x Giganteus Phytotechnology Applied to the Trace Elements Contaminated Soils. Int. Biodeterior. Biodegrad. 2020, 155, 105103. [Google Scholar] [CrossRef]
- Pidlisnyuk, V.; Mamirova, A.; Pranaw, K.; Stadnik, V.; Kuráň, P.; Trögl, J.; Shapoval, P. Miscanthus × Giganteus Phytoremediation of Soil Contaminated with Trace Elements as Influenced by the Presence of Plant Growth-Promoting Bacteria. Agronomy 2022, 12, 771. [Google Scholar] [CrossRef]
- Nurzhanova, A.A.; Pidlisnyuk, V.; Berzhanova, R.; Nurmagambetova, A.S.; Terletskaya, N.; Omirbekova, N.; Berkinbayev, G.; Mamirova, A. PGPR-Driven Phytoremediation and Physiobiochemical Response of Miscanthus × Giganteus to Stress Induced by the Trace Elements. Environ. Sci. Pollut. Res. 2023, 30, 96098–96113. [Google Scholar] [CrossRef]
- Hasan, A.; Tabassum, B.; Hashim, M.; Khan, N. Role of Plant Growth Promoting Rhizobacteria (PGPR) as a Plant Growth Enhancer for Sustainable Agriculture: A Review. Bacteria 2024, 3, 59–75. [Google Scholar] [CrossRef]
- Basu, A.; Prasad, P.; Das, S.N.; Kalam, S.; Sayyed, R.Z.; Reddy, M.S.; El Enshasy, H. Plant Growth Promoting Rhizobacteria (PGPR) as Green Bioinoculants: Recent Developments, Constraints, and Prospects. Sustainability 2021, 13, 1140. [Google Scholar] [CrossRef]
- Fenta, L. Isolation and Characterization of Phosphate Solubilizing Bacteria from Tomato (Solanum L.) Rhizosphere and Their Effect on Growth and Phosphorus Uptake of the Host Plant under Green House Experiment. Bachelor’s Thesis, Addis Ababa University, Addis Ababa, Ethiopia, 2012. [Google Scholar]
- Bechtaoui, N.; Raklami, A.; Benidire, L.; Tahiri, A.; Göttfert, M.; Oufdou, K. Effects of PGPR Co-Inoculation on Growth, Phosphorus Nutrition and Phosphatase/Phytase Activities of Faba Bean under Different Phosphorus Availability Conditions. Pol. J. Environ. Stud. 2020, 29, 1557–1565. [Google Scholar] [CrossRef] [PubMed]
- FAO/WHO. Human Vitamin and Mineral Requirements; Food and Agriculture Organization of the United Nations, Food and Nutrition Division: Bangkok, Thailand, 2001; p. 303. [Google Scholar]
- Lopes, M.J.d.S.; Dias-Filho, M.B.; Gurgel, E.S.C. Successful Plant Growth-Promoting Microbes: Inoculation Methods and Abiotic Factors. Front. Sustain. Food Syst. 2021, 5, 606454. [Google Scholar] [CrossRef]
- Pal, G.; Kumar, K.; Verma, A.; Verma, S.K. Seed Inhabiting Bacterial Endophytes of Maize Promote Seedling Establishment and Provide Protection against Fungal Disease. Microbiol. Res. 2022, 255, 126926. [Google Scholar] [CrossRef]
- Pandey, A.; Das, N.; Kumar, B.; Rinu, K.; Trivedi, P. Phosphate Solubilization by Penicillium Spp. Isolated from Soil Samples of Indian Himalayan Region. World J. Microbiol. Biotechnol. 2008, 24, 97–102. [Google Scholar] [CrossRef]
- Shahwar, D.; Mushtaq, Z.; Mushtaq, H.; Alqarawi, A.A.; Park, Y.; Alshahrani, T.S.; Faizan, S. Role of Microbial Inoculants as Bio Fertilizers for Improving Crop Productivity: A Review. Heliyon 2023, 9, e16134. [Google Scholar] [CrossRef]
- Jana, S.K.; Islam, M.M.; Hore, S.; Mandal, S. Rice Seed Endophytes Transmit into the Plant Seedling, Promote Plant Growth and Inhibit Fungal Phytopathogens. Plant Growth Regul. 2023, 99, 373–388. [Google Scholar] [CrossRef]
- Sunitha Kumari, K.; Devi, S.N.P.; Ranjithkumar, R.; Djearamane, S.; Tey, L.-H.; Wong, L.S.; Kayarohanam, S.; Arumugam, N.; Almansour, A.I.; Perumal, K. Organic Remobilization of Zinc and Phosphorus Availability to Plants by Application of Mineral Solubilizing Bacteria Pseudomonas aeruginosa. Heliyon 2023, 9, e22128. [Google Scholar] [CrossRef]
- Abdelkefi, N.; Louati, I.; Mechichi, H.-Z.; Sayahi, N.; El-Sayed, W.S.; Nayal, A.E.; Ismail, W.; Hanin, M.; Mechichi, T. Enhanced Salt Stress Tolerance in Tomato Plants Following Inoculation with Newly Isolated Plant Growth-Promoting Rhizobacteria. Sci. Hortic. 2024, 328, 112921. [Google Scholar] [CrossRef]
- Pidlisnyuk, V.; Mamirova, A.; Newton, R.A.; Stefanovska, T.; Zhukov, O.; Tsygankova, V.; Shapoval, P. The Role of Plant Growth Regulators in Miscanthus × Giganteus Growth on Trace Elements-Contaminated Soils. Agronomy 2022, 12, 2999. [Google Scholar] [CrossRef]
- Kumar, A.; Maurya, B.R.; Raghuwanshi, R. Isolation and Characterization of PGPR and Their Effect on Growth, Yield and Nutrient Content in Wheat (Triticum aestivum L.). Biocatal. Agric. Biotechnol. 2014, 3, 121–128. [Google Scholar] [CrossRef]
- Pidlisnyuk, V.; Mamirova, A.; Newton, R.A.; Grycová, B.; Klemencová, K.; Leštinský, P.; Ust’ak, S.; Shapoval, P. Miscanthus Phytotechnology of Cu- or Zn-Spiked Soils Supported by Contaminated Miscanthus Biochar—Is This a Viable Option for Valorization? Environ. Sci. Pollut. Res. 2025, 32, 7737–7759. [Google Scholar] [CrossRef]
- Umair Hassan, M.; Aamer, M.; Umer Chattha, M.; Haiying, T.; Shahzad, B.; Barbanti, L.; Nawaz, M.; Rasheed, A.; Afzal, A.; Liu, Y.; et al. The Critical Role of Zinc in Plants Facing the Drought Stress. Agriculture 2020, 10, 396. [Google Scholar] [CrossRef]
- Janati, W.; Mikou, K.; El Ghadraoui, L.; Errachidi, F. Isolation and Characterization of Phosphate Solubilizing Bacteria Naturally Colonizing Legumes Rhizosphere in Morocco. Front. Microbiol. 2022, 13, 958300. [Google Scholar] [CrossRef]
- Sharma, P.; Kumawat, K.C.; Kaur, S.; Kaur, N. Assessment of Zinc Solubilization by Endophytic Bacteria in Legume Rhizosphere. Ind. J. Appl. Res. 2014, 4, 439–441. [Google Scholar] [CrossRef]
- Chaffai, R.; Ganesan, M.; Cherif, A. Plant Growth-Promoting Rhizobacteria (PGPR) and Plant Growth-Promoting Fungi (PGPF) for Alleviating Abiotic Stress in Plants. In Plant Adaptation to Abiotic Stress: From Signaling Pathways and Microbiomes to Molecular Mechanisms; Chaffai, R., Ganesan, M., Cherif, A., Eds.; Springer Nature: Singapore, 2024; pp. 457–496. ISBN 978-981-97-0672-3. [Google Scholar]
- Pikovskaya, R. Mobilization of phosphorus in soil in connection with vital activity of some microbial species. Microbiology 1948, 17, 362–370. [Google Scholar]
- ALKahtani, M.D.F.; Fouda, A.; Attia, K.A.; Al-Otaibi, F.; Eid, A.M.; Ewais, E.E.-D.; Hijri, M.; St-Arnaud, M.; Hassan, S.E.-D.; Khan, N.; et al. Isolation and Characterization of Plant Growth Promoting Endophytic Bacteria from Desert Plants and Their Application as Bioinoculants for Sustainable Agriculture. Agronomy 2020, 10, 1325. [Google Scholar] [CrossRef]
- Varga, T.; Hixson, K.K.; Ahkami, A.H.; Sher, A.W.; Barnes, M.E.; Chu, R.K.; Battu, A.K.; Nicora, C.D.; Winkler, T.E.; Reno, L.R.; et al. Endophyte-Promoted Phosphorus Solubilization in Populus. Front. Plant Sci. 2020, 11, 567918. [Google Scholar] [CrossRef]
- Youseif, S.H. Genetic Diversity of Plant Growth Promoting Rhizobacteria and Their Effects on the Growth of Maize Plants under Greenhouse Conditions. Ann. Agric. Sci. 2018, 63, 25–35. [Google Scholar] [CrossRef]
- Luziatelli, F.; Ficca, A.G.; Cardarelli, M.; Melini, F.; Cavalieri, A.; Ruzzi, M. Genome Sequencing of Pantoea Agglomerans C1 Provides Insights into Molecular and Genetic Mechanisms of Plant Growth-Promotion and Tolerance to Heavy Metals. Microorganisms 2020, 8, 153. [Google Scholar] [CrossRef]
- Shariati J., V.; Malboobi, M.A.; Tabrizi, Z.; Tavakol, E.; Owlia, P.; Safari, M. Comprehensive Genomic Analysis of a Plant Growth-Promoting Rhizobacterium Pantoea Agglomerans Strain P5. Sci. Rep. 2017, 7, 15610. [Google Scholar] [CrossRef] [PubMed]
- Vasseur-Coronado, M.; Vlassi, A.; du Boulois, H.D.; Schuhmacher, R.; Parich, A.; Pertot, I.; Puopolo, G. Ecological Role of Volatile Organic Compounds Emitted by Pantoea Agglomerans as Interspecies and Interkingdom Signals. Microorganisms 2021, 9, 1186. [Google Scholar] [CrossRef] [PubMed]
- Chanu, P.H.; Yadav, J. Exploring Microbial Solutions: A Comprehensive Study on Isolating, Characterizing, and Selecting Zinc-Solubilizing Fungi from Rhizospheric Soil. Int. J. Plant Soil. Sci. 2024, 36, 369–377. [Google Scholar] [CrossRef]
- Wijerathna, R.M.N.; Wijeweera, A.A.; Wijethunga, A.M.; Mapa, M.M.S.T. Determination of Oil Quality and Antifungal Effect of Selected Citronella Accessions (Cymbopogon Nardus, Cymbopogon Winterianus) to Formulate an Anti-Dandruff Shampoo. Biol. Med. Nat. Prod. Chem. 2023, 12, 485–498. [Google Scholar] [CrossRef]
- Krishnappa, C.; Balamurugan, A.; Velmurugan, S.; Kumar, S.; Sampathrajan, V.; Kundu, A.; Javed, M.; Chouhan, V.; Ganesan, P.; Kumar, A. Rice Foliar-Adapted Pantoea Species: Promising Microbial Biostimulants Enhancing Rice Resilience against Foliar Pathogens, Magnaporthe Oryzae and Xanthomonas Oryzae Pv. Oryzae. Microb. Pathog. 2024, 186, 106445. [Google Scholar] [CrossRef]
- Ning, Y.; Xiao, Z.; Weinmann, M.; Li, Z. Phosphate Uptake is Correlated with the Root Length of Celery Plants Following the Association Between Arbuscular Mycorrhizal Fungi, Pseudomonas Sp. and Biochar with Different Phosphate Fertilization Levels. Agronomy 2019, 9, 824. [Google Scholar] [CrossRef]
- Saxena, J.; Rana, G.; Pandey, M. Impact of Addition of Biochar along with Bacillus Sp. on Growth and Yield of French Beans. Sci. Hortic. 2013, 162, 351–356. [Google Scholar] [CrossRef]
- Hosseini, E.; Zarei, M.; Sepehri, M.; Safarzadeh, S. Do Bagasse Biochar and Microbial Inoculants Positively Affect Barley Grain Yield and Nutrients, and Microbial Activity? J. Plant Nutr. 2021, 45, 522–539. [Google Scholar] [CrossRef]
- Lehmann, J.; Rillig, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar Effects on Soil Biota—A Review. Soil. Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Wang, Y.; Li, W.; Du, B.; Li, H. Effect of Biochar Applied with Plant Growth-Promoting Rhizobacteria (PGPR) on Soil Microbial Community Composition and Nitrogen Utilization in Tomato. Pedosphere 2021, 31, 872–881. [Google Scholar] [CrossRef]
- Jabborova, D.; Wirth, S.; Kannepalli, A.; Narimanov, A.; Desouky, S.; Davranov, K.; Sayyed, R.Z.; El Enshasy, H.; Malek, R.A.; Syed, A.; et al. Co-Inoculation of Rhizobacteria and Biochar Application Improves Growth and Nutrientsin Soybean and Enriches Soil Nutrients and Enzymes. Agronomy 2020, 10, 1142. [Google Scholar] [CrossRef]
- Ren, H.; Huang, B.; Fernández-García, V.; Miesel, J.; Yan, L.; Lv, C. Biochar and Rhizobacteria Amendments Improve Several Soil Properties and Bacterial Diversity. Microorganisms 2020, 8, 502. [Google Scholar] [CrossRef] [PubMed]
- Dayoub, E.B.; Tóth, Z.; Soós, G.; Anda, A. Chemical and Physical Properties of Selected Biochar Types and a Few Application Methods in Agriculture. Agronomy 2024, 14, 2540. [Google Scholar] [CrossRef]
- Pidlisnyuk, V.; Newton, R.A.; Mamirova, A. Miscanthus Biochar Value Chain—A Review. J. Environ. Manag. 2021, 290, 112611. [Google Scholar] [CrossRef]
- Sharma, S.; Negi, M.; Sharma, U.; Kumar, P.; Chauhan, A.; Shavnam; Katoch, V.; Sharma, R. A Critique of the Effectiveness of Biochar for Managing Soil Health and Soil Biota. Appl. Soil. Ecol. 2023, 191, 105065. [Google Scholar] [CrossRef]
- ISO 18400-206:2018; Soil Quality—Sampling—Part 206: Collection, Handling and Storage of Soil under Aerobic Conditions for the Assessment of Microbiological Processes, Biomass and Diversity in the Laboratory. International Organization for Standardization: Geneva, Switzerland, 2018.
- McPherson, M.R.; Wang, P.; Marsh, E.L.; Mitchell, R.B.; Schachtman, D.P. Isolation and Analysis of Microbial Communities in Soil, Rhizosphere, and Roots in Perennial Grass Experiments. J. Vis. Exp. 2018, 137, 57932. [Google Scholar] [CrossRef]
- Hartmann, A.; Schmid, M.; van Tuinen, D.; Berg, G. Plant-Driven Selection of Microbes. Plant Soil. 2009, 321, 235–257. [Google Scholar] [CrossRef]
- Khan, M.F.; Liao, J.; Liu, Z.; Chugh, G. Bacterial Cytochrome P450 Involvement in the Biodegradation of Fluorinated Pyrethroids. J. Xenobiotics 2025, 15, 58. [Google Scholar] [CrossRef] [PubMed]
- Mohite, B. Isolation and Characterization of Indole Acetic Acid (IAA) Producing Bacteria from Rhizospheric Soil and Its Effect on Plant Growth. J. Soil. Sci. Plant Nutr. 2013, 13, 638–649. [Google Scholar] [CrossRef]
- Mamarasulov, B.; Davranov, K.; Jahan, M.S.; Jabborova, D.; Nasif, O.; Ansari, M.J.; Danish, S.; Datta, R. Characterization, Enzymatic and Biochemical Properties of Endophytic Bacterial Strains of the Medicinal Plant Ajuga Turkestanica (Rgl.) Brig (Lamiaceae). J. King Saud. Univ.-Sci. 2022, 34, 102183. [Google Scholar] [CrossRef]
- Ali, B.; Wang, X.; Saleem, M.H.; Sumaira; Hafeez, A.; Afridi, M.S.; Khan, S.; Zaib-Un-Nisa; Ullah, I.; Amaral Júnior, A.T.d.; et al. PGPR-Mediated Salt Tolerance in Maize by Modulating Plant Physiology, Antioxidant Defense, Compatible Solutes Accumulation and Bio-Surfactant Producing Genes. Plants 2022, 11, 345. [Google Scholar] [CrossRef]
- Khianngam, S.; Meetum, P.; Chiangmai, P.N.; Tanasupawat, S. Identification and Optimisation of Indole-3-Acetic Acid Production of Endophytic Bacteria and Their Effects on Plant Growth. Trop. Life Sci. Res. 2023, 34, 219–239. [Google Scholar] [CrossRef]
- Boubekri, K.; Soumare, A.; Mardad, I.; Lyamlouli, K.; Hafidi, M.; Ouhdouch, Y.; Kouisni, L. The Screening of Potassium- and Phosphate-Solubilizing Actinobacteria and the Assessment of Their Ability to Promote Wheat Growth Parameters. Microorganisms 2021, 9, 470. [Google Scholar] [CrossRef]
- Shalaby, M.; Elbagory, M.; EL-Khateeb, N.; Mehesen, A.; EL-Sheshtawy, O.; Elsakhawy, T.; Omara, A.E.-D. Potential Impacts of Certain N2-Fixing Bacterial Strains and Mineral N Doses for Enhancing the Growth and Productivity of Maize Plants. Plants 2023, 12, 3830. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro Evaluating Antimicrobial Activity: A Review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]
- Patel, M.; Mistry, J.; Desai, S.; Patel, S.; Desai, S. Isolation and Characterization of Lipase Producing Bacteria from Vegetable Oil Spillage Site. Int. J. Curr. Microbiol. App. Sci. 2016, 5, 214–232. [Google Scholar] [CrossRef]
- Ojovan, B.; Catana, R.; Neagu, S.; Cojoc, R.; Lucaci, A.I.; Marutescu, L.; Florescu, L.; Ruginescu, R.; Enache, M.; Moldoveanu, M. Metabolic Potential of Some Functional Groups of Bacteria in Aquatic Urban Systems. Fermentation 2021, 7, 242. [Google Scholar] [CrossRef]
- Ntabo, R.M.; Nyamache, A.K.; Lwande, W.; Kabii, J.; Nonoh, J. Enzymatic Activity of Endophytic Bacterial Isolates from Selected Mangrove Plants in Kenya. Open Microbiol. J. 2018, 12, 354–363. [Google Scholar] [CrossRef]
- Malleswari, D.; Bagyanarayana, G. Plant Growth-Promoting Activities and Molecular Characterization of Rhizobacterial Strains Isolated from Medicinal and Aromatic Plants. J. Pharm. Biol. Sci. 2013, 6, 30–37. [Google Scholar] [CrossRef]
- Kasana, R.C.; Salwan, R.; Dhar, H.; Dutt, S.; Gulati, A. A Rapid and Easy Method for the Detection of Microbial Cellulases on Agar Plates Using Gram’s Iodine. Curr. Microbiol. 2008, 57, 503–507. [Google Scholar] [CrossRef] [PubMed]
- Bučková, M.; Godočíková, J.; Zámocký, M.; Polek, B. Screening of Bacterial Isolates from Polluted Soils Exhibiting Catalase and Peroxidase Activity and Diversity of Their Responses to Oxidative Stress. Curr. Microbiol. 2010, 61, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, N.M.; Patel, A.; Mehta, S.; Patel, N. Isolation and Screening of Cellulolytic Bacteria Inhabiting Different Environment and Optimization of Cellulase Production. Univers. J. Environ. Res. Technol. 2013, 3, 39–49. [Google Scholar]
- Wilson, K. Preparation of Genomic DNA from Bacteria. Curr. Protoc. Mol. Biol. 2001, 56, 2.4.1–2.4.5. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Tamura, K.; Nei, M. Estimation of the Number of Nucleotide Substitutions in the Control Region of Mitochondrial DNA in Humans and Chimpanzees. Mol. Biol. Evol. 1993, 10, 512–526. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- La Pierre, K.J.; Simms, E.L.; Tariq, M.; Zafar, M.; Porter, S.S. Invasive Legumes Can Associate with Many Mutualists of Native Legumes, but Usually Do Not. Ecol. Evol. 2017, 7, 8599–8611. [Google Scholar] [CrossRef]
- Weisburg, W.G.; Barns, S.M.; Pelletier, D.A.; Lane, D.J. 16S Ribosomal DNA Amplification for Phylogenetic Study. J. Bacteriol. 1991, 173, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic Local Alignment Search Tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Zahra, S.T.; Tariq, M.; Abdullah, M.; Azeem, F.; Ashraf, M.A. Dominance of Bacillus Species in the Wheat (Triticum aestivum L.) Rhizosphere and Their Plant Growth Promoting Potential under Salt Stress Conditions. PeerJ 2023, 11, e14621. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Schwartz, S.; Wagner, L.; Miller, W. A Greedy Algorithm for Aligning DNA Sequences. J. Comput. Biol. 2000, 7, 203–214. [Google Scholar] [CrossRef]
- Muhire, B.M.; Varsani, A.; Martin, D.P. SDT: A Virus Classification Tool Based on Pairwise Sequence Alignment and Identity Calculation. PLoS ONE 2014, 9, e108277. [Google Scholar] [CrossRef]
- Zhang, Z.; Ma, R.; Tao, Y.; Wang, Z.; Yang, Y. Effects of Fe and Zn Alone and Combined Treatment on Triticum aestivum L. Seed Germination. BMC Plant Biol. 2025, 25, 430. [Google Scholar] [CrossRef]
- Iswandi, A.; Bossier, P.; Vandenabeele, J.; Verstraete, W. Effect of Seed Inoculation with the Rhizopseudomonad Strain 7NSK2 on the Root Microbiota of Maize (Zea mays) and Barley (Hordeum vulgare). Biol. Fert. Soils 1987, 3, 153–158. [Google Scholar] [CrossRef]
- Tricker, P.J.; ElHabti, A.; Schmidt, J.; Fleury, D. The Physiological and Genetic Basis of Combined Drought and Heat Tolerance in Wheat. J. Exp. Bot. 2018, 69, 3195–3210. [Google Scholar] [CrossRef]
- Nawaz, F.; Zulfiqar, B.; Ahmad, K.S.; Majeed, S.; Shehzad, M.A.; Javeed, H.M.R.; Tahir, M.N.; Ahsan, M. Pretreatment with Selenium and Zinc Modulates Physiological Indices and Antioxidant Machinery to Improve Drought Tolerance in Maize (Zea Mays L.). S. Afr. J. Bot. 2021, 138, 209–216. [Google Scholar] [CrossRef]
- Aghaie, P.; Tafreshi, S.A.H. Central Role of 70-kDa Heat Shock Protein in Adaptation of Plants to Drought Stress. Cell Stress. Chaperones 2020, 25, 1071–1081. [Google Scholar] [CrossRef]
- Khan, N.; Ali, S.; Tariq, H.; Latif, S.; Yasmin, H.; Mehmood, A.; Shahid, M.A. Water Conservation and Plant Survival Strategies of Rhizobacteria under Drought Stress. Agronomy 2020, 10, 1683. [Google Scholar] [CrossRef]
- Flexas, J.; Bota, J.; Loreto, F.; Cornic, G.; Sharkey, T.D. Diffusive and Metabolic Limitations to Photosynthesis under Drought and Salinity in C3 Plants. Plant Biol. 2004, 6, 269–279. [Google Scholar] [CrossRef]
- Naz, R.; Gul, F.; Zahoor, S.; Nosheen, A.; Yasmin, H.; Keyani, R.; Shahid, M.; Hassan, M.N.; Siddiqui, M.H.; Batool, S.; et al. Interactive Effects of Hydrogen Sulphide and Silicon Enhance Drought and Heat Tolerance by Modulating Hormones, Antioxidant Defence Enzymes and Redox Status in Barley (Hordeum vulgare L.). Plant Biol. 2022, 24, 684–696. [Google Scholar] [CrossRef]
- Wang, D.; Cao, Z.; Wang, W.; Zhu, W.; Hao, X.; Fang, Z.; Liu, S.; Wang, X.; Zhao, C.; Tang, Y. Genome-Wide Characterization of OFP Family Genes in Wheat (Triticum aestivum L.) Reveals That TaOPF29a-A Promotes Drought Tolerance. BioMed Res. Int. 2020, 2020, 9708324. [Google Scholar] [CrossRef]
- Kumar Arora, N.; Fatima, T.; Mishra, J.; Mishra, I.; Verma, S.; Verma, R.; Verma, M.; Bhattacharya, A.; Verma, P.; Mishra, P.; et al. Halo-Tolerant Plant Growth Promoting Rhizobacteria for Improving Productivity and Remediation of Saline Soils. J. Adv. Res. 2020, 26, 69–82. [Google Scholar] [CrossRef]
- Mahapatra, S.; Yadav, R.; Ramakrishna, W. Bacillus Subtilis Impact on Plant Growth, Soil Health and Environment: Dr. Jekyll and Mr. Hyde. J. Appl. Microbiol. 2022, 132, 3543–3562. [Google Scholar] [CrossRef] [PubMed]
- Sabki, M.H.; Ong, P.Y.; Ibrahim, N.; Lee, C.T.; Klemeš, J.J.; Li, C.; Gao, Y. A Review on Abiotic Stress Tolerance and Plant Growth Metabolite Framework by Plant Growth-Promoting Bacteria for Sustainable Agriculture. Chem. Eng. Trans. 2021, 83, 367–372. [Google Scholar] [CrossRef]
- Majeed, A.; Abbasi, M.K.; Hameed, S.; Imran, A.; Rahim, N. Isolation and Characterization of Plant Growth-Promoting Rhizobacteria from Wheat Rhizosphere and Their Effect on Plant Growth Promotion. Front. Microbiol. 2015, 6, 198. [Google Scholar] [CrossRef] [PubMed]
- Sheirdil, R.A.; Hayat, R.; Zhang, X.-X.; Abbasi, N.A.; Ali, S.; Ahmed, M.; Khattak, J.Z.K.; Ahmad, S. Exploring Potential Soil Bacteria for Sustainable Wheat (Triticum aestivum L.) Production. Sustainability 2019, 11, 3361. [Google Scholar] [CrossRef]
- Cherif-Silini, H.; Silini, A.; Yahiaoui, B.; Ouzari, I.; Boudabous, A. Phylogenetic and Plant-Growth-Promoting Characteristics of Bacillus Isolated from the Wheat Rhizosphere. Ann. Microbiol. 2016, 66, 1087–1097. [Google Scholar] [CrossRef]
- Delfim, J.; Dijoo, Z.K. Bacillus Thuringiensis as a Biofertilizer and Plant Growth Promoter. In Microbiota and Biofertilizers, Vol 2: Ecofriendly Tools for Reclamation of Degraded Soil Environs; Dar, G.H., Bhat, R.A., Mehmood, M.A., Hakeem, K.R., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 251–265. ISBN 978-3-030-61010-4. [Google Scholar]
- Ibarra-Villarreal, A.L.; Gándara-Ledezma, A.; Godoy-Flores, A.D.; Herrera-Sepúlveda, A.; Díaz-Rodríguez, A.M.; Parra-Cota, F.I.; de los Santos-Villalobos, S. Salt-Tolerant Bacillus Species as a Promising Strategy to Mitigate the Salinity Stress in Wheat (Triticum Turgidum Subsp. Durum). J. Arid. Environ. 2021, 186, 104399. [Google Scholar] [CrossRef]
- Ali, B.; Hafeez, A.; Ahmad, S.; Javed, M.A.; Sumaira; Afridi, M.S.; Dawoud, T.M.; Almaary, K.S.; Muresan, C.C.; Marc, R.A.; et al. Bacillus Thuringiensis PM25 Ameliorates Oxidative Damage of Salinity Stress in Maize via Regulating Growth, Leaf Pigments, Antioxidant Defense System, and Stress Responsive Gene Expression. Front. Plant Sci. 2022, 13, 921668. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Gao, J.; Zhang, M.; Chen, X.; Moe, T.S.; Du, Y.; Yang, F.; Xue, J.; Zhang, X. Isolation and Characterization of Plant Growth-Promoting Endophytic Bacteria Bacillus Stratosphericus LW-03 from Lilium Wardii. 3 Biotech. 2020, 10, 305. [Google Scholar] [CrossRef] [PubMed]
- Huu Dat, T.; Thi Kim, N.; Viet Cuong, P. Optimization of Indole-3-Acetic Acid Production by Bacillus subtilisTIB6 Using Responses Surface Methodology. Int. J. Dev. Res. 2015, 5, 4036–4042. [Google Scholar]
- Choudhury, P.; Jawed, A.; Saha, P. Optimization of Phytostimulatory Potential in Bacillus Toyonensis Isolated from Tea Plant Rhizosphere Soil of Nilgiri Hills, India. Int. J. Eng. Sci. Invent. 2017, 6, 13–18. [Google Scholar]
- Apine, O.A.; Jadhav, J.P. Optimization of Medium for Indole-3-acetic Acid Production Using Pantoea Agglomerans Strain PVM. J. Appl. Microbiol. 2011, 110, 1235–1244. [Google Scholar] [CrossRef]
- Chandra, S.; Askari, K.; Kumari, M. Optimization of Indole Acetic Acid Production by Isolated Bacteria from Stevia Rebaudiana Rhizosphere and Its Effects on Plant Growth. J. Genet. Eng. Biotechnol. 2018, 16, 581–586. [Google Scholar] [CrossRef]
- Khan, M.S.; Zaidi, A.; Wani, P.A.; Ahemad, M.; Oves, M. Functional Diversity Among Plant Growth-Promoting Rhizobacteria: Current Status. In Microbial Strategies for Crop Improvement; Khan, M.S., Zaidi, A., Musarrat, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 105–132. ISBN 978-3-642-01979-1. [Google Scholar]
- Gontia-Mishra, I.; Sapre, S.; Kachare, S.; Tiwari, S. Molecular Diversity of 1-Aminocyclopropane-1-Carboxylate (ACC) Deaminase Producing PGPR from Wheat (Triticum aestivum L.) Rhizosphere. Plant Soil. 2017, 414, 213–227. [Google Scholar] [CrossRef]
- Ahmad, I.; Ahmad, M.; Hussain, A.; Jamil, M. Integrated Use of Phosphate-Solubilizing Bacillus Subtilis Strain IA6 and Zinc-Solubilizing Bacillus Sp. Strain IA16: A Promising Approach for Improving Cotton Growth. Folia Microbiol. 2021, 66, 115–125. [Google Scholar] [CrossRef]
- Ramesh, A.; Sharma, S.K.; Sharma, M.P.; Yadav, N.; Joshi, O.P. Inoculation of Zinc Solubilizing Bacillus aryabhattai Strains for Improved Growth, Mobilization and Biofortification of Zinc in Soybean and Wheat Cultivated in Vertisols of Central India. Appl. Soil. Ecol. 2014, 73, 87–96. [Google Scholar] [CrossRef]
- Mehmood, S.; Khan, A.A.; Shi, F.; Tahir, M.; Sultan, T.; Munis, M.F.H.; Kaushik, P.; Alyemeni, M.N.; Chaudhary, H.J. Alleviation of Salt Stress in Wheat Seedlings via Multifunctional Bacillus Aryabhattai PM34: An In-Vitro Study. Sustainability 2021, 13, 8030. [Google Scholar] [CrossRef]
- Dhaked, B.S.; Triveni, S.; Reddy, R.S.; Padmaja, G. Isolation and Screening of Potassium and Zinc Solubilizing Bacteria from Different Rhizosphere Soil. Int. J. Curr. Microbiol. App. Sci. 2017, 6, 1271–1281. [Google Scholar] [CrossRef]
- Singh, S.; Chhabra, R.; Sharma, A.; Bisht, A. Harnessing the Power of Zinc-Solubilizing Bacteria: A Catalyst for a Sustainable Agrosystem. Bacteria 2024, 3, 15–29. [Google Scholar] [CrossRef]
- Sehrawat, A.; Sindhu, S.S. Zinc-Solubilizing Microorganisms: Contributions in Nutrient Availability and Implications for Crop Productivity in Sustainable Agriculture. In Plant Holobiome Engineering for Climate-Smart Agriculture; Sayyed, R.Z., Ilyas, N., Eds.; Springer Nature: Singapore, 2024; pp. 183–213. ISBN 978-981-99-9388-8. [Google Scholar]
- Haroon, M.; Khan, S.T.; Malik, A. Zinc-Solubilizing Bacteria: An Option to Increase Zinc Uptake by Plants. In Microbial Biofertilizers and Micronutrient Availability: The Role of Zinc in Agriculture and Human Health; Khan, S.T., Malik, A., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 207–238. ISBN 978-3-030-76609-2. [Google Scholar]
- Shakeel, M.; Hafeez, F.Y.; Malik, I.R.; Rauf, A.; Jan, F.; Khan, I.; Ijaz, I.; Elsadek, M.F.; Ali, M.A.; Rashid, K.; et al. Zinc Solubilizing Bacteria Synergize the Effect of Zinc Sulfate on Growth, Yield and Grain Zinc Content of Rice (Oryza sativa). Cereal Res. Commun. 2024, 52, 961–971. [Google Scholar] [CrossRef]
- Rahman, A.; Ahmad, M.A.; Mehmood, S.; Rauf, A.; Iqbal, A.; Ali, B.; Ullah, M.; Ali, M.; Mohamed, H.I.; Uddin, I. Isolation and Screening of Zn (Zn) Solubilizing Rhizosphere Bacteria from Different Vegetations for Their Ability to Improve Growth, Zn Uptake, and Expression of Zn Transporter Genes in Tomato. Curr. Microbiol. 2024, 81, 83. [Google Scholar] [CrossRef]
- Luo, D.; Shi, J.; Li, M.; Chen, J.; Wang, T.; Zhang, Q.; Yang, L.; Zhu, N.; Wang, Y. Consortium of Phosphorus-Solubilizing Bacteria Promotes Maize Growth and Changes the Microbial Community Composition of Rhizosphere Soil. Agronomy 2024, 14, 1535. [Google Scholar] [CrossRef]
- Alemneh, A.A.; Cawthray, G.R.; Zhou, Y.; Ryder, M.H.; Denton, M.D. Ability to Produce Indole Acetic Acid Is Associated with Improved Phosphate Solubilising Activity of Rhizobacteria. Arch. Microbiol. 2021, 203, 3825–3837. [Google Scholar] [CrossRef]
- Kumar, V.; Prasher, I.B. Phosphate Solubilization and Indole-3-Acetic Acid (IAA) Produced by Colletotrichum Gloeosporioides and Aspergillus Fumigatus Strains Isolated from the Rhizosphere of Dillenia indica L. Folia Microbiol. 2023, 68, 219–229. [Google Scholar] [CrossRef]
- Kaur, H.; Mir, R.A.; Hussain, S.J.; Prasad, B.; Kumar, P.; Aloo, B.N.; Sharma, C.M.; Dubey, R.C. Prospects of Phosphate Solubilizing Microorganisms in Sustainable Agriculture. World J. Microbiol. Biotechnol. 2024, 40, 291. [Google Scholar] [CrossRef]
- Berza, B.; Sekar, J.; Vaiyapuri, P.; Pagano, M.C.; Assefa, F. Evaluation of Inorganic Phosphate Solubilizing Efficiency and Multiple Plant Growth Promoting Properties of Endophytic Bacteria Isolated from Root Nodules Erythrina Brucei. BMC Microbiol. 2022, 22, 276. [Google Scholar] [CrossRef]
- Wang, J.; Li, R.; Zhang, H.; Wei, G.; Li, Z. Beneficial Bacteria Activate Nutrients and Promote Wheat Growth under Conditions of Reduced Fertilizer Application. BMC Microbiol. 2020, 20, 38. [Google Scholar] [CrossRef]
- Peng, J.; Ma, J.; Wei, X.; Zhang, C.; Jia, N.; Wang, X.; Wang, E.T.; Hu, D.; Wang, Z. Accumulation of Beneficial Bacteria in the Rhizosphere of Maize (Zea mays L.) Grown in a Saline Soil in Responding to a Consortium of Plant Growth Promoting Rhizobacteria. Ann. Microbiol. 2021, 71, 40. [Google Scholar] [CrossRef]
- Riseh, R.S.; Vatankhah, M.; Hassanisaadi, M.; Barka, E.A. Unveiling the Role of Hydrolytic Enzymes from Soil Biocontrol Bacteria in Sustainable Phytopathogen Management. Front. Biosci.-Landmark 2024, 29, 105. [Google Scholar] [CrossRef]
- Admassie, M.; Woldehawariat, Y.; Alemu, T. In Vitro Evaluation of Extracellular Enzyme Activity and Its Biocontrol Efficacy of Bacterial Isolates from Pepper Plants for the Management of Phytophthora Capsici. BioMed Res. Int. 2022, 2022, 6778352. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, R.; You, M.P.; Barbetti, M.J.; Chen, Y. Pathogen Biocontrol Using Plant Growth-Promoting Bacteria (PGPR): Role of Bacterial Diversity. Microorganisms 2021, 9, 1988. [Google Scholar] [CrossRef]
- Rivera-Hernández, G.; Tijerina-Castro, G.D.; Cortés-Pérez, S.; Ferrera-Cerrato, R.; Alarcón, A. Evaluation of Functional Plant Growth-Promoting Activities of Culturable Rhizobacteria Associated to Tunicate Maize (Zea mays var. tunicata A. St. Hil), a Mexican Exotic Landrace Grown in Traditional Agroecosystems. Front. Microbiol. 2024, 15, 1478807. [Google Scholar] [CrossRef]
- Mishra, P.; Mishra, J.; Dwivedi, S.K.; Arora, N.K. Microbial Enzymes in Biocontrol of Phytopathogens. In Microbial Enzymes: Roles and Applications in Industries; Arora, N.K., Mishra, J., Mishra, V., Eds.; Springer: Singapore, 2020; pp. 259–285. ISBN 978-981-15-1710-5. [Google Scholar]
- Zalila-Kolsi, I.; Ben-Mahmoud, A.; Al-Barazie, R. Bacillus Amyloliquefaciens: Harnessing Its Potential for Industrial, Medical, and Agricultural Applications—A Comprehensive Review. Microorganisms 2023, 11, 2215. [Google Scholar] [CrossRef]
- Sritongon, N.; Boonlue, S.; Mongkolthanaruk, W.; Jogloy, S.; Riddech, N. The Combination of Multiple Plant Growth Promotion and Hydrolytic Enzyme Producing Rhizobacteria and Their Effect on Jerusalem Artichoke Growth Improvement. Sci. Rep. 2023, 13, 5917. [Google Scholar] [CrossRef]
- Azman, N.A.; Sijam, K.; Hata, E.M.; Othman, R.; Saud, H.M. Screening of Bacteria as Antagonist Against Xanthomonas Oryzae Pv. Oryzae, the Causal Agent of Bacterial Leaf Blight of Paddy and as Plant Growth Promoter. J. Exp. Agric. Int. 2017, 16, 1–15. [Google Scholar] [CrossRef][Green Version]
- Fadiji, A.E.; Babalola, O.O. Elucidating Mechanisms of Endophytes Used in Plant Protection and Other Bioactivities with Multifunctional Prospects. Front. Bioeng. Biotechnol. 2020, 8, 467. [Google Scholar] [CrossRef]
- Jacob, J.; Krishnan, G.V.; Thankappan, D.; Bhaskaran Nair Saraswathy Amma, D.K. 4-Endophytic Bacterial Strains Induced Systemic Resistance in Agriculturally Important Crop Plants. In Microbial Endophytes; Kumar, A., E.K, R., Eds.; Woodhead Publishing: Cambridge, UK, 2020; pp. 75–105. ISBN 978-0-12-819654-0. [Google Scholar]
- Egamberdieva, D.; Shurigin, V.; Alaylar, B.; Wirth, S.; Bellingrath-Kimura, S.D. Bacterial Endophytes from Horseradish (Armoracia rusticana G. Gaertn.,B.Mey.&Scherb.) with Antimicrobial Efficacy against Pathogens. Plant Soil. Environ. 2020, 66, 309–316. [Google Scholar] [CrossRef]
- Sebihi, F.Z.; Benguedouar, A.; Benhizia, Y.; Sanchez, J.; Gallego, E. Evaluation of Multi-Trait Plant Growth Promoting Pseudomonas Fluorescens Isolated from Constantine Wheat Rhizosphere Soil (Algeria) and Screening There Antifungal Activity against Two Species of Fusarium. Adv. Environ. Biol. 2016, 10, 102–116. [Google Scholar]
- Adeleke, B.S.; Ayangbenro, A.S.; Babalola, O.O. In vitro Screening of Sunflower Associated Endophytic Bacteria With Plant Growth-Promoting Traits. Front. Sustain. Food Syst. 2022, 6, 903114. [Google Scholar] [CrossRef]








| Component | Unit | LB | TNB | Pikovskaya | B&R | Ashby’s | SDA |
|---|---|---|---|---|---|---|---|
| Ref. | − | [60] | [61] | [36] | [43] | [28] | [44] |
| Agarose | g | 20 | − | − | 15 | − | 15.0 |
| Glucose | g | − | − | 10 | 10 | − | 40.0 |
| Peptone | g | − | − | − | 1.0 | − | 10.0 |
| Sucrose | g | − | − | − | − | 20 | − |
| Tryptone | g | 10 | 10 | − | − | − | − |
| Yeast extract | g | 5 | 5 | 0.5 | 1.0 | − | − |
| CaCl2 | g | − | − | − | − | 0.02 | − |
| CaCO3 | g | − | − | − | − | 5 | − |
| Ca3(PO4)2 | g | − | − | 5 | − | − | − |
| FeCl3 | g | − | − | − | − | 0.01 | − |
| FeSO4 ×·7H2O | mg | − | − | 0.1 | − | − | − |
| KCl | g | − | − | 0.2 | − | − | − |
| KH2PO4 | g | − | − | − | − | 0.4 | − |
| K2HPO4 | g | − | − | − | 0.18 | 0.1 | − |
| L-tryptophan | g | − | 0.5 | − | − | − | − |
| MgCl2 | g | − | − | − | 0.2 | − | − |
| MgSO4 ×·7H2O | g | − | − | 0.1 | − | 0.2 | − |
| MnSO4 ×·7H2O | mg | − | − | 0.1 | − | − | − |
| NaCl | g | 5 | 0.5 | 0.2 | − | 0.1 | − |
| Na2MoO4 | g | − | − | − | − | 0.002 | − |
| (NH4)2SO4 | g | − | − | 0.5 | 0.5 | − | − |
| dH2O | L | 1 | 1 | 1 | 1 | 1 | 1 |
| pH | − | 7.0 | 7.0 | 7.0 | 7.2 | 6.9 | 5.6 |
| Component | Unit | Simmons | TSIA | SMA | EYA | CMC |
|---|---|---|---|---|---|---|
| Ref. | − | [67] | [68] | [69,70] | [70] | [71] |
| Agarose | g | 15.0 | 12.0 | 15.0 | 25.0 | 20.0 |
| Beef extract | g | − | 3.0 | − | − | − |
| Bromo thymol blue | g | 0.08 | − | − | − | − |
| Casein | g | − | − | 5.0 | − | − |
| CMC | g | − | − | − | − | 0.2 |
| Dextrose | g | − | 1.0 | − | − | − |
| Glucose | g | − | − | 1.0 | − | 2.0 |
| Lactose | g | − | 10.0 | − | − | − |
| Peptone | g | − | 20 | − | − | 1.0 |
| Phenol red | g | − | 0.024 | − | − | − |
| Proteose peptone | g | − | − | − | 40.0 | − |
| Skim milk | percent | − | − | 7.0 | − | − |
| Sucrose | g | − | 10.0 | − | − | − |
| Yeast extract | g | − | 3.0 | 2.5 | − | − |
| C34H32ClFeN4O4 | g | − | − | − | 0.005 | − |
| FeSO4 ×·7H2O | g | − | 0.01 | − | − | 0.01 |
| KCl | g | − | − | − | − | 0.3 |
| KH2PO4 | g | − | − | − | 1.0 | 1.0 |
| K2HPO4 | g | 1.0 | − | − | − | − |
| MgSO4 ×·7H2O | g | 0.2 | − | − | 0.1 | 0.3 |
| NaCl | g | 5.0 | 2.0 | − | 2.0 | 0.1 |
| dH2O | L | 1 | 1 | 1 | 1 | 1 |
| pH | − | 7.0 | 7.0 | 7.0 | 7.2 | 6.9 |
| Parameter | AS8 | AS23 | AS31 |
|---|---|---|---|
| Citrate assimilation | + | + | + |
| Carbohydrate fermentation | + | + | + |
| Catalase activity | + | + | + |
| Amylolytic activity | +++ | + | ++ |
| Protease activity | + | − | − |
| Lipase activity | ++ | − | + |
| Cellulolytic activity | ++ | − | +++ |
| Treatment | SL, cm | % to Ctrl | RL, cm | % to Ctrl | VI | % to Ctrl |
|---|---|---|---|---|---|---|
| Day 7 | ||||||
| Ctrl | 0.40 ± 0.14 b | 100 | − | 100 | 24.0 ± 8.49 b | 100 |
| AS8 | 0.50 ± 0.08 b | − | 0.18 ± 0.10 b | − | 45.0 ± 10.4 b | − |
| AS23 | 4.22 ± 0.67 a | 1 055 | 1.12 ± 0.26 a | − | 558 ± 45.5 a | 2 325 |
| AS31 | 5.25 ± 0.17 a | 1 313 | 1.38 ± 0.17 a | − | 580 ± 51.2 a | 2 415 |
| p-value | <0.001 | <0.01 | <0.001 | |||
| Day 14 | ||||||
| Ctrl | 3.40 ± 1.27 d | 100 | 0.46 ± 0.11 c | 100 | 40.0 ± 6.53 d | 100 |
| AS8 | 6.78 ± 0.36 c | 199 | 1.04 ± 0.18 b | 227 | 784 ± 66.6 c | 1 960 |
| AS23 | 12.7 ± 0.72 b | 374 | 2.46 ± 0.33 a | 534 | 1 540 ± 105 b | 3 850 |
| AS31 | 16.6 ± 0.73 a | 488 | 2.83 ± 0.29 a | 616 | 1 737 ± 70.2 a | 4 343 |
| p-value | <0.001 | <0.001 | <0.001 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batykova, Z.; Pidlisnyuk, V.; Kistaubayeva, A.; Ust’ak, S.; Savitskaya, I.; Saidullayeva, L.; Mamirova, A. Isolation and Screening of the Novel Multi-Trait Strains for Future Implications in Phytotechnology. Microorganisms 2025, 13, 1902. https://doi.org/10.3390/microorganisms13081902
Batykova Z, Pidlisnyuk V, Kistaubayeva A, Ust’ak S, Savitskaya I, Saidullayeva L, Mamirova A. Isolation and Screening of the Novel Multi-Trait Strains for Future Implications in Phytotechnology. Microorganisms. 2025; 13(8):1902. https://doi.org/10.3390/microorganisms13081902
Chicago/Turabian StyleBatykova, Zhuldyz, Valentina Pidlisnyuk, Aida Kistaubayeva, Sergey Ust’ak, Irina Savitskaya, Laila Saidullayeva, and Aigerim Mamirova. 2025. "Isolation and Screening of the Novel Multi-Trait Strains for Future Implications in Phytotechnology" Microorganisms 13, no. 8: 1902. https://doi.org/10.3390/microorganisms13081902
APA StyleBatykova, Z., Pidlisnyuk, V., Kistaubayeva, A., Ust’ak, S., Savitskaya, I., Saidullayeva, L., & Mamirova, A. (2025). Isolation and Screening of the Novel Multi-Trait Strains for Future Implications in Phytotechnology. Microorganisms, 13(8), 1902. https://doi.org/10.3390/microorganisms13081902







