Bacterial Diversity Dynamics in Sandy Loam Soils in Tanzania Under Varying Fertilizer-Derived Uranium Concentrations
Abstract
1. Introduction
2. Materials and Methods
2.1. Location of the Study Area and Collection of Soil Samples
2.2. Bacteria DNA Extraction and Gene Sequencing
2.3. Bioinformatics Analyses for the Soil Bacteriota Composition
2.4. Determination of U Concentration in Soil
2.5. Statistical Analysis
3. Results
3.1. Effect of Soil pH and U Content in Different Fertilizer Application Plots
3.2. Effect of Soil N and P Content in Different Fertilizer Application Plots
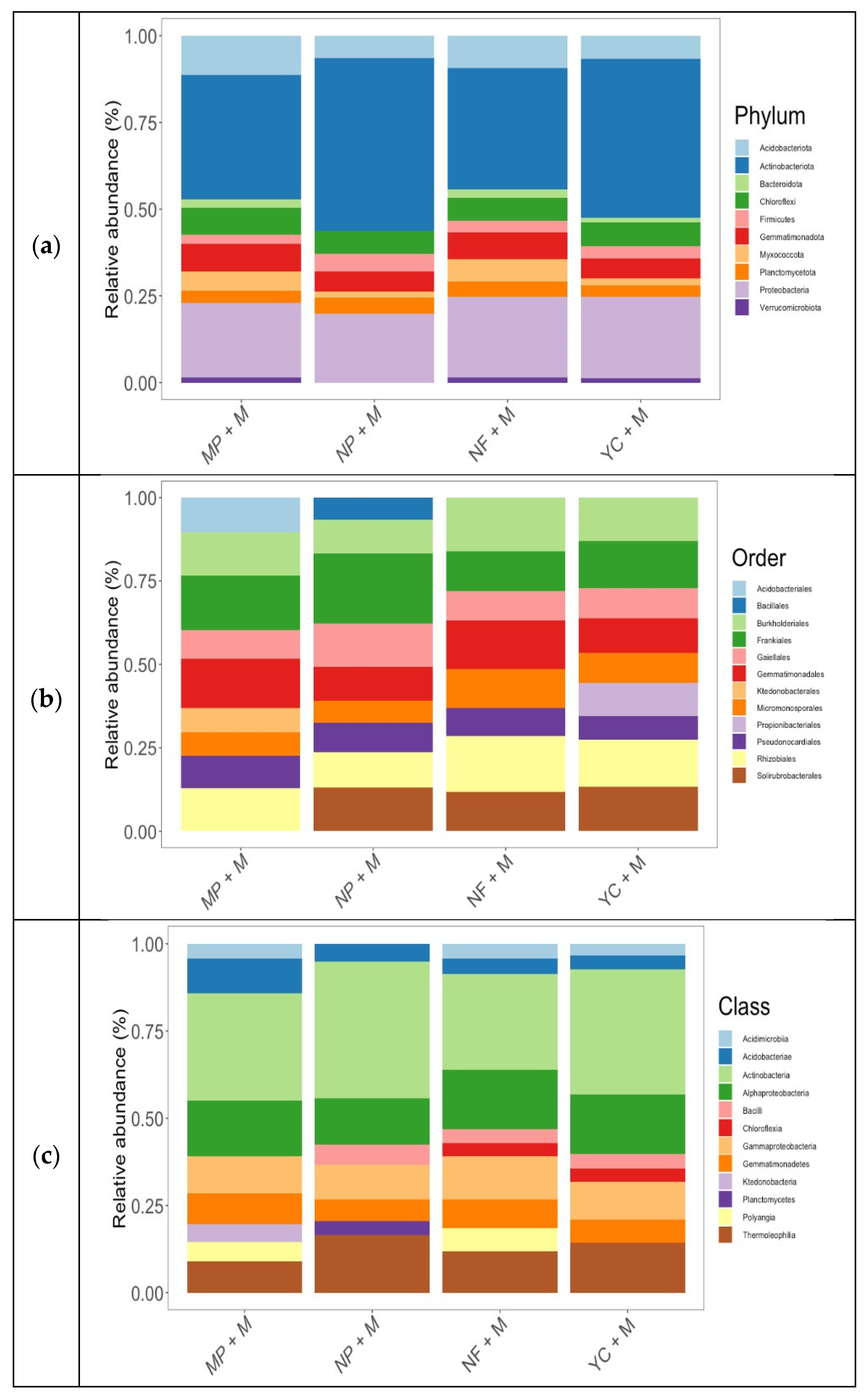
3.3. Abundance of Soil Bacteria at Phylum Level in Maize Plots
3.4. Abundance of Soil Bacteria at Order Level in Maize Plots
3.5. Abundance of Soil Bacteria at Class Level in Maize Plots
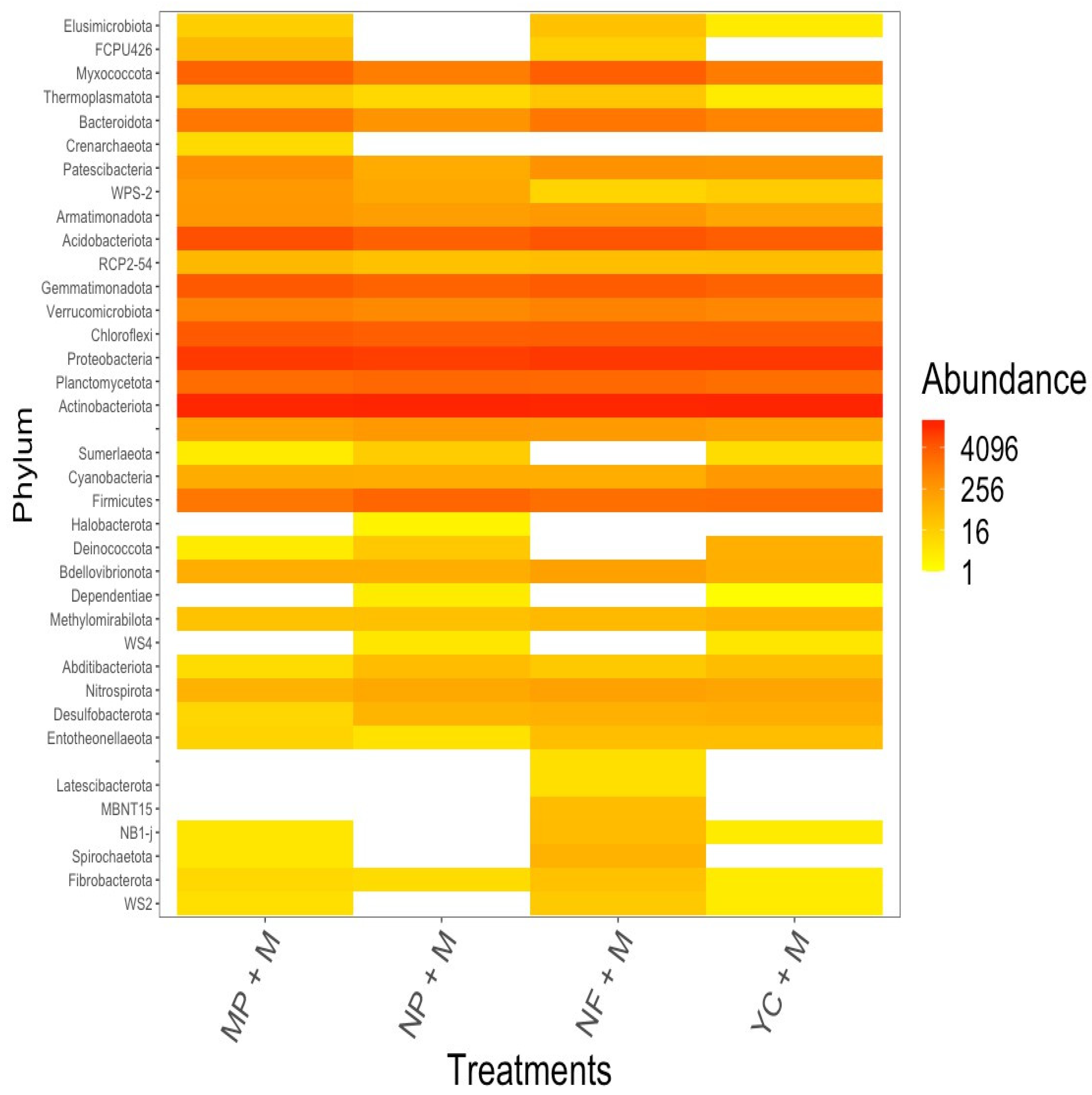
3.6. Composition of Phylum Community Variation Within Treatments
3.7. Venn Diagram Analysis for Unique Phyla, Classes, and Orders of Bacteria
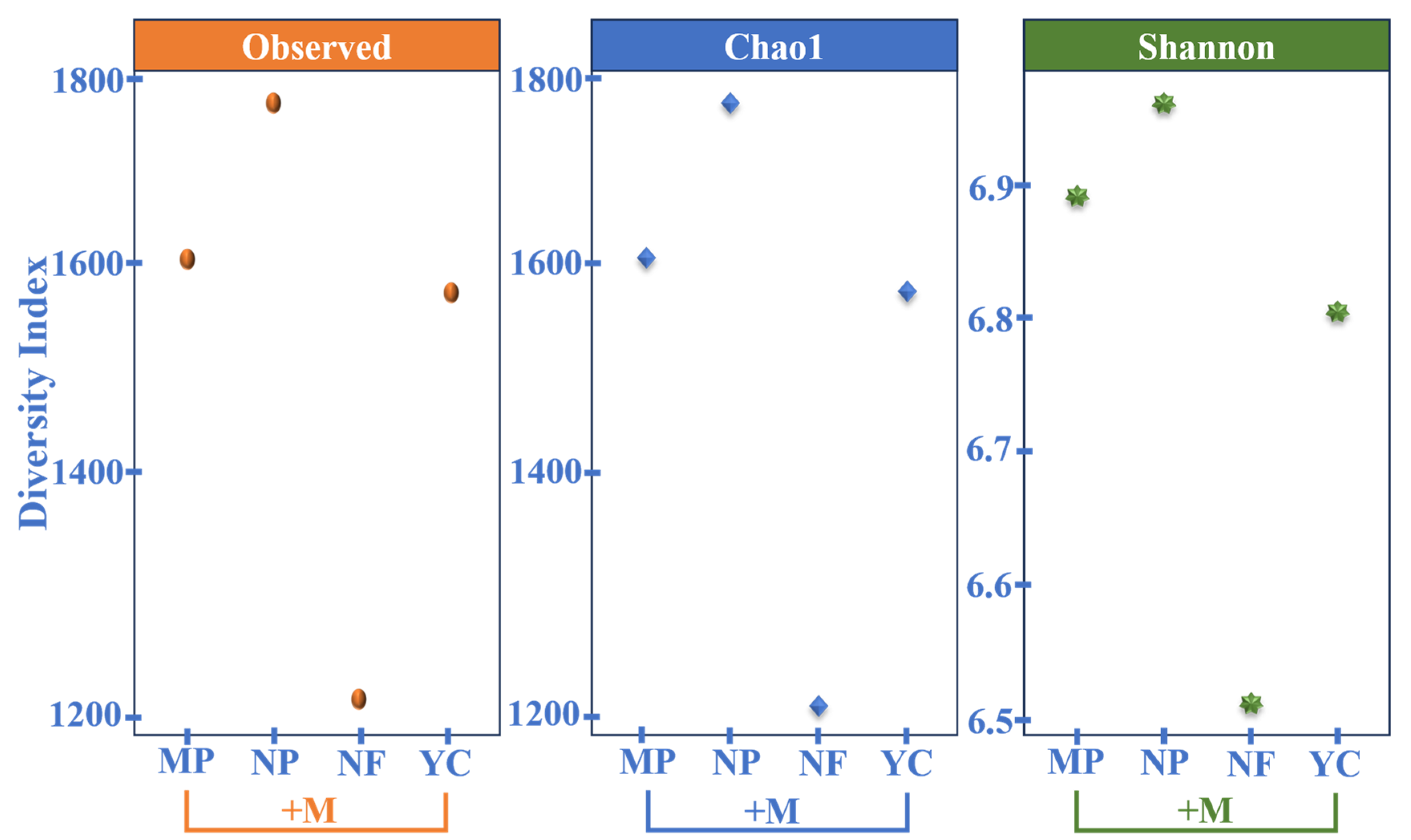
3.8. Observed, Chao1, and Shannon Diversity Indices Indicating Phylum-Level Diversity Across Treatments
4. Discussion
4.1. Rhizosphere Change in U Content and Soil pH Following Application of Different Types of Fertilizer
4.2. Distribution of Soil Bacteria in Plots Fertilized with Different Kinds of Fertilizer
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lobulu, J.; Shimelis, H.; Laing, M.; Mushongi, A.A. Maize Production Constraints, Traits Preference and Current Striga Control Options in Western Tanzania: Farmers’ Consultation and Implications for Breeding. Acta Agric. Scand. B Soil. Plant Sci. 2019, 69, 734–746. [Google Scholar] [CrossRef]
- Lisuma, J.B.; Semoka, J.M.R.; Semu, E. Maize Yield Response and Nutrient Uptake after Micronutrient Application on a Volcanic Soil. Agron. J. 2006, 98, 402–406. [Google Scholar] [CrossRef]
- Verbeeck, M.; Salaets, P.; Smolders, E. Trace Element Concentrations in Mineral Phosphate Fertilizers Used in Europe: A Balanced Survey. Sci. Total Environ. 2020, 712, 136419. [Google Scholar] [CrossRef]
- Mwalongo, D.A.; Haneklaus, N.H.; Lisuma, J.B.; Mpumi, N.; Amasi, A.I.; Mwimanzi, J.M.; Chuma, F.M.; Kivevele, T.T.; Mtei, K.M. Uranium Dissemination with Phosphate Fertilizers Globally: A Systematic Review with Focus on East Africa. Sustainability 2024, 16, 1496. [Google Scholar] [CrossRef]
- Haneklaus, N.H. Unconventional Uranium Resources from Phosphates. In Encyclopedia of Nuclear Energy; Greenspan, E., Ed.; Elsevier: Oxford, UK, 2021; pp. 286–291. ISBN 978-0-12-819732-5. [Google Scholar] [CrossRef]
- Haneklaus, N.H.; Mwalongo, D.A.; Lisuma, J.B.; Amasi, A.I.; Mwimanzi, J.; Bituh, T.; Ćirić, J.; Nowak, J.; Ryszko, U.; Rusek, P.; et al. Rare Earth Elements and Uranium in Minjingu Phosphate Fertilizer Products: Plant Food for Thought. Resour. Conserv. Recycl. 2024, 207, 107694. [Google Scholar] [CrossRef]
- Wu, F.; Wei, P.; Li, X.; Huang, M.; Zhou, L.; Liu, Z. Research Progress of Rhizosphere Effect in the Phytoremediation of Uranium-Contaminated Soil. J. Radioanal. Nucl. Chem. 2022, 331, 5493–5505. [Google Scholar] [CrossRef]
- Antunes, S.C.; Pereira, R.; Marques, S.M.; Castro, B.B.; Gonçalves, F. Impaired Microbial Activity Caused by Metal Pollution: A Field Study in a Deactivated Uranium Mining Area. Sci. Total Environ. 2011, 410–411, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Suriya, J.; Chandra Shekar, M.; Nathani, N.M.; Suganya, T.; Bharathiraja, S.; Krishnan, M. Assessment of Bacterial Community Composition in Response to Uranium Levels in Sediment Samples of Sacred Cauvery River. Appl. Microbiol. Biotechnol. 2017, 101, 831–841. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, H.; Wang, R.; Zhou, L.; Li, N.; He, Y.; Yang, X.; Lai, J.; Chen, K.; Zhu, W. Non-Targeted Metabolomics and 16s RDNA Reveal the Impact of Uranium Stress on Rhizosphere and Non-Rhizosphere Soil of Ryegrass. J. Environ. Radioact. 2023, 258, 107090. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, D.; Xiong, R.; Liu, S.; Hu, R.; Chen, P.; Wu, X.; Zou, H.; Hu, N.; Ding, D.; et al. Soil-Dependent Responses of Bacterial Communities, Phosphorus and Carbon Turnover to Uranium Stress in Different Soil Ecosystems. J. Hazard. Mater. 2025, 493, 138383. [Google Scholar] [CrossRef]
- Chen, H.; Sheng, Y.; Wang, S.; Chen, Y.; Qiao, Z.; Guo, H.; Dong, H. Uranium Contamination Mediating Soil and Ore Microbial Community Assembly at Four Mining Sites, South China. Front. Microbiol. 2025, 16, 1553072. [Google Scholar] [CrossRef]
- Li, Q.; Xiong, Z.; Xiang, P.; Zhou, L.; Zhang, T.; Wu, Q.; Zhao, C. Effects of Uranium Mining on Soil Bacterial Communities and Functions in the Qinghai-Tibet Plateau. Chemosphere 2024, 347, 140715. [Google Scholar] [CrossRef]
- Xiang, W.; Li, Z.; Xu, Z.; Yanxia, W.; Aixia, L.; Jian, Z.; Guiqiang, H. Exploring Functional Microbiota for Uranium Sequestration in Zoige Uranium Mine Soil. Microbiol Spectr 2025, 13, e02517-24. [Google Scholar] [CrossRef]
- Rastogi, G.; Osman, S.; Vaishampayan, P.A.; Andersen, G.L.; Stetler, L.D.; Sani, R.K. Microbial Diversity in Uranium Mining-Impacted Soils as Revealed by High-Density 16S Microarray and Clone Library. Microb. Ecol. 2010, 59, 94–108. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Zhang, Q.; Chen, X.; Dong, F.; Chen, H.; Liu, M.; Ali, I. Speciation Distribution of Heavy Metals in Uranium Mining Impacted Soils and Impact on Bacterial Community Revealed by High-Throughput Sequencing. Front. Microbiol. 2019, 10, 1867. [Google Scholar] [CrossRef] [PubMed]
- Sowmya, S.; Rekha, P.D.; Yashodhara, I.; Karunakara, N.; Arun, A.B. Uranium Tolerant Phosphate Solubilizing Bacteria Isolated from Gogi, a Proposed Uranium Mining Site in South India. Appl. Geochem. 2020, 114, 104523. [Google Scholar] [CrossRef]
- Mondani, L.; Benzerara, K.; Carrière, M.; Christen, R.; Mamindy-Pajany, Y.; Février, L.; Marmier, N.; Achouak, W.; Nardoux, P.; Berthomieu, C.; et al. Influence of Uranium on Bacterial Communities: A Comparison of Natural Uranium-Rich Soils with Controls. PLoS ONE 2011, 6, e25771. [Google Scholar] [CrossRef]
- Babich, T.L.; Semenova, E.M.; Sokolova, D.S.; Tourova, T.P.; Bidzhieva, S.K.; Loiko, N.G.; Avdonin, G.I.; Lutsenko, N.I.; Nazina, T.N. Phylogenetic Diversity and Potential Activity of Bacteria and Fungi in the Deep Subsurface Horizons of an Uranium Deposit. Microbiology 2021, 90, 607–620. [Google Scholar] [CrossRef]
- Yamaguchi, N.; Kawasaki, A.; Iiyama, I. Distribution of Uranium in Soil Components of Agricultural Fields after Long-Term Application of Phosphate Fertilizers. Sci. Total Environ. 2009, 407, 1383–1390. [Google Scholar] [CrossRef]
- Sun, Y.; Amelung, W.; Wu, B.; Haneklaus, S.; Schnug, E.; Bol, R. Fertilizer P-Derived Uranium Continues to Accumulate at Rothamsted Long-Term Experiments. Sci. Total Environ. 2022, 820, 153118. [Google Scholar] [CrossRef]
- Haneklaus, N.; Sun, Y.; Bol, R.; Lottermoser, B.; Schnug, E. To Extract, or Not to Extract Uranium from Phosphate Rock, That Is the Question. Environ. Sci. Technol. 2017, 51, 753–754. [Google Scholar] [CrossRef]
- Xiao, S.; Chen, X.; Qi, X.; Tian, J.; Dong, F.; Huang, S.; Guo, J. Effect of the Uranium Contamination on Soil Enzyme Activities and Microbial Functional Diversity. J. Nucl. Agric. Sci. 2020, 34, 896. [Google Scholar] [CrossRef]
- Mumtaz, S.; Streten, C.; Parry, D.L.; McGuinness, K.A.; Lu, P.; Gibb, K.S. Soil Uranium Concentration at Ranger Uranium Mine Land Application Areas Drives Changes in the Bacterial Community. J. Environ. Radioact. 2018, 189, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Zhong, J.; Lv, Y.; Liu, X.; Li, Y.; Zhang, M.; Yan, X.; Sun, W. Response and Dynamic Change of Microbial Community during Bioremediation of Uranium Tailings by Bacillus sp. Minerals 2021, 11, 967. [Google Scholar] [CrossRef]
- Michael, A.; Varnava, N.; Ioannidis, I.; Pashalidis, I. Uranium Recovery from Phosphate Rocks/Minerals–A Comprehensive Review. Sustain. Chem. Environ. 2024, 5, 100055. [Google Scholar] [CrossRef]
- IAEA. Recovery of Uranium from Phosphate Ores; IAEA: Vienna, Austria, 2025. [Google Scholar] [CrossRef]
- Tulsidas, H.; Gabriel, S.; Kiegiel, K.; Haneklaus, N. Uranium Resources in EU Phosphate Rock Imports. Resour. Policy 2019, 61, 151–156. [Google Scholar] [CrossRef]
- Shang, D.; Geissler, B.; Mew, M.; Satalkina, L.; Zenk, L.; Tulsidas, H.; Barker, L.; El-Yahyaoui, A.; Hussein, A.; Taha, M.; et al. Unconventional Uranium in China’s Phosphate Rock: Review and Outlook. Renew. Sustain. Energy Rev. 2021, 140, 110740. [Google Scholar] [CrossRef]
- Steiner, G.; Geissler, B.; Haneklaus, N. Making Uranium Recovery from Phosphates Great Again? Environ. Sci. Technol. 2020, 54, 1287–1289. [Google Scholar] [CrossRef]
- Haneklaus, N.; Kaggwa, M.; Misihairabgwi, J.; Abu El-Magd, S.; Ahmadi, N.; Ait Brahim, J.; Amasi, A.; Balláné Kovács, A.; Bartela, Ł.; Bellefqih, H.; et al. The Phosphorus Negotiation Game (P-Game): First Evaluation of a Serious Game to Support Science-Policy Decision Making Played in More than 20 Countries Worldwide. Discov. Sustain. 2025, 6, 1. [Google Scholar] [CrossRef]
- Haneklaus, N.; Bayok, A.; Fedchenko, V. Phosphate Rocks and Nuclear Proliferation. Sci. Glob. Secur. 2017, 25, 143–158. [Google Scholar] [CrossRef]
- Mwalongo, D.A.; Haneklaus, N.H.; Lisuma, J.B.; Kivevele, T.T.; Mtei, K.M. Uranium in Phosphate Rocks and Mineral Fertilizers Applied to Agricultural Soils in East Africa. Environ. Sci. Pollut. Res. 2023, 30, 33898–33906. [Google Scholar] [CrossRef]
- QIAGEN. DNeasy® Powersoil® Pro Kit Handbook; QIAGEN: Sydney, Australia, 2021. [Google Scholar]
- Lear, G.; Dickie, I.; Banks, J.; Boyer, S.; Buckley, H.; Buckley, T.; Cruickshank, R.; Dopheide, A.; Handley, K.; Hermans, S.; et al. Methods for the Extraction, Storage, Amplification and Sequencing of DNA from Environmental Samples. N. Z. J. Ecol. 2018, 42, 10. [Google Scholar] [CrossRef]
- Guenay-Greunke, Y.; Bohan, D.A.; Traugott, M.; Wallinger, C. Handling of Targeted Amplicon Sequencing Data Focusing on Index Hopping and Demultiplexing Using a Nested Metabarcoding Approach in Ecology. Sci. Rep. 2021, 11, 19510. [Google Scholar] [CrossRef] [PubMed]
- Mwalongo, D.A.; Haneklaus, N.H.; Carvalho, F.P.; Lisuma, J.B.; Kivevele, T.T.; Mtei, K.M. Influence of Phosphate Fertilizers on the Radioactivity of Agricultural Soils and Tobacco Plants in Kenya, Tanzania, and Uganda. Environ. Sci. Pollut. Res. 2023, 30, 83004–83023. [Google Scholar] [CrossRef] [PubMed]
- IAEA. IAEA Soil 7-Trace Elements in Soil (Certified Reference Materials); IAEA: Vienna, Austria, 2000; pp. 1–4. [Google Scholar]
- NIST Standard Reference Material 2711. 2011. Available online: https://www.govinfo.gov/content/pkg/GOVPUB-C13-a732e2729c519dc64544760f2a769dc5/pdf/GOVPUB-C13-a732e2729c519dc64544760f2a769dc5.pdf (accessed on 12 April 2025).
- Russel, J.; Oksanen, J. GitHub-MicEco: Various Functions for Analysis of Microbial Community Data. Available online: https://github.com/Russel88/MicEco (accessed on 12 June 2023).
- Vandenhove, H.; Van Hees, M.; Wouters, K.; Wannijn, J. Can We Predict Uranium Bioavailability Based on Soil Parameters? Part 1: Effect of Soil Parameters on Soil Solution Uranium Concentration. Environ. Pollut. 2007, 145, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Manoj, S.; Thirumurugan, M.; Elango, L. Determination of Distribution Coefficient of Uranium from Physical and Chemical Properties of Soil. Chemosphere 2020, 244, 125411. [Google Scholar] [CrossRef]
- Cui, Q.; Zhang, Z.; Beiyuan, J.; Cui, Y.; Chen, L.; Chen, H.; Fang, L. A Critical Review of Uranium in the Soil-Plant System: Distribution, Bioavailability, Toxicity, and Bioremediation Strategies. Crit. Rev. Environ. Sci. Technol. 2023, 53, 340–365. [Google Scholar] [CrossRef]
- Islam, E.; Sar, P. Molecular Assessment on Impact of Uranium Ore Contamination in Soil Bacterial Diversity. Int. Biodeterior. Biodegrad. 2011, 65, 1043–1051. [Google Scholar] [CrossRef]
- Yan, X.; Luo, X. Radionuclides Distribution, Properties, and Microbial Diversity of Soils in Uranium Mill Tailings from Southeastern China. J. Environ. Radioact. 2015, 139, 85–90. [Google Scholar] [CrossRef]
- Sanyal, S.K.; Etschmann, B.; Hore, S.B.; Shuster, J.; Brugger, J. Microbial Adaptations and Biogeochemical Cycling of Uranium in Polymetallic Tailings. J. Hazard. Mater. 2024, 465, 133334. [Google Scholar] [CrossRef]
- Saif, S.; Khan, M.S.; Zaidi, A.; Ahmad, E. Role of Phosphate-Solubilizing Actinomycetes in Plant Growth Promotion: Current Perspective. In Phosphate Solubilizing Microorganisms: Principles and Application of Microphos Technology; Khan, M.S., Zaidi, A., Musarrat, J., Eds.; Springer International Publishing: Cham, Germany, 2014; pp. 137–156. ISBN 978-3-319-08216-5. [Google Scholar]
- Solans, M.; Messuti, M.I.; Reiner, G.; Boenel, M.; Vobis, G.; Wall, L.G.; Scervino, J.M. Exploring the Response of Actinobacteria to the Presence of Phosphorus Salts Sources: Metabolic and Co-Metabolic Processes. J. Basic. Microbiol. 2019, 59, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Farda, B.; Djebaili, R.; Vaccarelli, I.; Del Gallo, M.; Pellegrini, M. Actinomycetes from Caves: An Overview of Their Diversity, Biotechnological Properties, and Insights for Their Use in Soil Environments. Microorganisms 2022, 10, 453. [Google Scholar] [CrossRef]
- Abdellatif, A.A.M.; Gebily, D.A.S.; Elmaghraby, M.M.K.; Sahu, P.K.; Thakur, B.; Kaur, S. Transforming Roles of Actinobacteria in Sustainable Agriculture: From Soil Health and Plant Productivity Perspective. In Metabolomics, Proteomics and Gene Editing Approaches in Biofertilizer Industry: Volume II; Kaur, S., Dwibedi, V., Sahu, P.K., Eds.; Springer Nature: Singapore, 2024; pp. 299–338. ISBN 978-981-97-2910-4. [Google Scholar]
- Kavya, T.; Govindasamy, V.; Suman, A.; Abraham, G. Plant–Actinobacteria Interactions for Biotic and Abiotic Stress Management in Crops. In Plant Holobiome Engineering for Climate-Smart Agriculture; Sayyed, R.Z., Ilyas, N., Eds.; Springer Nature: Singapore, 2024; pp. 441–463. ISBN 978-981-99-9388-8. [Google Scholar]
- O’Connor, A.; Mcclean, S. The Role of Universal Stress Proteins in Bacterial Infections. Curr. Med. Chem. 2017, 24, 3970–3979. [Google Scholar] [CrossRef]
- Nabi, B.; Kumawat, M.; Ahlawat, N.; Ahlawat, S. Molecular, Structural, and Functional Diversity of Universal Stress Proteins (USPs) in Bacteria, Plants, and Their Biotechnological Applications. Protein J. 2024, 43, 437–446. [Google Scholar] [CrossRef]
- Bergkemper, F.; Schöler, A.; Engel, M.; Lang, F.; Krüger, J.; Schloter, M.; Schulz, S. Phosphorus Depletion in Forest Soils Shapes Bacterial Communities towards Phosphorus Recycling Systems. Environ. Microbiol. 2016, 18, 1988–2000. [Google Scholar] [CrossRef]
- Dai, Z.; Liu, G.; Chen, H.; Chen, C.; Wang, J.; Ai, S.; Wei, D.; Li, D.; Ma, B.; Tang, C.; et al. Long-Term Nutrient Inputs Shift Soil Microbial Functional Profiles of Phosphorus Cycling in Diverse Agroecosystems. ISME J. 2020, 14, 757–770. [Google Scholar] [CrossRef] [PubMed]
- Mason, L.M.; Eagar, A.; Patel, P.; Blackwood, C.B.; DeForest, J.L. Potential Microbial Bioindicators of Phosphorus Mining in a Temperate Deciduous Forest. J. Appl. Microbiol. 2021, 130, 109–122. [Google Scholar] [CrossRef]
- Dahe, Z.; Shengjie, Z.; Sumit, K.; Heng, Z.; Qiong, X.; Wurunze, S.; Jian, Z.; Hua, X. Comparative Genomic Insights into the Evolution of Halobacteria-Associated “Candidatus Nanohaloarchaeota”. mSystems 2022, 7, e00669-22. [Google Scholar] [CrossRef]
- Oren, A. Novel Insights into the Diversity of Halophilic Microorganisms and Their Functioning in Hypersaline Ecosystems. npj Biodivers. 2024, 3, 18. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, S.; Mei, B.; Jia, L.; Liao, J.; Zhu, W. Construction of Dopamine Supported Mg(Ca)Al Layered Double Hydroxides with Enhanced Adsorption Properties for Uranium. Sci. Total Environ. 2023, 881, 163525. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Reich, P.B.; Khachane, A.N.; Campbell, C.D.; Thomas, N.; Freitag, T.E.; Abu Al-Soud, W.; Sørensen, S.; Bardgett, R.D.; Singh, B.K. It Is Elemental: Soil Nutrient Stoichiometry Drives Bacterial Diversity. Environ. Microbiol. 2017, 19, 1176–1188. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, H.; Yang, X.; Yao, H.; Deng, X.; Wang, Y.; An, S.; Kuzyakov, Y.; Chang, S.X. Plant and Soil Elemental C:N:P Ratios Are Linked to Soil Microbial Diversity during Grassland Restoration on the Loess Plateau, China. Sci. Total Environ. 2022, 806, 150557. [Google Scholar] [CrossRef] [PubMed]
- Craig, H.; Antwis, R.E.; Cordero, I.; Ashworth, D.; Robinson, C.H.; Osborne, T.Z.; Bardgett, R.D.; Rowntree, J.K.; Simpson, L.T. Nitrogen Addition Alters Composition, Diversity, and Functioning of Microbial Communities in Mangrove Soils: An Incubation Experiment. Soil. Biol. Biochem. 2021, 153, 108076. [Google Scholar] [CrossRef]
- Beltran-Garcia, M.J.; Martínez-Rodríguez, A.; Olmos-Arriaga, I.; Valdes-Salas, B.; Di Mascio, P.; White, J.F. Nitrogen Fertilization and Stress Factors Drive Shifts in Microbial Diversity in Soils and Plants. Symbiosis 2021, 84, 379–390. [Google Scholar] [CrossRef]
- Ling, N.; Chen, D.; Guo, H.; Wei, J.; Bai, Y.; Shen, Q.; Hu, S. Differential Responses of Soil Bacterial Communities to Long-Term N and P Inputs in a Semi-Arid Steppe. Geoderma 2017, 292, 25–33. [Google Scholar] [CrossRef]
- Wang, Y.C.; Ni, J.J.; Guo, H.W.; Kravchenko, E. Influences of Phosphorus-Modified Biochar on Bacterial Community and Diversity in Rhizosphere Soil. Environ. Sci. Pollut. Res. 2024, 31, 1681–1691. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Zhang, Y.; Luo, X.; Yu, L. Effects of Uranium on Soil Microbial Biomass Carbon, Enzymes, Plant Biomass and Microbial Diversity in Yellow Soils. Radioprotection 2016, 51, 207–212. [Google Scholar] [CrossRef]

| Treatment Code | Fertilizer/Treatment Component | Fertilizer Formulation | Uranium Concentration (mg kg−1) |
|---|---|---|---|
| T1 | Not fertilized (NF + M) | No fertilizer used | - |
| T2 | YaraMila Cereal (YC + M) | 23N:10P:5K + 3S + 2MgO + 0.3Zn | 38.84 ± 1.24 |
| T3 | Nafaka Plus (NP + M) | N: 9%, P2O5: 16%, K2O: 6%, CaO: 25%, S: 5%, MgO: 2%, Zn: 0.5%, B: 0.1% | 147.65 ± 8.61 |
| T4 | Minjingu Powder (MP + M) | P2O5: 28% | 159.67 ± 10.48 |
| Measured Uranium Mass Concentration | |||
| Reference Material | Uranium Concentration (mg kg−1) | Bias (%) | |
| Measured | Reference | ||
| IAEA Soil 7 | 2.78 ± 0.43 | 2.6 ± 0.6 | 5.8% |
| NIST 2711a | 3.10 | 2.96 | 5.1% |
| Treatments (T) | Soil pH Before Fertilization | Soil pH After Fertilization | Soil U (mg kg−1) Before Fertilization | Soil U (mg kg−1) After Fertilization |
|---|---|---|---|---|
| T1 = NF + M | 5.21 ± 0.01 a | 5.21 ± 0.01 c | 2.04 ± 0.01 a | 2.04 ± 0.02 c |
| T2 = YC + M | 5.22 ± 0.00 a | 5.22 ± 0.01 bc | 2.02 ± 0.02 a | 2.04 ± 0.01 c |
| T3 = NP + M | 5.22 ± 0.00 a | 5.26 ± 0.01 a | 2.01 ± 0.02 a | 3.93 ± 0.28 a |
| T4 = MP + M | 5.22 ± 0.00 a | 5.24 ± 0.01 ab | 2.02 ± 0.01 a | 3.06 ± 0.15 b |
| F-statistics | 0.27 ns | 0.02 * | 0.48 ns | 0.00 *** |
| Treatments (Ts) | Soil N Before Fertilization (%) | Soil N After Fertilization (%) | Soil P Before Fertilization (mg kg−1) | Soil P After Fertilization (mg kg−1) |
|---|---|---|---|---|
| T1 = NF + M | 0.03 ± 0.00 a | 0.02 ± 0.01 c | 39.24 ± 0.00 d | 37.52 ± 0.01 d |
| T2 = YC + M | 0.04 ± 0.00 a | 0.07 ± 0.01 a | 39.97 ± 0.00 b | 40.59 ± 0.01 c |
| T3 = NP + M | 0.04 ± 0.00 a | 0.05 ± 0.01 b | 40.18 ± 0.00 a | 41.23 ± 0.01 a |
| T4 = MP + M | 0.04 ± 0.00 a | 0.04 ± 0.01 b | 39.91 ± 0.00 c | 40.74 ± 0.01 b |
| F-statistics | 1.53 ns | 20.31 *** | 6864 *** | 105,647 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mwalongo, D.A.; Lisuma, J.B.; Haneklaus, N.H.; Maged, A.; Brink, H.; Carvalho, F.P.; Wacławek, S.; Mpumi, N.; Amasi, A.I.; Mwimanzi, J.M.; et al. Bacterial Diversity Dynamics in Sandy Loam Soils in Tanzania Under Varying Fertilizer-Derived Uranium Concentrations. Microorganisms 2025, 13, 1886. https://doi.org/10.3390/microorganisms13081886
Mwalongo DA, Lisuma JB, Haneklaus NH, Maged A, Brink H, Carvalho FP, Wacławek S, Mpumi N, Amasi AI, Mwimanzi JM, et al. Bacterial Diversity Dynamics in Sandy Loam Soils in Tanzania Under Varying Fertilizer-Derived Uranium Concentrations. Microorganisms. 2025; 13(8):1886. https://doi.org/10.3390/microorganisms13081886
Chicago/Turabian StyleMwalongo, Dennis A., Jacob B. Lisuma, Nils H. Haneklaus, Ali Maged, Hendrik Brink, Fernando P. Carvalho, Stanisław Wacławek, Nelson Mpumi, Aloyce I. Amasi, Jerome M. Mwimanzi, and et al. 2025. "Bacterial Diversity Dynamics in Sandy Loam Soils in Tanzania Under Varying Fertilizer-Derived Uranium Concentrations" Microorganisms 13, no. 8: 1886. https://doi.org/10.3390/microorganisms13081886
APA StyleMwalongo, D. A., Lisuma, J. B., Haneklaus, N. H., Maged, A., Brink, H., Carvalho, F. P., Wacławek, S., Mpumi, N., Amasi, A. I., Mwimanzi, J. M., Chuma, F. M., Kivevele, T. T., & Mtei, K. M. (2025). Bacterial Diversity Dynamics in Sandy Loam Soils in Tanzania Under Varying Fertilizer-Derived Uranium Concentrations. Microorganisms, 13(8), 1886. https://doi.org/10.3390/microorganisms13081886









