Impact of Phosphogypsum on Viability of Trichuris suis Eggs in Anaerobic Digestion of Swine Manure
Abstract
1. Introduction
- -
- The reduction of phosphogypsum to calcium sulfide;
- -
- The effect of alkaline leaching on sulfate reduction;
- -
- Sulfate reduction using mixed cultures;
- -
- Sulfate leaching and transformation.
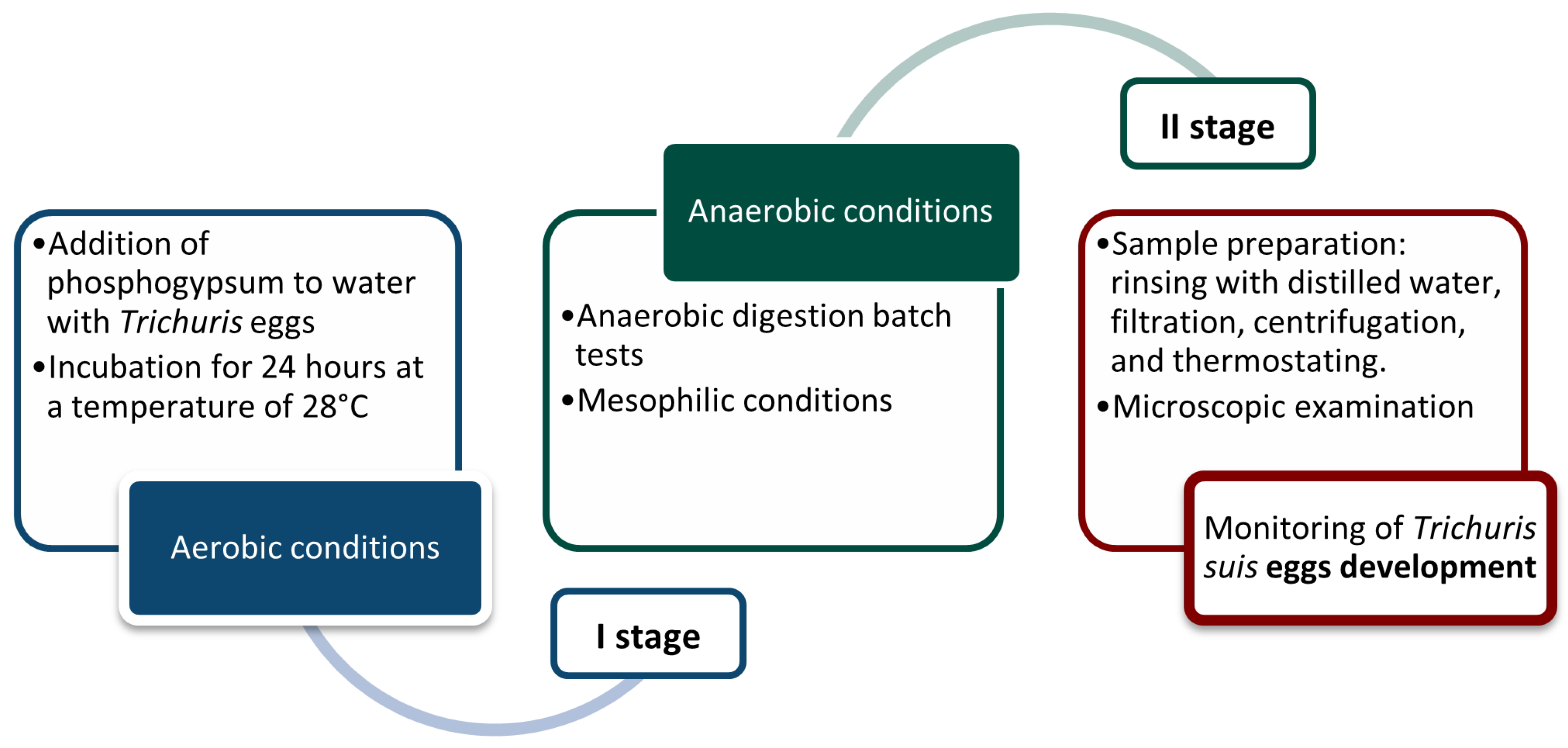
2. Materials and Methods
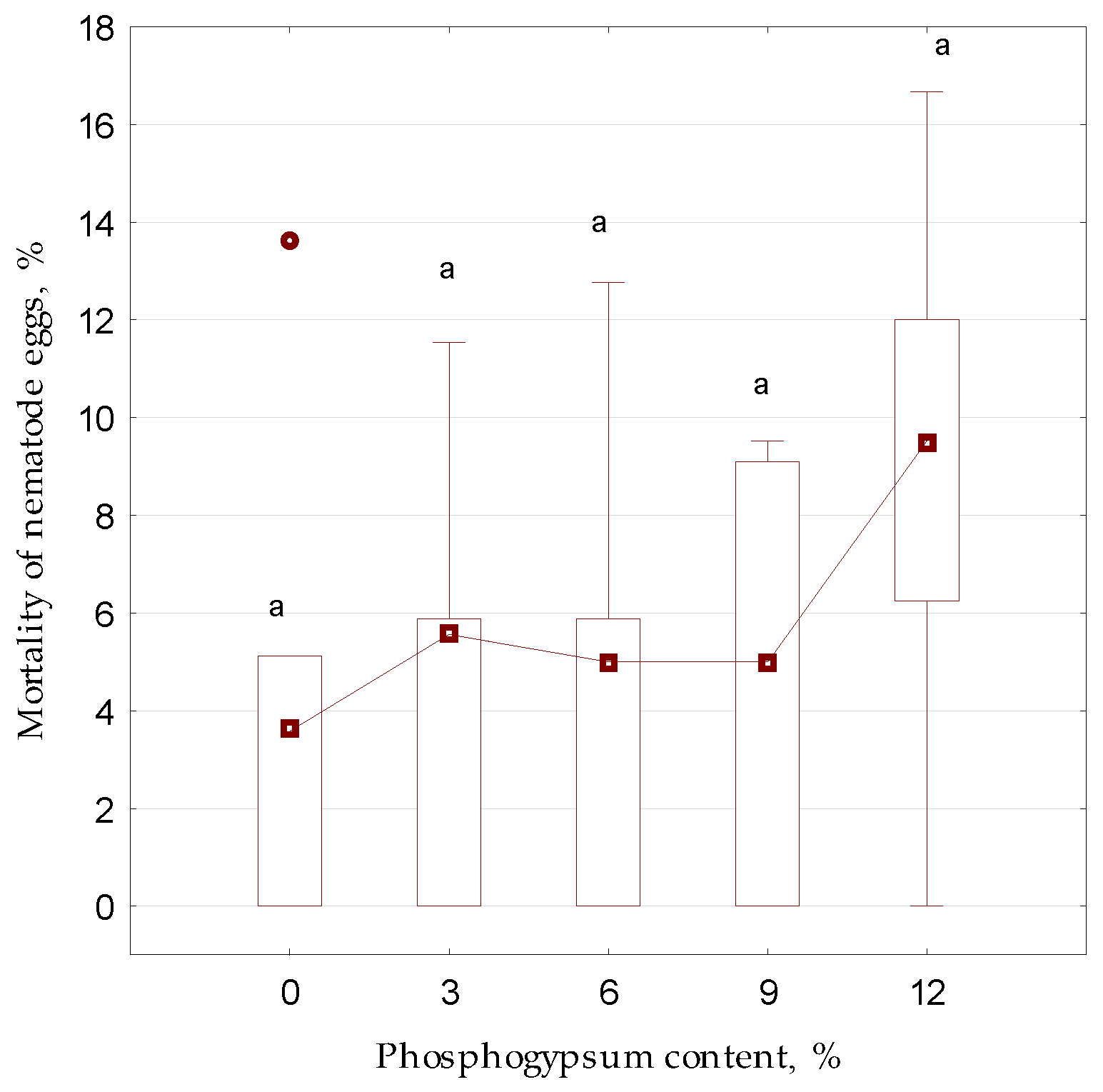
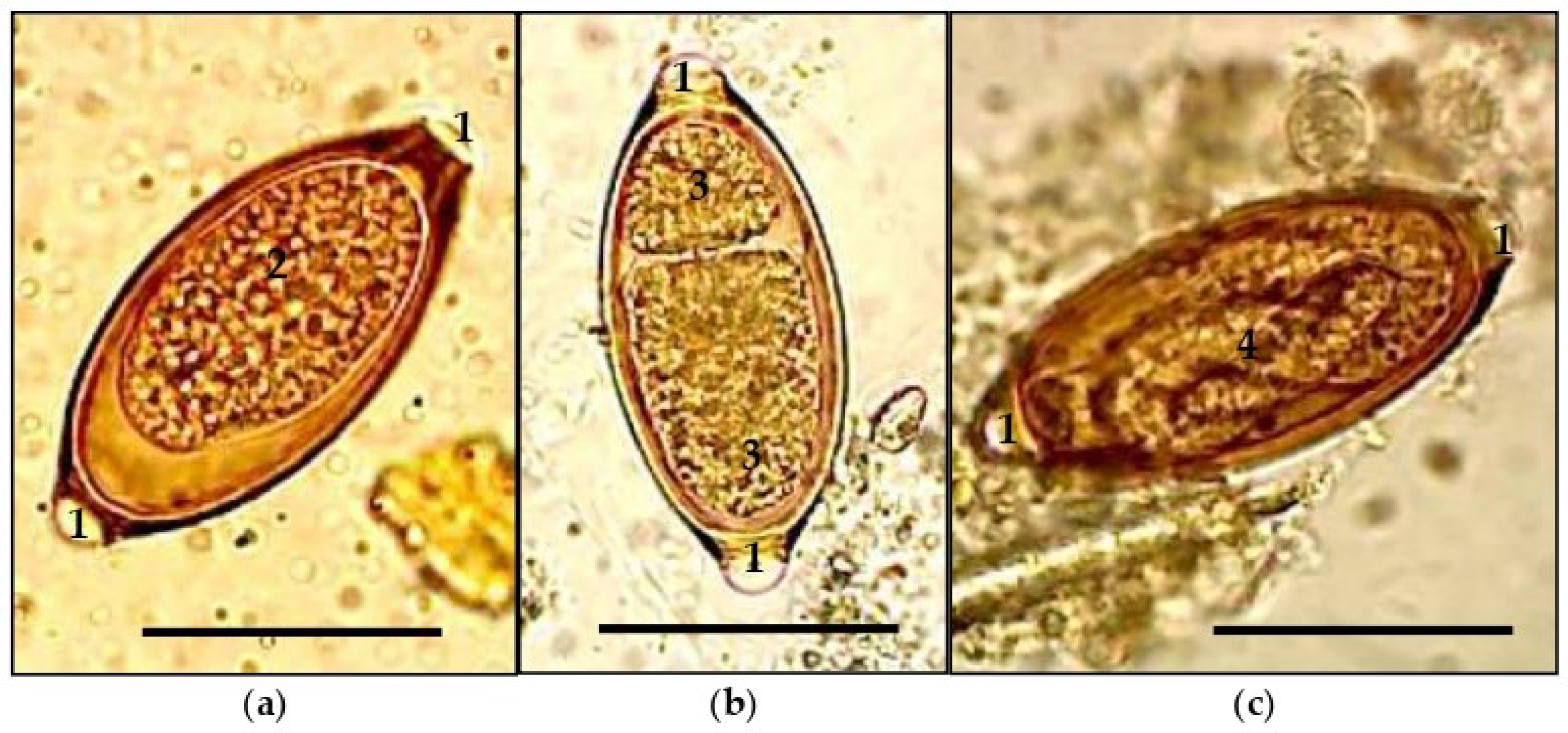
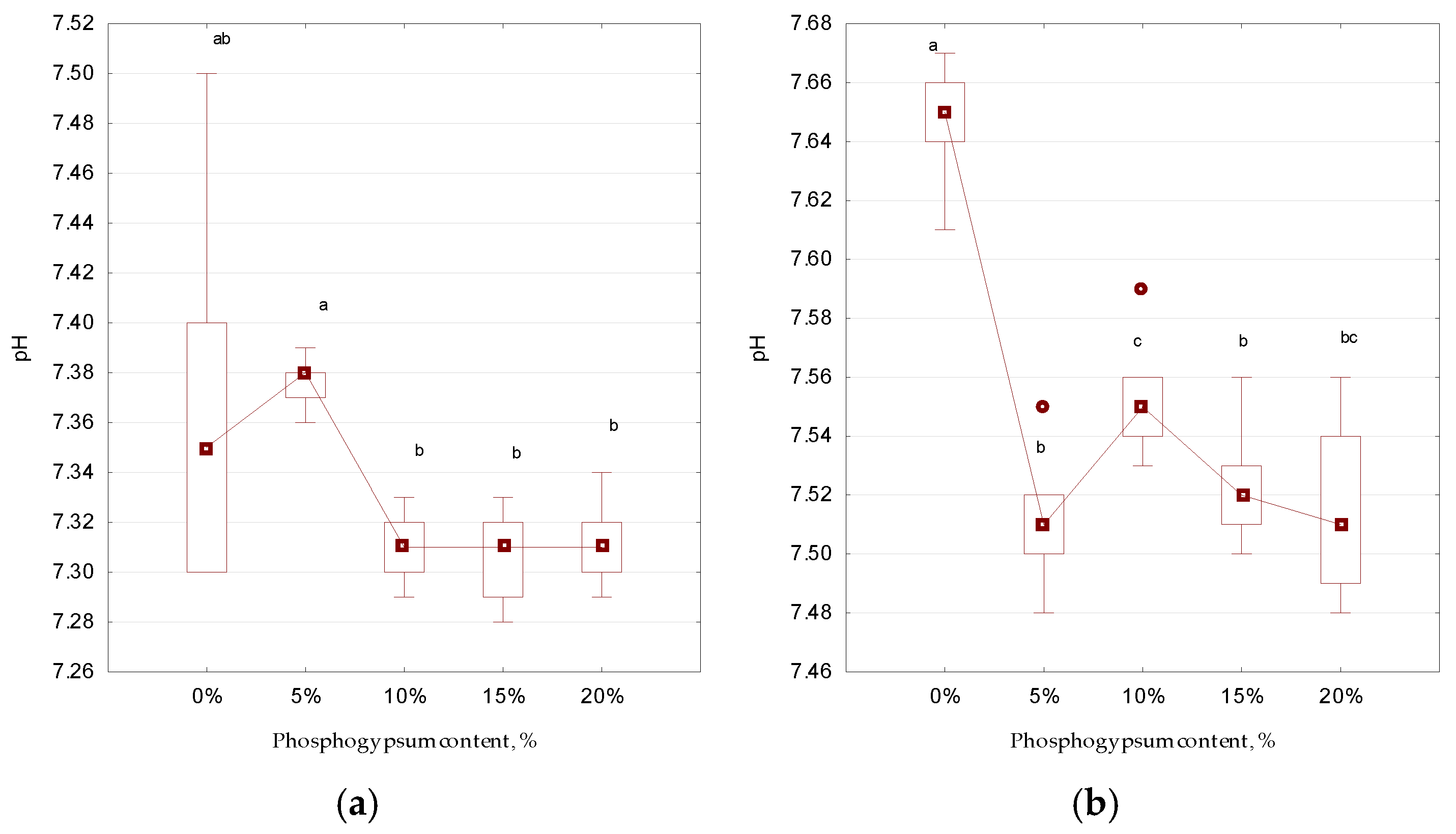
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kiyasudeen, K.S.; Ibrahim, M.H.; Quaik, S.; Ismail, S.A. Introduction to organic wastes and its management. In Prospects of Organic Waste Management and the Significance of Earthworms; Kiyasudeen, K.S., Ibrahim, M.H., Quaik, S., Ismail, S.A., Eds.; Springer: Cham, Switzerland, 2015; pp. 1–21. [Google Scholar] [CrossRef]
- Matiz-Villamil, A.; Méndez-Carranza, K.J.; Pascagaza-Pulido, A.F.; Rendón-Rendón, T.; Noriega-Noriega, J.; Pulido-Villamarín, A. Trends in the management of organic swine farm waste by composting: A systematic review. Heliyon 2023, 9, e18208. [Google Scholar] [CrossRef]
- Pittman, J.; Shepherd, G.; Thacker, B.; Myers, G. Trichuris suis in finishing pigs: Case report and review. J. Swine Health Prod. 2010, 18, 306–313. [Google Scholar] [CrossRef]
- Bünger, M.; Renzhammer, R.; Joachim, A.; Hinney, B.; Brunthaler, R.; Al Hossan, M.; Matt, J.; Nedorost, N.; Weissenbacher-Lang, C.; Schwarz, L. Trichurosis on a conventional swine fattening farm with extensive husbandry—A case report. Pathogens 2022, 11, 775. [Google Scholar] [CrossRef]
- Yevstafieva, V.O.; Petrenko, M.O.; Melnychuk, V.V.; Vakulenko, Y.V.; Bakhur-Kavaliauskene, T.I.; Titarenko, O.V.; Shaferivskyi, B.S.; Pishchalenko, M.A.; Filonenko, S.V.; Sheiko, S.V. Effect of temperature on the survival rates of the embryonic states of development of Trichuris skrjabini nematodes parasitizing sheep. Acta Vet. Eurasia 2023, 49, 105–112. [Google Scholar] [CrossRef]
- Yevstafieva, V.; Petrenko, M.; Peleno, R.; Nikiforova, O.; Vakulenko, V.; Reshetylo, O.; Kone, M. Effect of disinfectants on viability of Trichuris skrjabini eggs. Regul. Mech. Biosyst. 2023, 14, 70–76. [Google Scholar] [CrossRef]
- Rusanowska, P.; Zieliński, M.; Kisielewska, M.; Dudek, M.; Paukszto, Ł.; Dębowski, M. Methane production, microbial community, and volatile fatty acids profiling during anaerobic digestion under different organic loading. Energies 2025, 18, 575. [Google Scholar] [CrossRef]
- Kabaivanova, L.; Hubenov, V.; Dimitrova, L.; Simeonov, I.; Wang, H.; Petrova, P. Archaeal and bacterial content in a two-stage anaerobic system for efficient energy production from agricultural wastes. Molecules 2022, 27, 1512. [Google Scholar] [CrossRef]
- Lee, H.; Fitamo, T.M.; Nesbø, C.L.; Guilford, N.G.H.; Kanger, K.; Yang, M.I.; Edwards, E.A. Microbial community dynamics of a sequentially fed anaerobic digester treating solid organic waste. bioRxiv 2022, 99, fiad017. [Google Scholar] [CrossRef]
- Boyko, O.O.; Hapich, H.V.; Mylostyvyi, R.V.; Izhboldina, O.O.; Chernysh, Y.; Chubur, V.; Roubík, H.; Brygadyrenko, V.V. Recycling and decontamination of organic waste in Ukraine: Current state, technologies and prospects for the biogas industry. Biosyst. Divers. 2024, 32, 260–269. [Google Scholar] [CrossRef]
- Yevstafieva, V.; Dolhin, O. Viability of exogenous stages of development of the causative agent of trichuriasis of dogs under the influence of temperature. Sci. Messenger LNU Vet. Med. Biotechnol. Ser. Vet. Sci. 2022, 107, 58–63. [Google Scholar] [CrossRef]
- Yevstafieva, V.A.; Yuskiv, I.D.; Melnychuk, V.V. An investigation of embryo and eggshell development in Trichuris suis (Nematoda, Trichuridae) under laboratory conditions. Vestn. Zool. 2016, 50, 173–178. [Google Scholar] [CrossRef]
- Martinez, J.; Dabert, P.; Barrington, S.; Burton, C. Livestock waste treatment systems for environmental quality, food safety, and sustainability. Bioresour. Technol. 2009, 100, 5527–5536. [Google Scholar] [CrossRef]
- Shakya, S.K.; Rawat, N.S.; Mishra, A.K.; Ranjan, R.; Singh, S.K.; Upadhyay, N.; Kakotiya, K.; Shakya, N.; Kumar, S. Livestock waste management practices to strengthen the farm profitability. J. Entomol. Zool. Stud. 2022, 10, 321–326. [Google Scholar] [CrossRef]
- Boyko, O.; Brygadyrenko, V. Nematicidal activity of inorganic food additives. Diversity 2022, 14, 663. [Google Scholar] [CrossRef]
- Boyko, O.; Brygadyrenko, V. Survival of nematode larvae Strongyloides papillosus and Haemonchus contortus under the influence of various groups of organic compounds. Diversity 2023, 15, 254. [Google Scholar] [CrossRef]
- Manser, N.D.; Wald, I.; Ergas, S.J.; Izurieta, R.; Mihelcic, J.R. Assessing the fate of Ascaris suum ova during mesophilic anaerobic digestion. Environ. Sci. Technol. 2015, 49, 3128–3135. [Google Scholar] [CrossRef]
- Patil, P.P.; Mutnuri, S. Study of helminth eggs (Ascaris suum) inactivation by anaerobic digestion and electrochemical treatment. Gates Open Res. 2024, 7, 93. [Google Scholar] [CrossRef]
- Shao, Z.; Guo, X.; Qu, Q.; Kang, K.; Su, Q.; Wang, C.; Qiu, L. Effects of chlorine disinfectants on the microbial community structure and the performance of anaerobic digestion of swine manure. Bioresour. Technol. 2021, 339, 125576. [Google Scholar] [CrossRef]
- Smith, S.R.; Lang, N.L.; Cheung, K.H.M.; Spanoudaki, K. Factors controlling pathogen destruction during anaerobic digestion of biowastes. Waste Manag. 2005, 25, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Jian, S.; Bi, J.; Li, Y.; Chang, Z.; He, J.; Ye, X. Anaerobic digestion in mesophilic and room temperature conditions: Digestion performance and soil-borne pathogen survival. J. Environ. Sci. 2016, 43, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Boyko, O.; Brygadyrenko, V.; Chernysh, Y.; Chubur, V.; Roubík, H. Possibilities of decontaminating organic waste from swine-farming complexes using anaerobic digestion. Biomass Convers. Biorefin. 2024, in press. [Google Scholar] [CrossRef]
- Huang, W.; Li, Y.; Wang, F.; Feng, L.; Wang, D.; Ma, Y.; Wu, Y.; Luo, J. Disinfectant sodium dichloroisocyanurate synergistically strengthened sludge acidogenic process and pathogens inactivation: Targeted upregulation of functional microorganisms and metabolic traits via self-adaptation. Water Res. 2023, 247, 120787. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wang, T.; Zhou, Y.; Zhao, M.; Shi, W.; Huang, Z.; Ruan, W. Insights into the phenol disinfectant on the methane performance from wastewater by mesophilic anaerobic digestion: Single and two stages analysis. Process Saf. Environ. Prot. 2023, 170, 19–27. [Google Scholar] [CrossRef]
- Singh, S.; Lin, H. Hydrogen sulfide in physiology and diseases of the digestive tract. Microorganisms 2015, 3, 866–889. [Google Scholar] [CrossRef] [PubMed]
- Buret, A.G.; Allain, T.; Motta, J.P.; Wallace, J.L. Effects of hydrogen sulfide on the microbiome: From toxicity to therapy. Antioxid. Redox Signal. 2022, 36, 211–219. [Google Scholar] [CrossRef]
- Seregina, T.A.; Lobanov, K.V.; Shakulov, R.S.; Mironov, A.S. Enhancement of the bactericidal effect of antibiotics by inhibition of enzymes involved in production of hydrogen sulfide in bacteria. Mol. Biol. 2022, 56, 638–648. [Google Scholar] [CrossRef]
- Jovliyev, Z. Composition, structure and physical-chemical properties of phosphogypsum. Galaxy Int. Interdiscip. Res. J. 2022, 10, 1105–1107. [Google Scholar]
- Plyatsuk, L.; Balintova, M.; Chernysh, Y.; Demcak, S.; Holub, M.; Yakhnenko, E. Influence of phosphogypsum dump on the soil ecosystem in the Sumy Region (Ukraine). Appl. Sci. 2019, 9, 5559. [Google Scholar] [CrossRef]
- Chernysh, Y.; Yakhnenko, O.; Chubur, V.; Roubík, H. Phosphogypsum recycling: A review of environmental issues, current trends, and prospects. Appl. Sci. 2021, 11, 1575. [Google Scholar] [CrossRef]
- Onopriienko, D.; Makarova, T.; Hapich, H.; Chernysh, Y.; Roubík, H. Agroecological transformation in the salt composition of soil under the phosphogypsum influence on irrigated lands in Ukraine. Agriculture 2024, 14, 408. [Google Scholar] [CrossRef]
- Alla, M.; Harrou, A.; Elhafiany, M.L.; Azerkane, D.; El Ouahabi, M.; Gharibi, E.K. Reduction of phosphogypsum to calcium sulfide (CaS) using metallic iron in a hydrochloric acid medium. Phosphorus Sulfur Silicon Relat. Elem. 2022, 197, 1026–1035. [Google Scholar] [CrossRef]
- Bounaga, A.; Alsanea, A.; Danouche, M.; Rittmann, B.E.; Zhou, C.; Boulif, R.; Zeroual, Y.; Benhida, R.; Lyamlouli, K. Effect of alkaline leaching of phosphogypsum on sulfate reduction activity and bacterial community composition using different sources of anaerobic microbial inoculum. Sci. Total Environ. 2023, 904, 166296. [Google Scholar] [CrossRef] [PubMed]
- Azabou, S.; Mechichi, T.; Sayadi, S. Sulfate reduction from phosphogypsum using a mixed culture of sulfate-reducing bacteria. Int. Biodeterior. Biodegrad. 2005, 56, 236–242. [Google Scholar] [CrossRef]
- Alsanea, A.; Bounaga, A.; Lyamlouli, K.; Zeroual, Y.; Boulif, R.; Zhou, C.; Rittmann, B. Sulfate leached from phosphogypsum is transformed in a hydrogen-based membrane biofilm reactor. ACS ES&T Eng. 2024, 5, 468–474. [Google Scholar] [CrossRef]
- Medennikov, O.A.; Egorova, M.A.; Shabelskaya, N.P.; Rajabov, A.; Sulima, S.I.; Sulima, E.V.; Khliyan, Z.D.; Monastyrskiy, D.I. Studying the process of phosphogypsum recycling into a calcium sulphide-based luminophor. Nanomaterials 2024, 14, 904. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Malik, Y.S.; Singh, G.; Dhar, P.; Singla, A.K. Aseptic collection, preservation, and dispatch of samples for disease diagnosis. In Core Competencies of a Veterinary Graduate; Verma, S., Malik, Y.S., Singh, G., Dhar, P., Singla, A.K., Eds.; Springer: Singapore, 2024; pp. 101–117. [Google Scholar] [CrossRef]
- Zajac, A.M.; Conboy, G.A. (Eds.) Veterinary Clinical Parasitology, 8th ed.; John Wiley and Sons: London, UK, 2011. [Google Scholar]
- Zhuravel, S.V.; Kravchuk, M.M.; Kropyvnytskyi, R.B.; Klymenko, T.V.; Trembitska, O.I.; Radko, V.H.; Nihorodova, S.A.; Dyachenko, M.O.; Zhuravel, S.S.; Polishchuk, V.O. Orhanichni dobryva [Organic fertilizers]; Polisskyi University Press: Zhytomyr, Ukranie, 2020; p. 200. (In Ukrainian) [Google Scholar]
- Zhou, J.; Zhang, R.; Liu, F.; Yong, X.; Wu, X.; Zheng, T.; Jiang, M.; Jia, H. Biogas production and microbial community shift through neutral pH control during the anaerobic digestion of pig manure. Bioresour. Technol. 2016, 217, 44–49. [Google Scholar] [CrossRef]
- Castro-Ramos, J.J.; Solís-Oba, A.; Solís-Oba, M.; Calderón-Vázquez, C.L.; Higuera-Rubio, J.M.; Castro-Rivera, R. Effect of the initial pH on the anaerobic digestion process of dairy cattle manure. AMB Express 2022, 12, 162. [Google Scholar] [CrossRef]
- Chubur, V.; Chernysh, Y.; Ferchau, E.; Zaffar, N. Effect of phosphogypsum addition on methane yield in biogas and digestate properties during anaerobic digestion. J. Eng. Sci. 2022, 9, H11–H18. [Google Scholar] [CrossRef]
- Khan, A.W.; Trottier, T.M. Effect of sulfur-containing compounds on anaerobic degradation of cellulose to methane by mixed cultures obtained from sewage sludge. Appl. Environ. Microbiol. 1978, 35, 1027–1034. [Google Scholar] [CrossRef]
- Maslova, O.; Senko, O.; Stepanov, N.; Gladchenko, M.; Gaydamaka, S.; Akopyan, A.; Eseva, E.; Anisimov, A.; Efremenko, E. Sulfur containing mixed wastes in anaerobic processing by new immobilized synthetic consortia. Bioresour. Technol. 2022, 362, 127794. [Google Scholar] [CrossRef]
- Mutegoa, E.; Sahini, M.G. Approaches to mitigation of hydrogen sulfide during anaerobic digestion process—A review. Heliyon 2023, 9, e19768. [Google Scholar] [CrossRef] [PubMed]
- Cheng, B.; Wang, Y.; Zhang, D.; Wu, D.; Zan, F.; Ma, J.; Miao, L.; Wang, Z.; Chen, G.; Guo, G. Thiosulfate pretreatment enhancing short-chain fatty acids production from anaerobic fermentation of waste activated sludge: Performance, metabolic activity and microbial community. Water Res. 2023, 238, 120013. [Google Scholar] [CrossRef] [PubMed]
- Chernysh, Y.; Chubur, V.; Roubik, H. Advancing circular bioeconomy: Trends, clusters, and roadmaps in biofuel production and waste valorisation. Agron. Res. 2024, 22, 774–793. [Google Scholar] [CrossRef]
- Hurley, L.C.; Sommerville, R.I. Reversible inhibition of hatching of infective eggs of Ascaris suum (Nematoda). Int. J. Parasitol. 1982, 12, 463–465. [Google Scholar] [CrossRef] [PubMed]
- Salo, M.; Mäkinen, J.; Yang, J.; Kurhila, M.; Koukkari, P. Continuous biological sulfate reduction from phosphogypsum waste leachate. Hydrometallurgy 2018, 180, 1–6. [Google Scholar] [CrossRef]
- Danouche, M.; Bounaga, A.; Boulif, R.; Zeroual, Y.; Benhida, R.; Lyamlouli, K. Optimization of sulfate leaching from phosphogypsum for efficient bioreduction in a batch bioreactor using a sulfate-reducing microbial consortium. Chem. Eng. J. 2023, 475, 146072. [Google Scholar] [CrossRef]
- Vejzagić, N.; Thamsborg, S.M.; Kringel, H.; Roepstorff, A.; Bruun, J.M.; Kapel, C.M.O. In vitro hatching of Trichuris suis eggs. Parasitol. Res. 2015, 114, 2705–2714. [Google Scholar] [CrossRef]

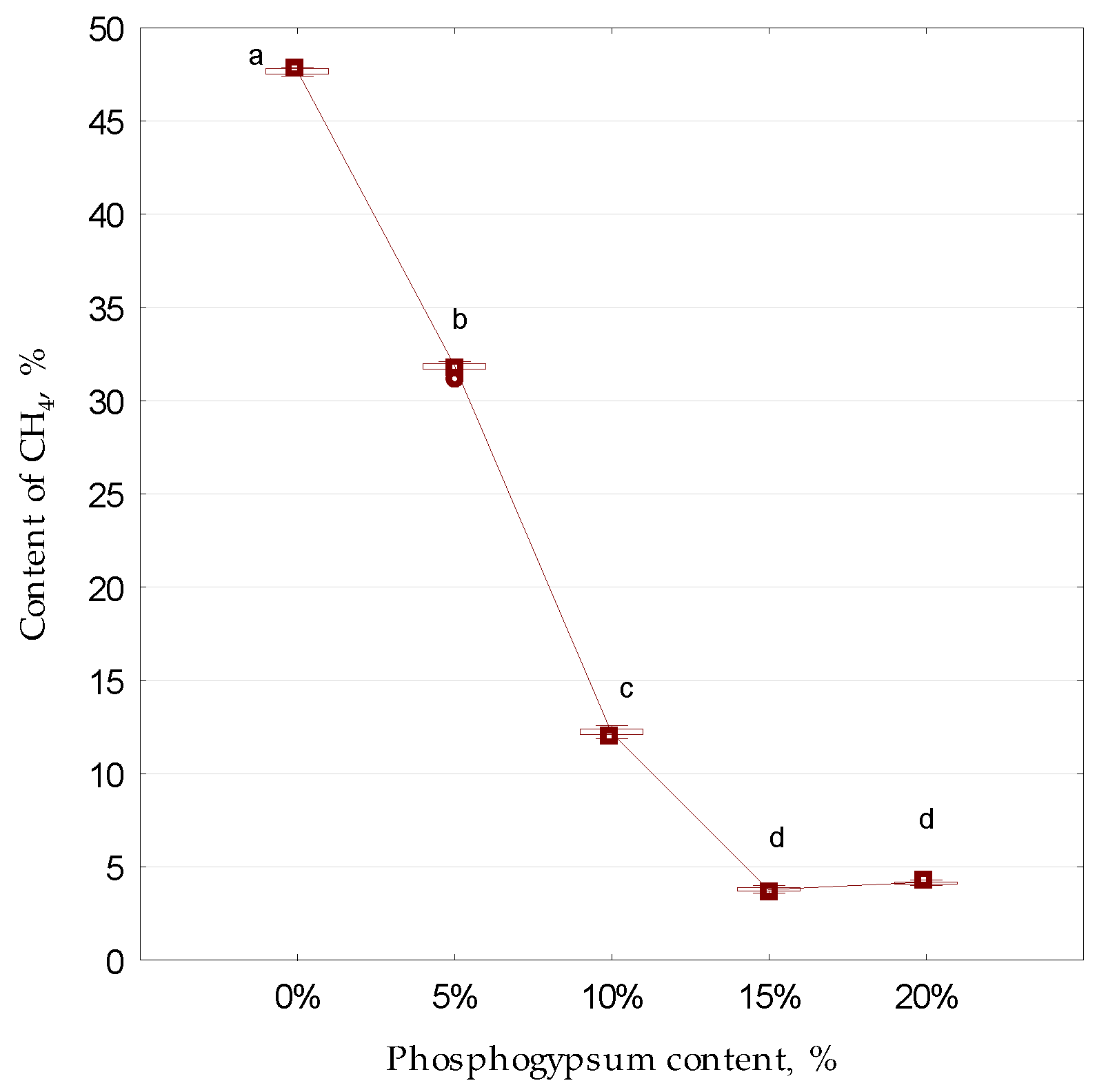
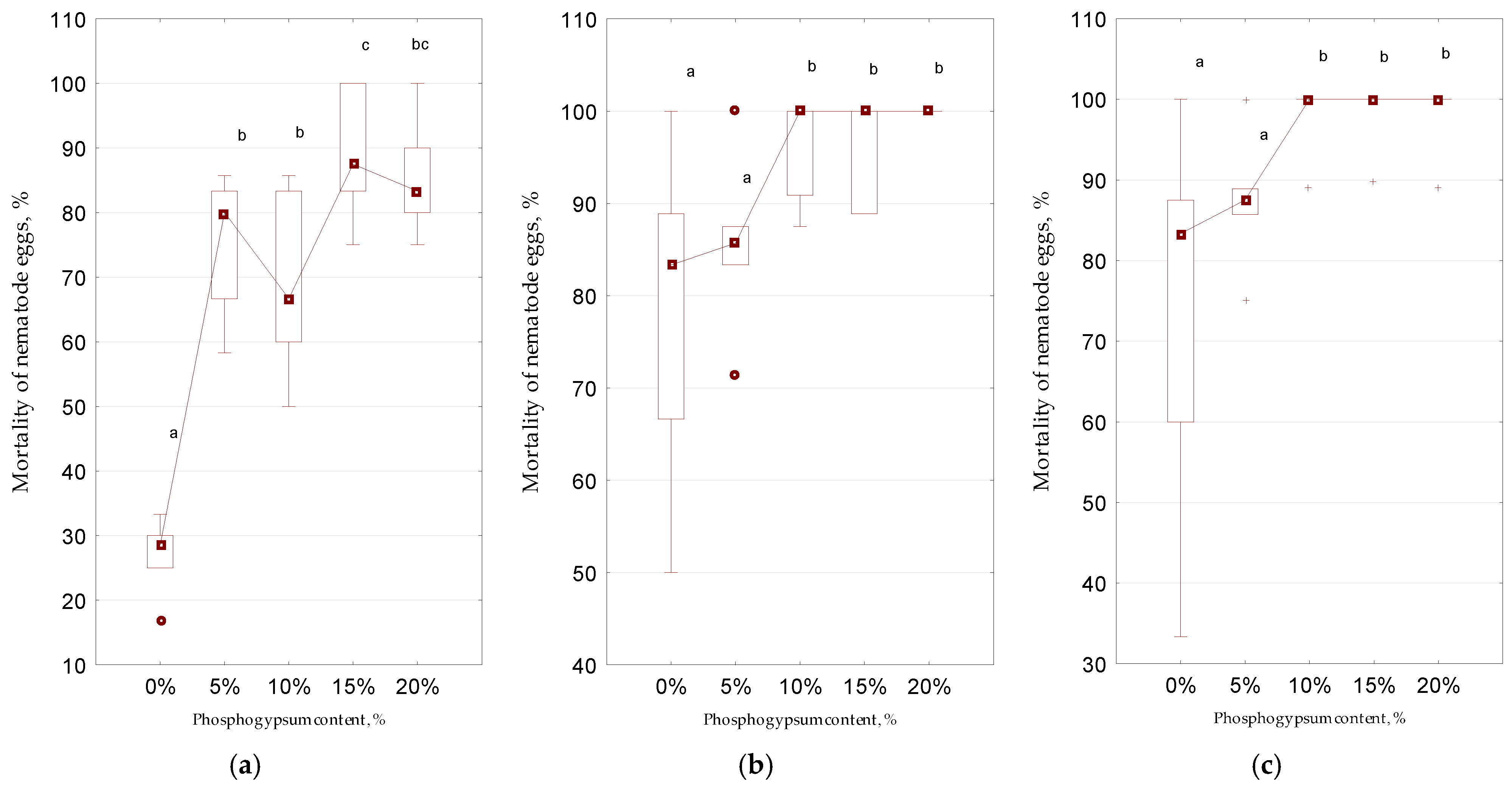

| Element | CaO | SO2 | SiO2 | Al2O3 | P2O5 | Fe2O3 | K2O | TiO2 | Na2O | MnO | MgO |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Concentrations, wt.% | 22.9–31.4 | 29.8–36.0 | 13.1–24.7 | 0.96–2.52 | 0.63–0.79 | 0.41–0.94 | 0.10–0.32 | 0.05–0.17 | 0.02–0.07 | 0.01 | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boyko, O.; Brygadyrenko, V.; Chernysh, Y.; Chubur, V.; Roubík, H. Impact of Phosphogypsum on Viability of Trichuris suis Eggs in Anaerobic Digestion of Swine Manure. Microorganisms 2025, 13, 1165. https://doi.org/10.3390/microorganisms13051165
Boyko O, Brygadyrenko V, Chernysh Y, Chubur V, Roubík H. Impact of Phosphogypsum on Viability of Trichuris suis Eggs in Anaerobic Digestion of Swine Manure. Microorganisms. 2025; 13(5):1165. https://doi.org/10.3390/microorganisms13051165
Chicago/Turabian StyleBoyko, Olexandra, Viktor Brygadyrenko, Yelizaveta Chernysh, Viktoriia Chubur, and Hynek Roubík. 2025. "Impact of Phosphogypsum on Viability of Trichuris suis Eggs in Anaerobic Digestion of Swine Manure" Microorganisms 13, no. 5: 1165. https://doi.org/10.3390/microorganisms13051165
APA StyleBoyko, O., Brygadyrenko, V., Chernysh, Y., Chubur, V., & Roubík, H. (2025). Impact of Phosphogypsum on Viability of Trichuris suis Eggs in Anaerobic Digestion of Swine Manure. Microorganisms, 13(5), 1165. https://doi.org/10.3390/microorganisms13051165










