Quantification and Comparison of Different Biofilm Components from Methicillin-Susceptible Staphylococcus aureus Treated with Tranexamic Acid Using an In Vitro Model
Abstract
1. Introduction
2. Method
Data Analysis
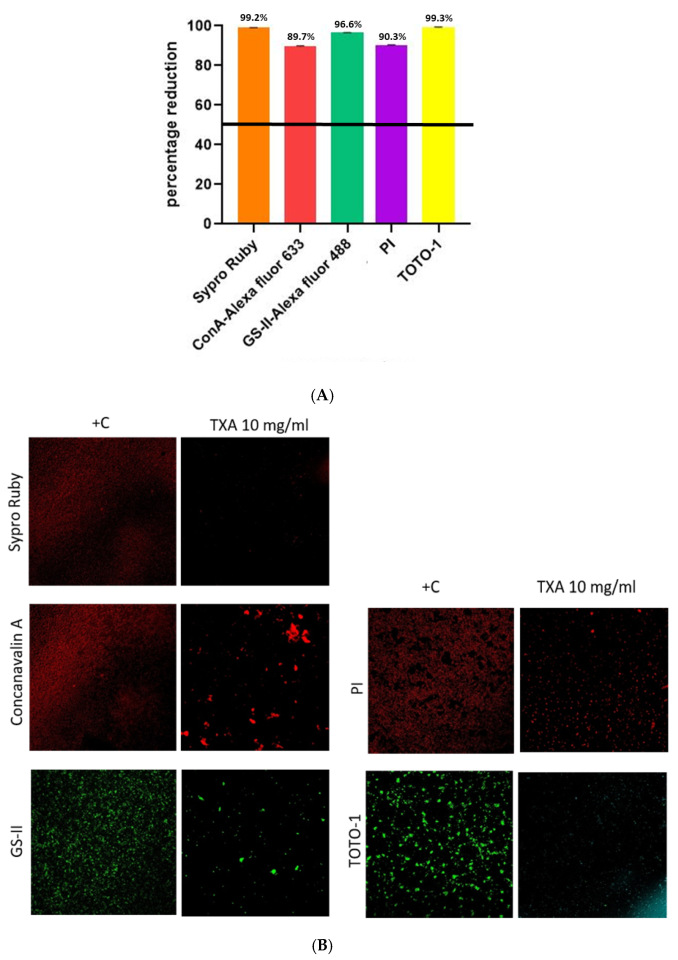
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shoji, M.M.; Chen, A.F. Biofilms in Periprosthetic Joint Infections: A Review of Diagnostic Modalities, Current Treatments, and Future Directions. J. Knee Surg. 2020, 33, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, G.; Ray, A.K. Quorum-sensing network-associated gene regulation in Gram-positive bacteria. Acta Microbiol. Et Immunol. Hung. 2017, 64, 439–453. [Google Scholar] [CrossRef] [PubMed]
- Di Domenico, E.G.; Oliva, A. The Current Knowledge on the Pathogenesis of Tissue and Medical Device-Related Biofilm Infections. Microorganisms 2022, 10, 1259. [Google Scholar] [CrossRef] [PubMed]
- Beam, E.; Osmon, D. Prosthetic Joint Infection Update. Infect. Dis. Clin. N. Am. 2018, 32, 843–859. [Google Scholar] [CrossRef]
- Jennings, J.D.; Solarz, M.K.; Haydel, C. Application of Tranexamic Acid in Trauma and Orthopedic Surgery. Orthop. Clin. N. Am. 2016, 47, 137–143. [Google Scholar] [CrossRef]
- Klement, M.R.; Padua, F.G.; Li, W.T.; Detweiler, M.; Parvizi, J. Tranexamic Acid Reduces the Rate of Periprosthetic Joint Infection After Aseptic Revision Arthroplasty. J. Bone Jt. Surg. 2020, 102, 1344–1350. [Google Scholar] [CrossRef]
- Yazdi, H.; Klement, M.R.; Hammad, M.; Inoue, D.; Xu, C.; Goswami, K.; Parvizi, J. Tranexamic Acid Is Associated with Reduced Periprosthetic Joint Infection After Primary Total Joint Arthroplasty. J. Arthroplast. 2020, 35, 840–844. [Google Scholar] [CrossRef]
- Imanishi, K.; Kobayashi, N.; Kamono, E.; Yukizawa, Y.; Takagawa, S.; Choe, H.; Kumagai, K.; Inaba, Y. Tranexamic acid administration for the prevention of periprosthetic joint infection and surgical site infection: A systematic review and meta-analysis. Arch. Orthop. Trauma Surg. 2023, 143, 6883–6899. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Z.; Li, J.; Huang, B.; Jiang, Z.; Pan, Y.; He, T.; Hu, Y.; Wang, L. Tranexamic acid protects against implant-associated infection by reducing biofilm formation. Sci. Rep. 2022, 12, 4840. [Google Scholar] [CrossRef]
- Zhang, F.; Dong, W.; Wang, F.; Yu, J.; Jiang, F.; Tang, J.; Qian, Y.; Shen, H. The Topical Tranexamic Acid Have Potential Hazard of Promoting Biofilm Formation of Staphylococcus aureus in Microenviroment of the Prosthetic Joint. Biomed Res. Inter. (Hindawi) 2021, 1, 5748069. [Google Scholar]
- Benjumea, A.; Díaz-Navarro, M.; Hafian, R.; Cercenado, E.; Sánchez-Somolinos, M.; Vaquero, J.; Chana, F.; Muñoz, P.; Guembe, M. Tranexamic Acid in Combination With Vancomycin or Gentamicin Has a Synergistic Effect Against Staphylococci. Front. Microbiol. 2022, 13, 935646. [Google Scholar] [CrossRef]
- Benjumea, A.; Díaz-Navarro, M.; Hafian, R.; Sánchez-Somolinos, M.; Vaquero, J.; Chana, F.; Muñoz, P.; Guembe, M. Effect of Tranexamic Acid against Staphylococcus spp. and Cutibacterium acnes Associated with Peri-Implant Infection: Results from an In Vitro Study. Microbiol. Spectr. 2022, 10, e0161221. [Google Scholar] [CrossRef] [PubMed]
- Benjumea, A.; Díaz-Navarro, M.; Gago-Campos, Á.S.; Visedo, A.; Hafian, R.; Cercenado, E.; Sánchez-Somolinos, M.; Muñoz, P.; Vaquero, J.; Chana, F.; et al. Validation of the antimicrobial effect of topically applied tranexamic acid using in vitro and in vivo models. Front. Microbiol. 2024, 15, 1367884, in the editorial office. [Google Scholar] [CrossRef] [PubMed]
- Mountcastle, S.E.; Vyas, N. Biofilm viability checker: An open-source tool for automated biofilm viability analysis from confocal microscopy images. Npj Biofilms Microbiomes 2021, 7, 44. [Google Scholar] [CrossRef] [PubMed]
- Lamret, F.; Lemaire, A.; Lagoutte, M.; Varin-Simon, J.; Abraham, L.; Colin, M.; Brauxet, M.; Velard, F.; Gangloff, S.C.; Reffuveille, F. Approaching prosthesis infection environment: Development of an innovative in vitro Staphylococcus aureus biofilm model. Biofilm 2023, 5, 100120. [Google Scholar] [CrossRef]
- Drago, L.; Agrappi, S.; Bortolin, M.; Toscano, M.; Romanò, C.L.; De Vecchi, E. How to Study Biofilms after Microbial Colonization of Materials Used in Orthopaedic Implants. Int. J. Mol. Sci. 2016, 17, 293. [Google Scholar] [CrossRef] [PubMed]
- Ravaioli, S.; Campoccia, D. Various biofilm matrices of the emerging pathogen Staphylococcus lugdunensis: Exopolysaccharides, proteins, eDNA and their correlation with biofilm mass. Biofouling 2020, 36, 86–100. [Google Scholar] [CrossRef]
- Márquez-Gómez, M.; Díaz-Navarro, M.; Visedo, A.; Hafian, R.; Matas, J.; Muñoz, P. An In Vitro Study to Assess the Best Strategy for the Chemical Debridement of Periprosthetic Joint Infection. Antibiotics 2023, 12, 1507. [Google Scholar] [CrossRef]
- Iorio, R.; Yu, S.; Anoushiravani, A.A.; Riesgo, A.M.; Park, B.; Vigdorchik, J.; Slover, J.; Long, W.J.; Schwarzkopf, R. Vancomycin Powder and Dilute Povidone-Iodine Lavage for Infection Prophylaxis in High-Risk Total Joint Arthroplasty. J. Arthroplast. 2020, 35, 1933–1936. [Google Scholar] [CrossRef]
- Coenye, T.; Nelis, H.J. In vitro and in vivo model systems to study microbial biofilm formation. J. Microbiol. Methods 2010, 83, 89–105. [Google Scholar] [CrossRef]
- Vandecandelaere, I.; Van Acker, H.; Coenye, T.A. Microplate-Based System as In Vitro Model of Biofilm Growth and Quantification. Methods Mol. Biol. 2016, 1333, 53–66. [Google Scholar]
- Maira-Litrán, T.; Bentancor, L.V.; Bozkurt-Guzel, C.; O’Malley, J.M.; Cywes-Bentley, C.; Pier, G.B. Synthesis and evaluation of a conjugate vaccine composed of Staphylococcus aureus poly-N-acetyl-glucosamine and clumping factor A. PLoS ONE 2012, 7, e43813. [Google Scholar] [CrossRef]
- Foulston, L.; Elsholz, A.K.; DeFrancesco, A.S.; Losick, R. The extracellular matrix of Staphylococcus aureus biofilms comprises cytoplasmic proteins that associate with the cell surface in response to decreasing pH. mBio 2014, 5, e01667-14. [Google Scholar] [CrossRef] [PubMed]
- Schlafer, S.; Meyer, R.L. Confocal microscopy imaging of the biofilm matrix. J. Microbiol. Methods 2017, 138, 50–59. [Google Scholar] [CrossRef]
| Component | Dye | Mean ± SD Occupied Area (%) | p | |
|---|---|---|---|---|
| 10 mg/mL TXA | +C | |||
| Extracellular proteins | Sypro Ruby | 0.15 ± 0.01 | 17.58 ± 1.22 | <0.001 |
| α-extracellular polysaccharides | ConA-Alexa fluor 633 | 1.69 ± 0.69 | 16.34 ± 4.71 | <0.001 |
| α-β-N-acetil glucosamine | GS-II-Alexa fluor 488 | 0.57 ± 0.28 | 16.77 ± 1.36 | <0.001 |
| Bacterial DNA | PI | 1.60 ± 0.81 | 16.55 ± 13.42 | <0.001 |
| eDNA | TOTO-1 | 0.07 ± 0.02 | 12.43 ± 6.23 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz-Navarro, M.; Benjumea, A.; Visedo, A.; Muñoz, P.; Vaquero, J.; Chana, F.; Guembe, M. Quantification and Comparison of Different Biofilm Components from Methicillin-Susceptible Staphylococcus aureus Treated with Tranexamic Acid Using an In Vitro Model. Microorganisms 2025, 13, 1874. https://doi.org/10.3390/microorganisms13081874
Díaz-Navarro M, Benjumea A, Visedo A, Muñoz P, Vaquero J, Chana F, Guembe M. Quantification and Comparison of Different Biofilm Components from Methicillin-Susceptible Staphylococcus aureus Treated with Tranexamic Acid Using an In Vitro Model. Microorganisms. 2025; 13(8):1874. https://doi.org/10.3390/microorganisms13081874
Chicago/Turabian StyleDíaz-Navarro, Marta, Antonio Benjumea, Andrés Visedo, Patricia Muñoz, Javier Vaquero, Francisco Chana, and María Guembe. 2025. "Quantification and Comparison of Different Biofilm Components from Methicillin-Susceptible Staphylococcus aureus Treated with Tranexamic Acid Using an In Vitro Model" Microorganisms 13, no. 8: 1874. https://doi.org/10.3390/microorganisms13081874
APA StyleDíaz-Navarro, M., Benjumea, A., Visedo, A., Muñoz, P., Vaquero, J., Chana, F., & Guembe, M. (2025). Quantification and Comparison of Different Biofilm Components from Methicillin-Susceptible Staphylococcus aureus Treated with Tranexamic Acid Using an In Vitro Model. Microorganisms, 13(8), 1874. https://doi.org/10.3390/microorganisms13081874






