BST-2 Promotes N Protein Degradation and Inhibits Viral Replication Through the MARCHF8/NDP52 Autophagy Pathway
Abstract
1. Introduction
2. Methods Summary
2.1. Cell Culture and Viruses
2.2. Reagents and Primers
2.3. Western Blot Analysis
2.4. RNA Extraction and qPCR
2.5. Confocal Immunofluorescence
2.6. Statistical Analysis
3. Results
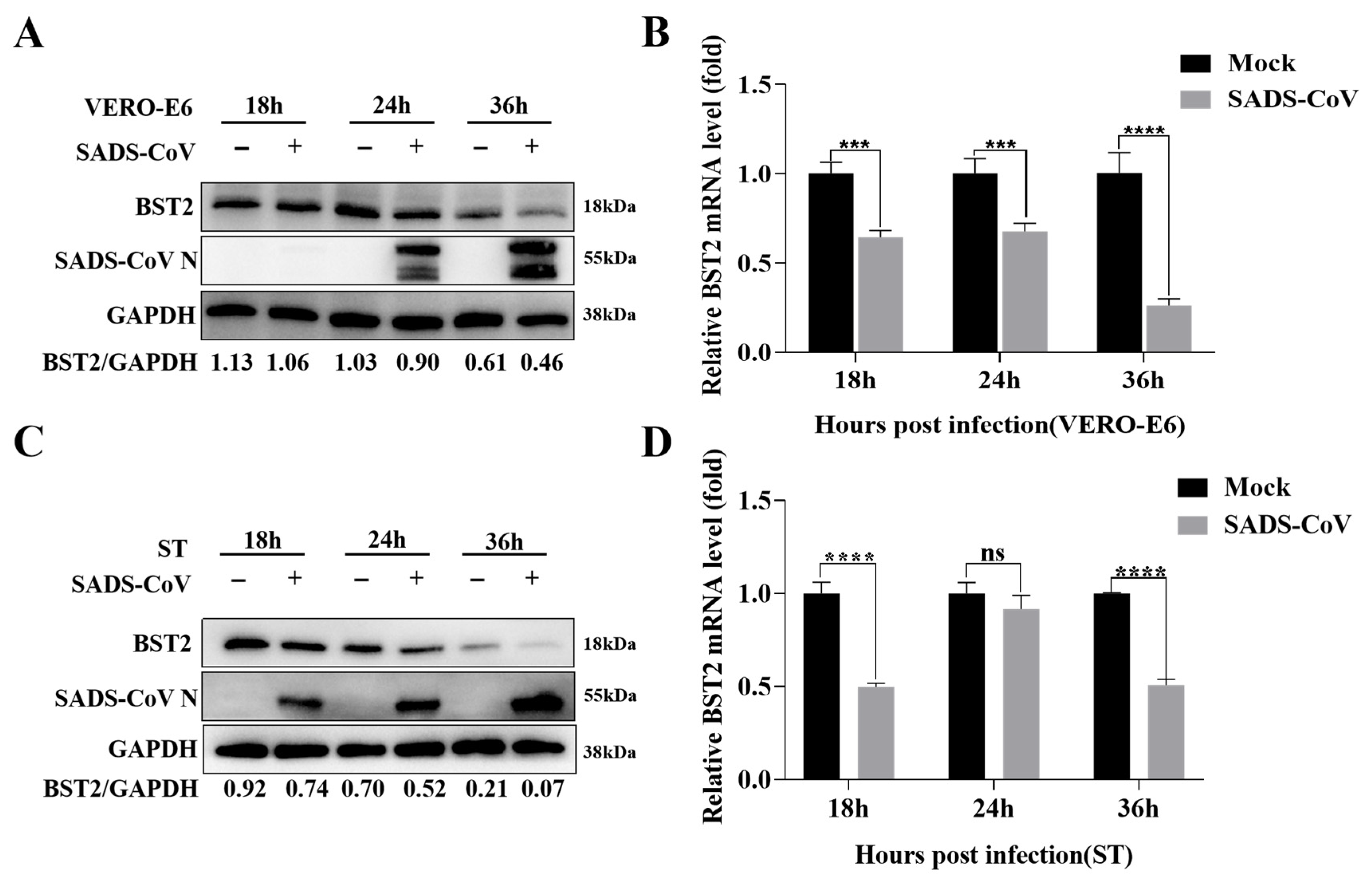
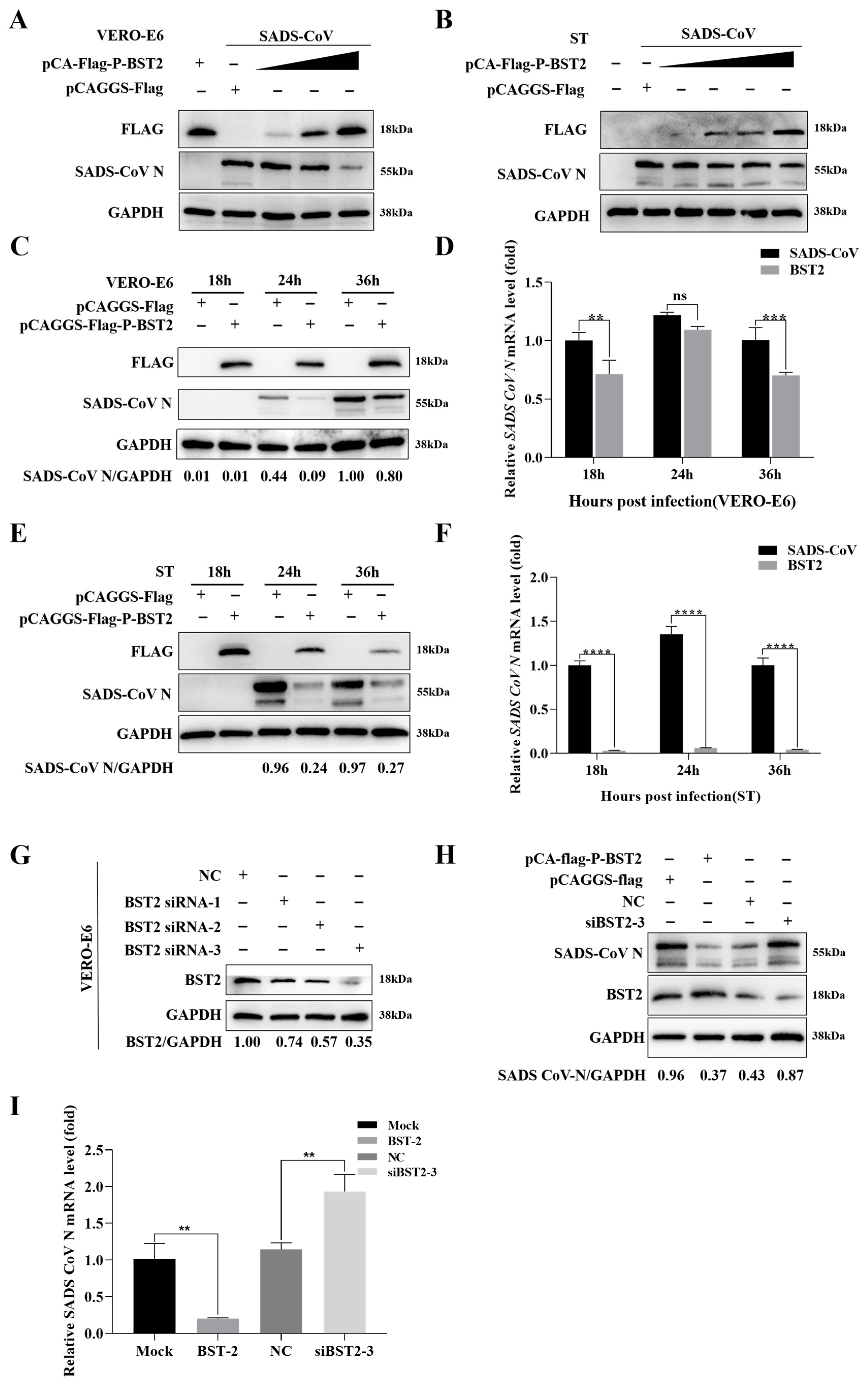
3.1. BST-2 Inhibits SADS-CoV Replication
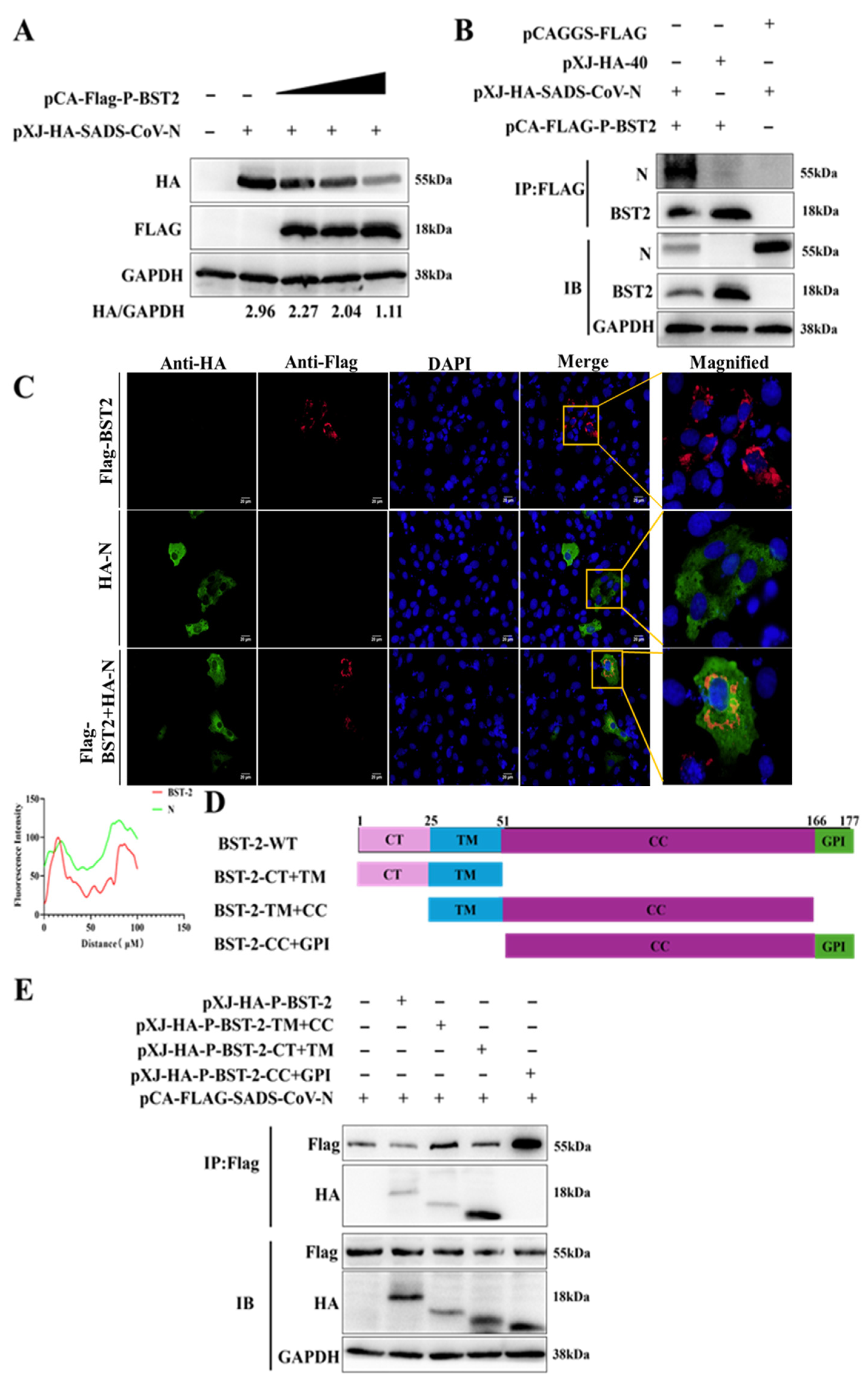
3.2. The BST-2 TM Domain Is Essential for the Inhibition of the SADS-CoV N Protein
3.3. BST-2 Facilitates Degradation of the SADS-CoV N Protein Through Activation of the BST-2/MARCH8/NDP52-Mediated Autophagosome Pathway
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, C.; Huang, W.; He, X.; Feng, Z.; Chen, Q. Research Advances on Swine Acute Diarrhea Syndrome Coronavirus. Animals 2024, 14, 448. [Google Scholar] [CrossRef]
- Cui, J.; Li, F.; Shi, Z.-L. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 2019, 17, 181–192. [Google Scholar] [CrossRef]
- Woo, P.C.; Lau, S.K.; Huang, Y.; Yuen, K.Y. Coronavirus diversity, phylogeny and interspecies jumping. Exp. Biol. Med. 2009, 234, 1117–1127. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.H.; Park, J.E. Swine Acute Diarrhea Syndrome Coronavirus: An Overview of Virus Structure and Virus-Host Interactions. Animals 2025, 15, 149. [Google Scholar] [CrossRef] [PubMed]
- McBride, R.; Van Zyl, M.; Fielding, B.C. The coronavirus nucleocapsid is a multifunctional protein. Viruses 2014, 6, 2991–3018. [Google Scholar] [CrossRef]
- Zhang, K.; Lin, S.; Li, J.; Deng, S.; Zhang, J.; Wang, S. Modulation of Innate Antiviral Immune Response by Porcine Enteric Coronavirus. Front. Microbiol. 2022, 13, 845137. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liang, Q.-Z.; Lu, W.; Yang, Y.-L.; Chen, R.; Huang, Y.-W.; Wang, B. A Comparative Analysis of Coronavirus Nucleocapsid (N) Proteins Reveals the SADS-CoV N Protein Antagonizes IFN-β Production by Inducing Ubiquitination of RIG-I. Front. Immunol. 2021, 12, 688758. [Google Scholar] [CrossRef]
- Tiwari, R.; de la Torre, J.C.; McGavern, D.B.; Nayak, D. Beyond Tethering the Viral Particles: Immunomodulatory Functions of Tetherin (BST-2). DNA Cell Biol. 2019, 38, 1170–1177. [Google Scholar] [CrossRef] [PubMed]
- Mahauad-Fernandez, W.D.; Okeoma, C.M. The role of BST-2/Tetherin in host protection and disease manifestation. Immun. Inflamm. Dis. 2016, 4, 4–23. [Google Scholar] [CrossRef]
- Le Tortorec, A.; Neil, S.J. Antagonism to and intracellular sequestration of human tetherin by the human immunodeficiency virus type 2 envelope glycoprotein. J. Virol. 2009, 83, 11966–11978. [Google Scholar] [CrossRef]
- Jouvenet, N.; Neil, S.J.D.; Zhadina, M.; Zang, T.; Kratovac, Z.; Lee, Y.; McNatt, M.; Hatziioannou, T.; Bieniasz, P.D. Broad-spectrum inhibition of retroviral and filoviral particle release by tetherin. J. Virol. 2009, 83, 1837–1844. [Google Scholar] [CrossRef]
- Yan, R.; Zhao, X.; Cai, D.; Liu, Y.; Block, T.M.; Guo, J.-T.; Guo, H. The Interferon-Inducible Protein Tetherin Inhibits Hepatitis B Virus Virion Secretion. J. Virol. 2015, 89, 9200–9212. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.H.; Maric, M.; Madison, M.N.; Maury, W.; Roller, R.J.; Okeoma, C.M. BST-2/tetherin-mediated restriction of chikungunya (CHIKV) VLP budding is counteracted by CHIKV non-structural protein 1 (nsP1). Virology 2013, 438, 37–49. [Google Scholar] [CrossRef]
- Petrosino, M.; Stellato, F.; Chiaraluce, R.; Consalvi, V.; La Penna, G.; Pasquo, A.; Proux, O.; Rossi, G.; Morante, S. Zn-Induced Interactions Between SARS-CoV-2 orf7a and BST2/Tetherin. ChemistryOpen 2021, 10, 1133–1141. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, K.; Wang, S.; Du, J. Multi-functional BST2/tetherin against HIV-1, other viruses and LINE-1. Front. Cell. Infect. Microbiol. 2022, 12, 979091. [Google Scholar] [CrossRef]
- Khan, N.; Geiger, J.D. Role of Viral Protein U (Vpu) in HIV-1 Infection and Pathogenesis. Viruses 2021, 13, 1466. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Tian, S.; Luo, M.; Xie, W.; Liu, T.; Duan, T.; Wu, Y.; Cui, J. Tetherin Suppresses Type I Interferon Signaling by Targeting MAVS for NDP52-Mediated Selective Autophagic Degradation in Human Cells. Mol. Cell 2017, 68, 308–322. [Google Scholar] [CrossRef] [PubMed]
- Kong, N.; Shan, T.; Wang, H.; Jiao, Y.; Zuo, Y.; Li, L.; Tong, W.; Yu, L.; Jiang, Y.; Zhou, Y.; et al. BST2 suppresses porcine epidemic diarrhea virus replication by targeting and degrading virus nucleocapsid protein with selective autophagy. Autophagy 2020, 16, 1737–1752. [Google Scholar] [CrossRef]
- Kirkin, V. History of the Selective Autophagy Research: How Did It Begin and Where Does It Stand Today? J. Mol. Biol. 2020, 432, 3–27. [Google Scholar] [CrossRef] [PubMed]
- Kirkin, V.; Rogov, V.V. A Diversity of Selective Autophagy Receptors Determines the Specificity of the Autophagy Pathway. Mol. Cell 2019, 76, 268–285. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, T.; Hu, J.; Liu, Y.; Jin, S.; Wu, J.; Guan, X.; Cui, J. Autophagy Activation Induces p62-Dependent Autophagic Degradation of Dengue Virus Capsid Protein During Infection. Front. Microbiol. 2022, 13, 889693. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Kong, N.; Zhang, Y.; Song, Y.; Qin, W.; Yang, X.; Ye, C.; Ye, M.; Tong, W.; Liu, C.; et al. N protein of PEDV plays chess game with host proteins by selective autophagy. Autophagy 2023, 19, 2338–2352. [Google Scholar] [CrossRef] [PubMed]
- Waisner, H.; Kalamvoki, M. The ICP0 Protein of Herpes Simplex Virus 1 (HSV-1) Downregulates Major Autophagy Adaptor Proteins Sequestosome 1 and Optineurin during the Early Stages of HSV-1 Infection. J. Virol. 2019, 93, 10-1128. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Luo, X.; Ren, M. Clearance or Hijack: Universal Interplay Mechanisms Between Viruses and Host Autophagy From Plants to Animals. Front. Cell. Infect. Microbiol. 2022, 11, 786348. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Zhou, L.; Shi, H.; Zhang, J.; Zhang, J.; Zhang, L.; Liu, D.; Feng, T.; Zeng, M.; Chen, J.; et al. Autophagy is induced by swine acute diarrhea syndrome coronavirus through the cellular IRE1-JNK-Beclin 1 signaling pathway after an interaction of viral membrane-associated papain-like protease and GRP78. PLOS Pathog. 2023, 19, e1011201. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Zhao, Y.; Peng, O.; Xia, Y.; Xu, Q.; Li, H.; Xue, C.; Cao, Y.; Zhang, H. Swine acute diarrhea syndrome coronavirus induces autophagy to promote its replication via the Akt/mTOR pathway. iScience 2022, 25, 105394. [Google Scholar] [CrossRef]
- Zhao, C.; Qin, Y.; Huang, H.; Chen, W.; Hu, Y.; Zhang, X.; Li, Y.; Lan, T.; Sun, W. PABPC4 Inhibits SADS-CoV Replication by Degrading the Nucleocapsid Protein Through Selective Autophagy. Vet. Sci. 2025, 12, 257. [Google Scholar] [CrossRef]


| Antibody or Chemical | Manufacturer | Country |
|---|---|---|
| anti-BST-2 | Proteintech | Wuhan, China |
| anti-GAPDH | Proteintech | Wuhan, China |
| anti-MARCHF8 | Proteintech | Wuhan, China |
| anti-NDP52 | Proteintech | Wuhan, China |
| anti-HA | Bioss | Beijing, China |
| anti-Flag | Bioss | Beijing, China |
| Chloroquine | Aladdin | Shanghai, China |
| MG132 | Aladdin | Shanghai, China |
| 3-Methyladenine | Aladdin | Shanghai, China |
| Name | Sequence (5′–3′) |
|---|---|
| pCAGGS-Flag-P-BST2 sense | gatgacgacgataaggaattcATGTCACCTAGTTTGT ATTCCTACAGC |
| pCAGGS-Flag-P-BST2 antisense | attaagatctgctagctcgagTCAGGTCAGCAGGGCA TTGA |
| si-BST2-1 sense | CCAUGGAUGACACUUGCAA |
| si-BST2-1 antisense | UUGCAAGUGUCAUCCAUGG |
| si-BST2-2 sense | GGGAGAUCACUACAUUGAA |
| si-BST2-2 antisense | UUCAAUGUAGUGAUCUCCC |
| si-BST2-3 sense | GGAGCGACUGAGAAGAGAA |
| si-BST2-3 antisense | UUCUCUUCUCAGUCGCUCC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, C.; Qin, Y.; Huang, H.; Li, Y.; Zhang, X.; Zhou, L.; Xie, L.; Zhou, Y.; Hu, Y.; Chen, W.; et al. BST-2 Promotes N Protein Degradation and Inhibits Viral Replication Through the MARCHF8/NDP52 Autophagy Pathway. Microorganisms 2025, 13, 1865. https://doi.org/10.3390/microorganisms13081865
Zhao C, Qin Y, Huang H, Li Y, Zhang X, Zhou L, Xie L, Zhou Y, Hu Y, Chen W, et al. BST-2 Promotes N Protein Degradation and Inhibits Viral Replication Through the MARCHF8/NDP52 Autophagy Pathway. Microorganisms. 2025; 13(8):1865. https://doi.org/10.3390/microorganisms13081865
Chicago/Turabian StyleZhao, Chenchen, Yan Qin, Haixin Huang, Yuying Li, Xinyu Zhang, Lin Zhou, Lulu Xie, Yimin Zhou, Yanqing Hu, Wei Chen, and et al. 2025. "BST-2 Promotes N Protein Degradation and Inhibits Viral Replication Through the MARCHF8/NDP52 Autophagy Pathway" Microorganisms 13, no. 8: 1865. https://doi.org/10.3390/microorganisms13081865
APA StyleZhao, C., Qin, Y., Huang, H., Li, Y., Zhang, X., Zhou, L., Xie, L., Zhou, Y., Hu, Y., Chen, W., Lan, T., & Sun, W.-C. (2025). BST-2 Promotes N Protein Degradation and Inhibits Viral Replication Through the MARCHF8/NDP52 Autophagy Pathway. Microorganisms, 13(8), 1865. https://doi.org/10.3390/microorganisms13081865





