Response of Chaetomium sp. to Nitrogen Input and Its Potential Role in Rhizosphere Enrichment of Lycium barbarum
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description and Experimental Design
2.2. DNA Extraction, PCR Amplification, and Sequencing
2.3. Sequencing and Statistical Analyses
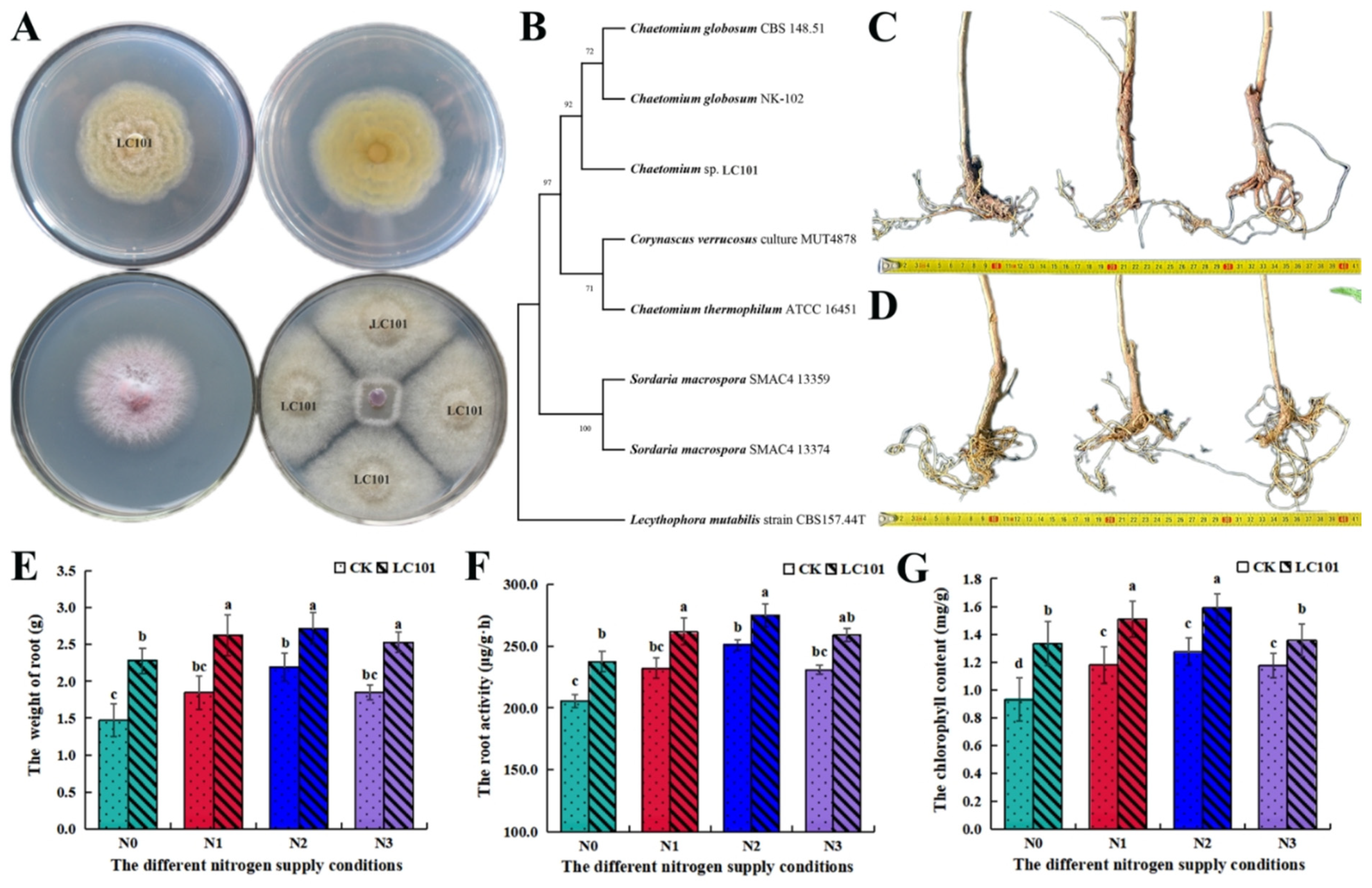
2.4. Isolation and Identification of Chaetomium sp. and Verification of Its Potential Function
2.5. Statistical Analysis
3. Results
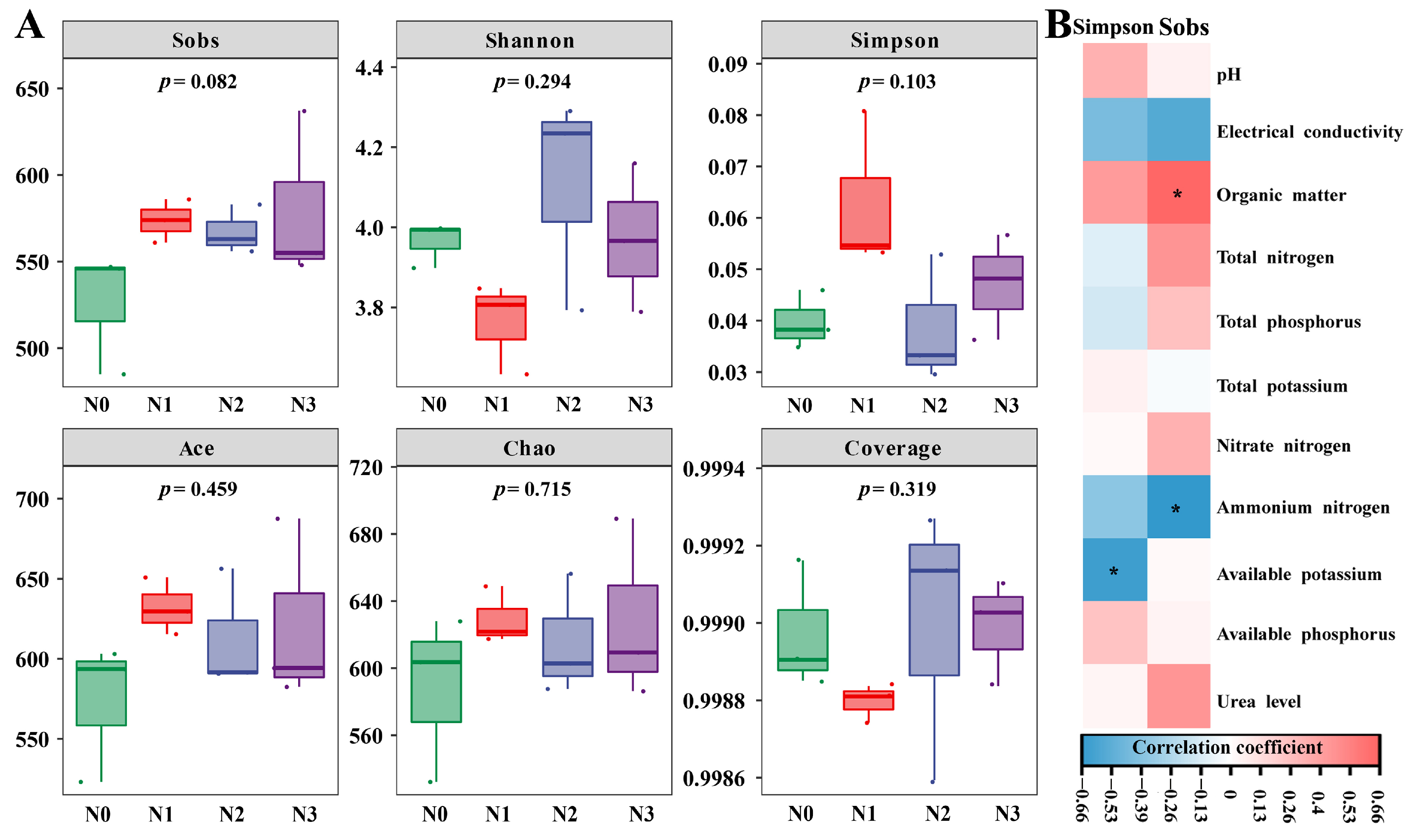
3.1. Effect of Nitrogen Supply Level on the Diversity of the Soil Fungal Community
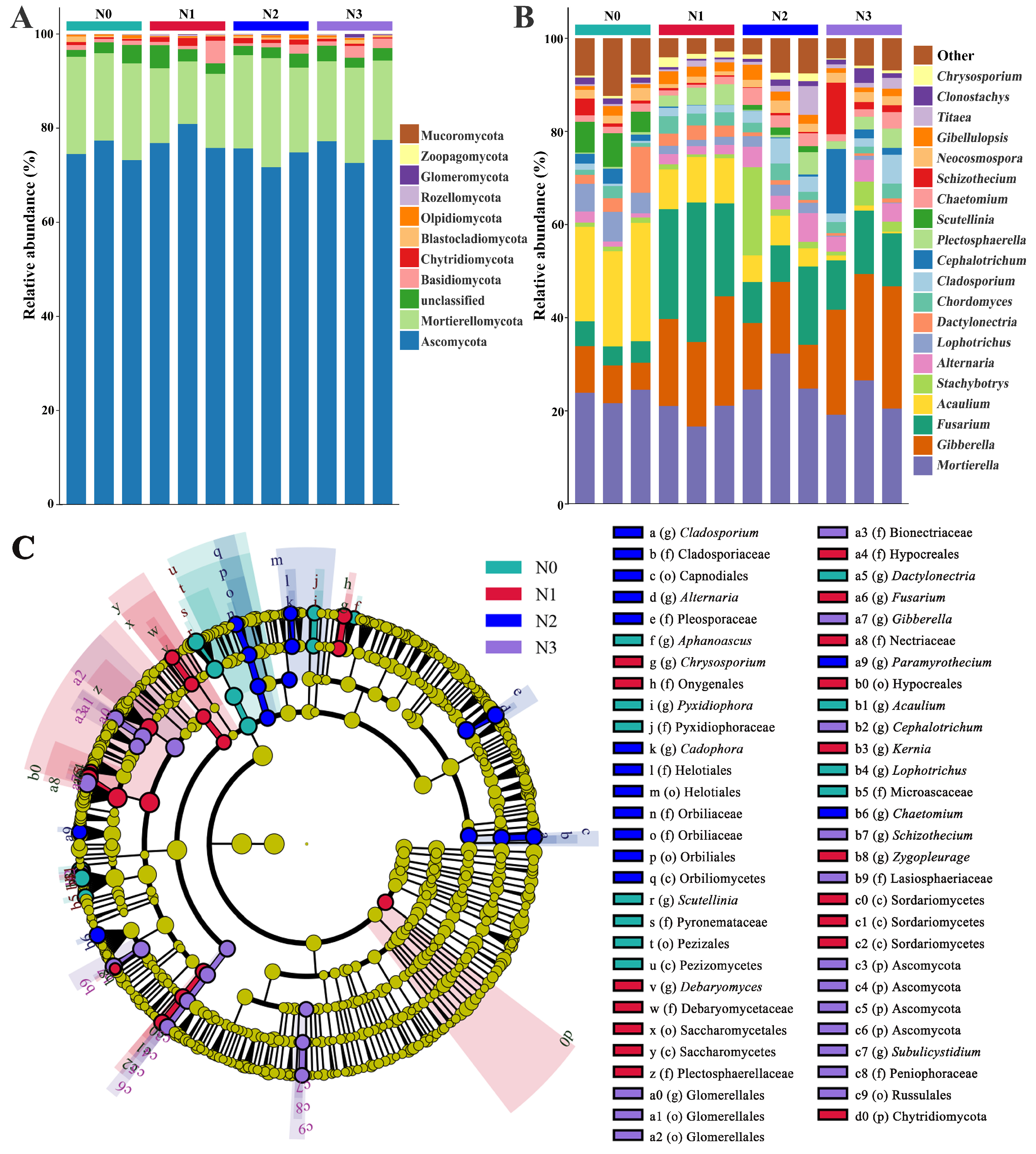
3.2. Effect of Nitrogen Supply Level on the Soil Fungal Community Composition
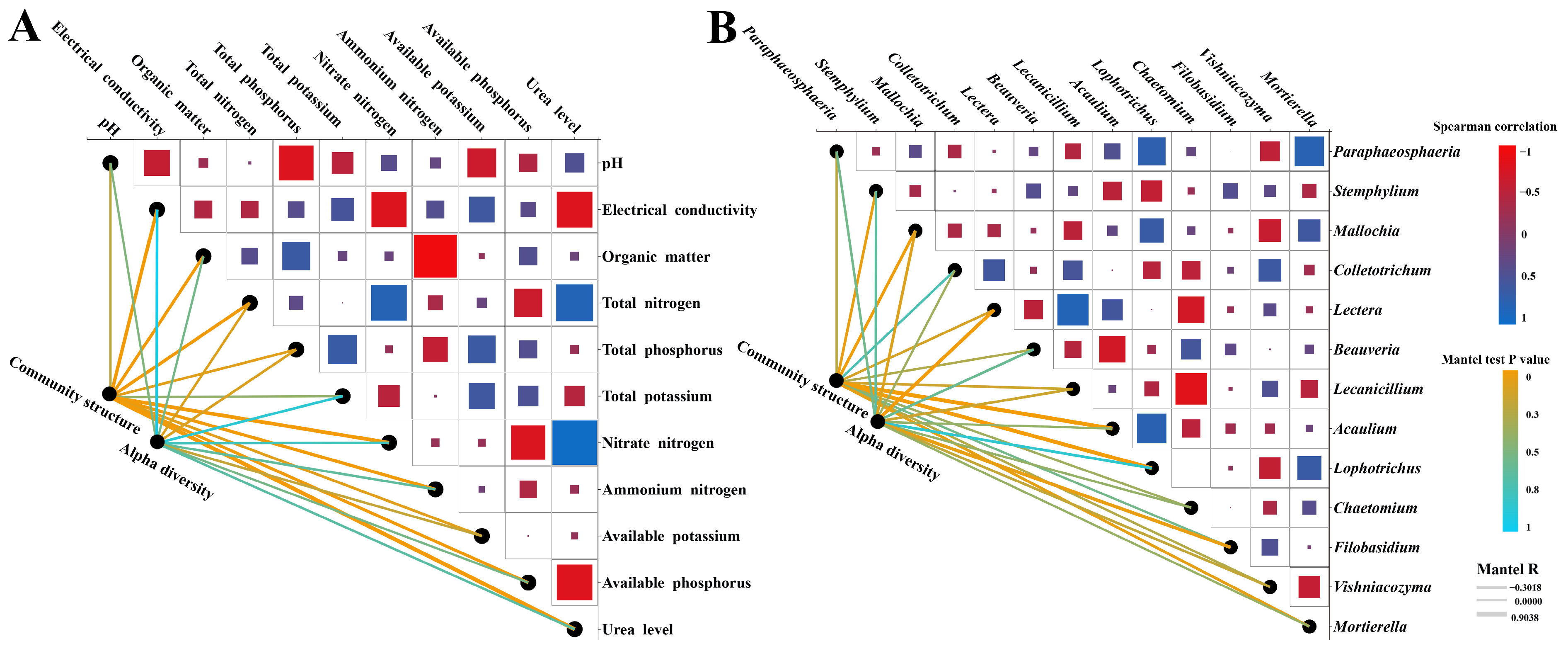
3.3. Correlations Between Soil Environmental Factors and Fungal Community Composition Under Different Nitrogen Application Levels
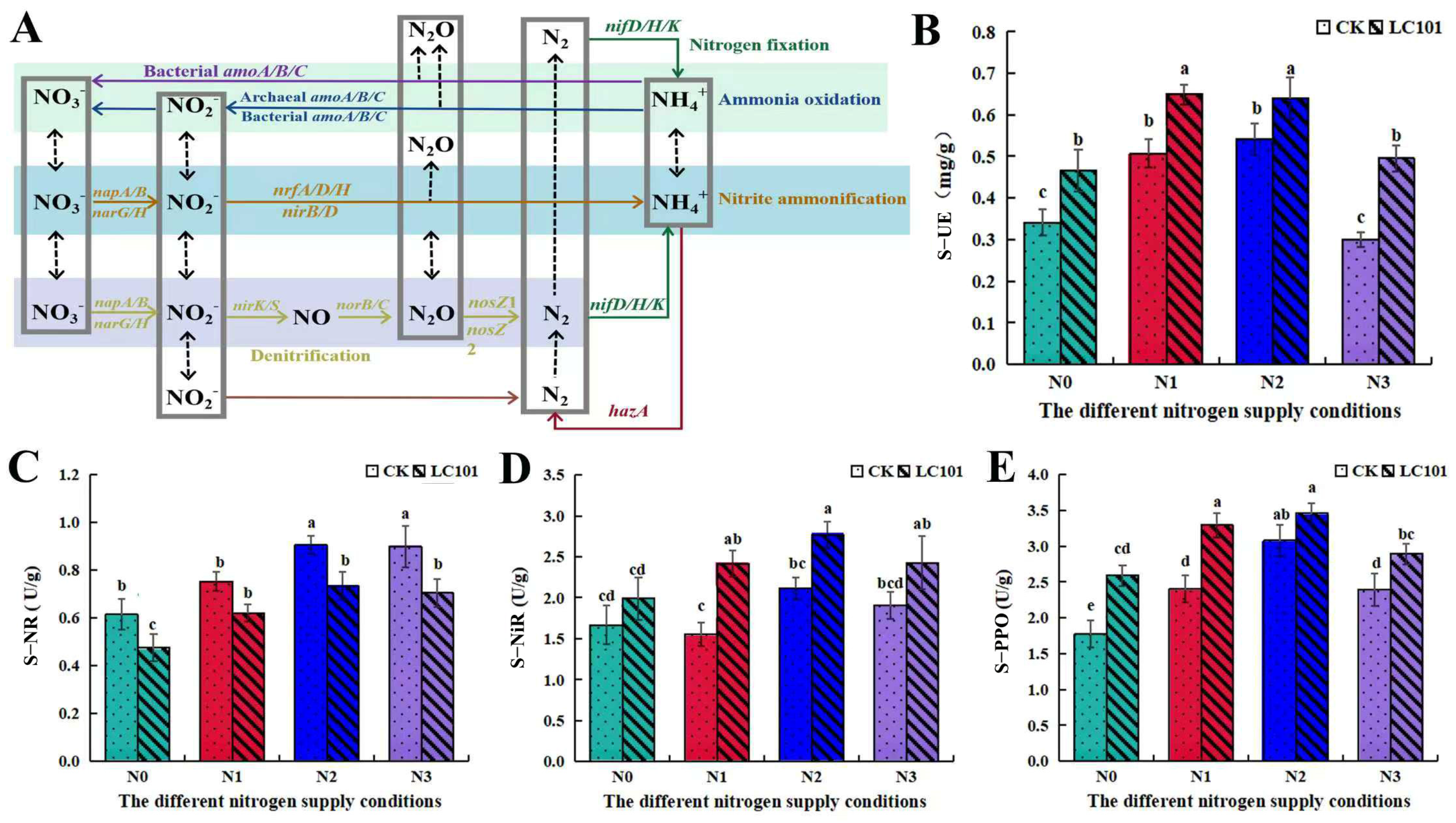
3.4. Isolation and Identification of Chaetomium sp. and Verification of Its Potential Function
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Y.; Zou, N.; Liang, X.; Zhou, X.; Guo, S.; Wang, Y.; Qin, X.; Tian, Y.; Lin, J. Effects of nitrogen input on soil bacterial community structure and soil nitrogen cycling in the rhizosphere soil of Lycium barbarum L. Front. Microbiol. 2023, 13, 1070817. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, X.; Chen, K.; Shi, J.; Wang, Y.; Luo, P.; Yang, J.; Wang, Y.; Han, X. Long-term fertilization altered microbial community structure in an aeolian sandy soil in northeast China. Front. Microbiol. 2022, 13, 979759. [Google Scholar] [CrossRef]
- Yang, H.; Liu, X. Nitrogen addition disrupts fungal network stability in semi-arid grasslands. Sci. Bull. 2024, 69, 1845–1847. [Google Scholar]
- Wang, Q.; Ma, M.; Jiang, X.; Zhou, B.; Guan, D.; Cao, F.; Chen, S.; Li, J. Long-term N fertilization altered 13C-labeled fungal community composition but not diversity in wheat rhizosphere of Chinese black soil. Soil Biol. Biochem. 2019, 135, 117–126. [Google Scholar] [CrossRef]
- Han, Y.; Feng, J.; Han, M.; Zhu, B. Responses of arbuscular mycorrhizal fungi to nitrogen addition: A meta-analysis. Glob. Chang. Biol. 2020, 26, 7229–7241. [Google Scholar] [CrossRef]
- Ali, S.; Liu, K.; Ahmed, W.; Jing, H.; Qaswar, M.; Kofi Anthonio, C.; Maitlo, A.A.; Lu, Z.; Liu, L.; Zhang, H. Nitrogen Mineralization, Soil Microbial Biomass and Extracellular Enzyme Activities Regulated by Long-Term N Fertilizer Inputs: A Comparison Study from Upland and Paddy Soils in a Red Soil Region of China. Agronomy 2021, 11, 2057. [Google Scholar] [CrossRef]
- Peng, S.; Zhang, X.; Wu, R.; Cai, Y.; Xing, W.; Ge, Z.; Mao, L. Seasonal dynamic responses of soil arbuscular mycorrhizal fungal community to nitrogen additions in a poplar plantation. J. Zhejiang AF Univ. 2023, 40, 792–800. [Google Scholar]
- Lu, Z.; Li, H.; Sayer, E.J.; Liu, Z.; Li, L.; Chen, Y.; Qin, G.; Li, J.; Zhou, J.; Huang, X.; et al. Enhanced abundance of generalist and litter saprotrophs explain increased tropical forest soil carbon with long-term nitrogen deposition. Funct. Ecol. 2023, 37, 2282–2296. [Google Scholar] [CrossRef]
- Liu, Z.; Gu, H.; Liang, A.; Li, L.; Yao, Q.; Xu, Y.; Liu, J.; Jin, J.; Liu, X.; Wang, G. Conservation tillage regulates the assembly, network structure and ecological function of the soil bacterial community in black soils. Plant Soil 2022, 472, 207–223. [Google Scholar] [CrossRef]
- Zhou, J.; Jiang, X.; Zhou, B.; Zhao, B.; Ma, M.; Guan, D.; Li, J.; Chen, S.; Cao, F.; Shen, D.; et al. Thirty four years of nitrogen fertilization decreases fungal diversity and alters fungal community composition in black soil in northeast China. Soil Biol. Biochem. 2016, 95, 135–143. [Google Scholar] [CrossRef]
- Castle, S.C.; Samac, D.A.; Gutknecht, J.L.; Sadowsky, M.J.; Rosen, C.J.; Schlatter, D.; Kinkel, L.L. Impacts of cover crops and nitrogen fertilization on agricultural soil fungal and bacterial communities. Plant Soil 2021, 466, 139–150. [Google Scholar] [CrossRef]
- Bharadwaj, P.; Devi, C.J.; Thakur, D. Unlocking Rhizosphere Dynamics: Exploring Mechanisms of Plant–Microbe Interactions for Enhanced Tea (Camellia sinensis (L.) O. Kuntze) Productivity. Curr. Microbiol. 2025, 82, 257. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-Y.; Teng, W.-K.; Zhao, L.; Han, B.-P.; Song, L.-R.; Shu, W.-S. Phylogenomics Uncovers Evolutionary Trajectory of Nitrogen Fixation in Cyanobacteria. Mol. Biol. Evol. 2022, 39, 139–150. [Google Scholar] [CrossRef]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Stackebrandt, E.; Goebel, B.M. Taxonomic Note: A Place for DNA-DNA Reassociation and 16S rRNA Sequence Analysis in the Present Species Definition in Bacteriology. Int. J. Syst. Evol. Microbiol. 1994, 44, 846–849. [Google Scholar] [CrossRef]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-Source, Platform-Independent, Community-Supported Software for Describing and Comparing Microbial Communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef] [PubMed]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Kozich James, J.; Westcott Sarah, L.; Baxter Nielson, T.; Highlander Sarah, K.; Schloss Patrick, D. Development of a Dual-Index Sequencing Strategy and Curation Pipeline for Analyzing Amplicon Sequence Data on the MiSeq Illumina Sequencing Platform. Appl. Environ. Microbiol. 2013, 79, 5112–5120. [Google Scholar] [CrossRef]
- McMurdie, P.J.; Holmes, S. phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Wellburn, A.R. The Spectral Determination of Chlorophylls a and b, as well as Total Carotenoids, Using Various Solvents with Spectrophotometers of Different Resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Zhao, P.; Zhu, Y.; Wang, W. Evaluation and improvement of spectrophotometric assays of TTC reduction: Maize (Zea mays) embryo as an example. Acta Physiol. Plant. 2010, 32, 815–819. [Google Scholar] [CrossRef]
- Yang, H.; Cheng, L.; Che, L.; Su, Y.; Li, Y. Nutrients addition decreases soil fungal diversity and alters fungal guilds and co-occurrence networks in a semi-arid grassland in northern China. Sci. Total Environ. 2024, 926, 172100. [Google Scholar] [CrossRef]
- Dai, T.; Wen, D.; Bates, C.T.; Wu, L.; Guo, X.; Liu, S.; Su, Y.; Lei, J.; Zhou, J.; Yang, Y. Nutrient supply controls the linkage between species abundance and ecological interactions in marine bacterial communities. Nat. Commun. 2022, 13, 175. [Google Scholar] [CrossRef]
- Li, X.; Wang, A.; Wan, W.; Luo, X.; Zheng, L.; He, G.; Huang, D.; Chen, W.; Huang, Q. High Salinity Inhibits Soil Bacterial Community Mediating Nitrogen Cycling. Appl. Environ. Microbiol. 2021, 87, e01366-21. [Google Scholar] [CrossRef]
- Ramond, J.-B.; Jordaan, K.; Díez, B.; Heinzelmann Sandra, M.; Cowan Don, A. Microbial Biogeochemical Cycling of Nitrogen in Arid Ecosystems. Microbiol. Mol. Biol. Rev. 2022, 86, e00109–e00121. [Google Scholar] [CrossRef]
- Barak, P.; Jobe, B.O.; Krueger, A.R.; Peterson, L.A.; Laird, D.A. Effects of long-term soil acidification due to nitrogen fertilizer inputs in Wisconsin. Plant Soil 1997, 197, 61–69. [Google Scholar] [CrossRef]
- Bowman, W.D.; Cleveland, C.C.; Halada, Ĺ.; Hreško, J.; Baron, J.S. Negative impact of nitrogen deposition on soil buffering capacity. Nat. Geosci. 2008, 1, 767–770. [Google Scholar] [CrossRef]
- Kim, Y.-C.; Gao, C.; Zheng, Y.; He, X.-H.; Yang, W.; Chen, L.; Wan, S.-Q.; Guo, L.-D. Arbuscular mycorrhizal fungal community response to warming and nitrogen addition in a semiarid steppe ecosystem. Mycorrhiza 2015, 25, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Yao, R.; Yang, J.; Zhu, W.; Li, H.; Yin, C.; Jing, Y.; Wang, X.; Xie, W.; Zhang, X. Impact of crop cultivation, nitrogen and fulvic acid on soil fungal community structure in salt-affected alluvial fluvo-aquic soil. Plant Soil 2021, 464, 539–558. [Google Scholar] [CrossRef]
- Dong, H.; Fan, S.; Sun, H.; Chen, C.; Wang, A.; Jiang, L.; Ma, D. Rhizosphere-Associated Microbiomes of Rice (Oryza sativa L.) Under Eff. Increased Nitrogen Fertilization. Front. Microbiol. 2021, 12, 730506. [Google Scholar] [CrossRef]
- Gallo, M.; Amonette, R.; Lauber, C.; Sinsabaugh, R.L.; Zak, D.R. Microbial Community Structure and Oxidative Enzyme Activity in Nitrogen-amended North Temperate Forest Soils. Microb. Ecol. 2004, 48, 218–229. [Google Scholar] [CrossRef]
- Yang, X.; Ma, L.; Ji, L.; Shi, Y.; Yi, X.; Yang, Q.; Ni, K.; Ruan, J. Long-term nitrogen fertilization indirectly affects soil fungi community structure by changing soil and pruned litter in a subtropical tea (Camellia sinensis L.) plantation in China. Plant Soil 2019, 444, 409–426. [Google Scholar] [CrossRef]
- She, W.; Bai, Y.; Zhang, Y.; Qin, S.; Feng, W.; Sun, Y.; Zheng, J.; Wu, B. Resource Availability Drives Responses of Soil Microbial Communities to Short-term Precipitation and Nitrogen Addition in a Desert Shrubland. Front. Microbiol. 2018, 9, 186. [Google Scholar] [CrossRef] [PubMed]
- Jörgensen, K.; Clemmensen, K.E.; Wallander, H.; Lindahl, B.D. Ectomycorrhizal fungi are more sensitive to high soil nitrogen levels in forests exposed to nitrogen deposition. N. Phytol. 2024, 242, 1725–1738. [Google Scholar] [CrossRef] [PubMed]
- Tedersoo, L.; Bahram, M.; Põlme, S.; Kõljalg, U.; Yorou, N.S.; Wijesundera, R.; Ruiz, L.V.; Vasco-Palacios, A.M.; Thu, P.Q.; Suija, A.; et al. Global diversity and geography of soil fungi. Science 2014, 346, 1256688. [Google Scholar] [CrossRef]
- Rosling, A.; Roose, T.; Herrmann, A.M.; Davidson, F.A.; Finlay, R.D.; Gadd, G.M. Approaches to modelling mineral weathering by fungi. Fungal Biol. Rev. 2009, 23, 138–144. [Google Scholar] [CrossRef]
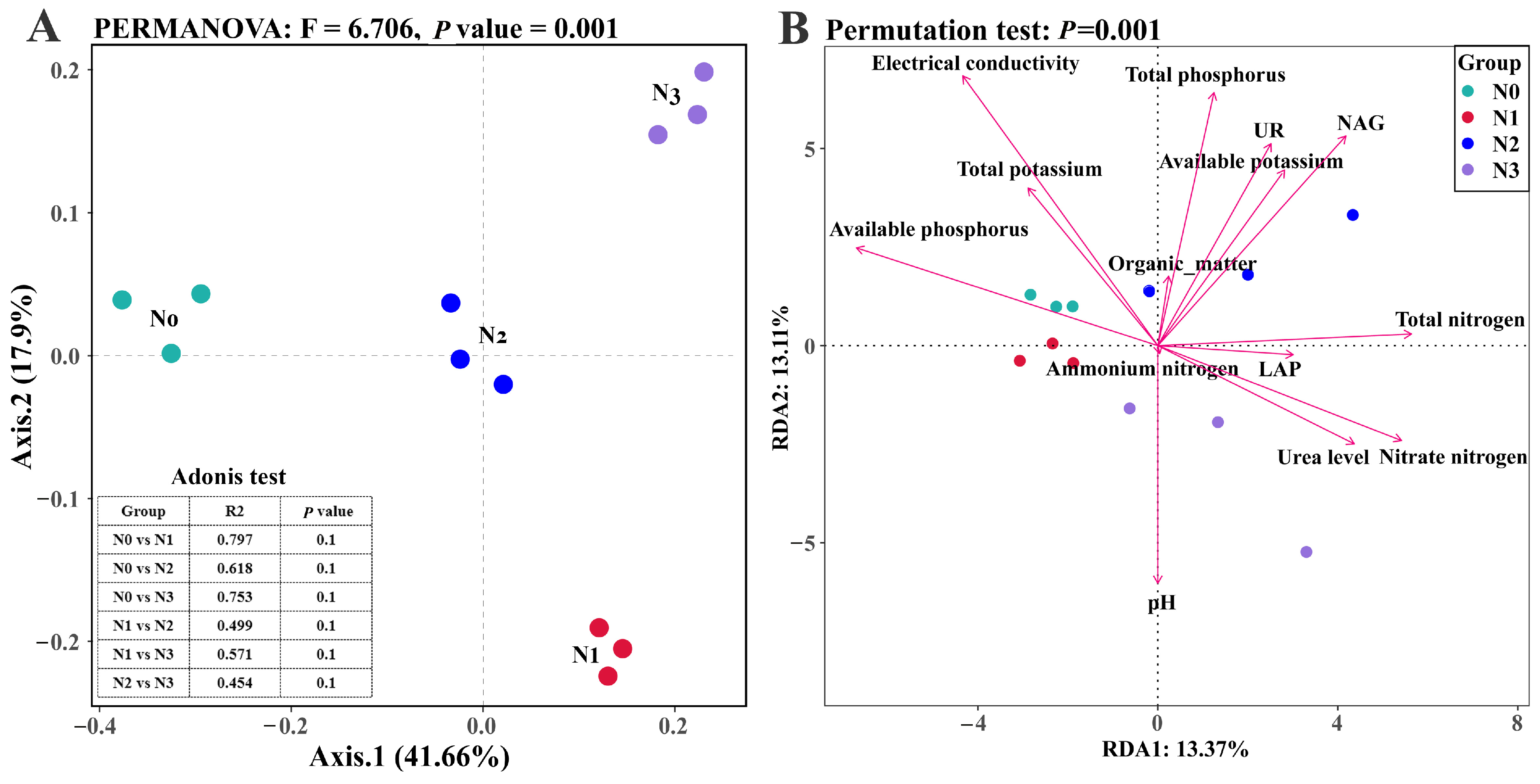
| Environmental Factors | Redundancy Analysis | |||
|---|---|---|---|---|
| RDA1 | RDA2 | R2 | p Value | |
| pH | −0.0001 | −1.0000 | 0.4616 | 0.0210 * |
| Electrical conductivity | −0.5348 | 0.8450 | 0.6205 | 0.0060 ** |
| Organic matter | 0.1351 | 0.9908 | 0.1363 | 0.6307 |
| Total nitrogen (N) | 0.9986 | 0.0526 | 0.4322 | 0.0275 * |
| Total phosphorus (P) | 0.1908 | 0.9816 | 0.5009 | 0.0090 ** |
| Total potassium (K) | −0.5846 | 0.8114 | 0.3779 | 0.1239 |
| Nitrate nitrogen | 0.9137 | −0.4064 | 0.4537 | 0.0275 * |
| Ammonium nitrogen | 0.1904 | −0.9817 | 0.0160 | 0.9440 |
| Available potassium | 0.5302 | 0.8479 | 0.4203 | 0.0405 * |
| Available phosphorus | −0.9377 | 0.3475 | 0.5470 | 0.0025 ** |
| NAG | 0.6175 | 0.7865 | 0.5182 | 0.0285 * |
| LAP | 0.9971 | −0.0759 | 0.2310 | 0.3323 |
| UR | 0.4403 | 0.8979 | 0.4372 | 0.0550 |
| Urea level | 0.8684 | −0.4958 | 0.3850 | 0.0935 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, R.; Wang, H.; Liang, X.; Zhou, X.; Wang, Y.; Tian, Y.; Shi, Z.; Li, Y. Response of Chaetomium sp. to Nitrogen Input and Its Potential Role in Rhizosphere Enrichment of Lycium barbarum. Microorganisms 2025, 13, 1864. https://doi.org/10.3390/microorganisms13081864
Wan R, Wang H, Liang X, Zhou X, Wang Y, Tian Y, Shi Z, Li Y. Response of Chaetomium sp. to Nitrogen Input and Its Potential Role in Rhizosphere Enrichment of Lycium barbarum. Microorganisms. 2025; 13(8):1864. https://doi.org/10.3390/microorganisms13081864
Chicago/Turabian StyleWan, Ru, Hezhen Wang, Xiaojie Liang, Xuan Zhou, Yajun Wang, Yehan Tian, Zhigang Shi, and Yuekun Li. 2025. "Response of Chaetomium sp. to Nitrogen Input and Its Potential Role in Rhizosphere Enrichment of Lycium barbarum" Microorganisms 13, no. 8: 1864. https://doi.org/10.3390/microorganisms13081864
APA StyleWan, R., Wang, H., Liang, X., Zhou, X., Wang, Y., Tian, Y., Shi, Z., & Li, Y. (2025). Response of Chaetomium sp. to Nitrogen Input and Its Potential Role in Rhizosphere Enrichment of Lycium barbarum. Microorganisms, 13(8), 1864. https://doi.org/10.3390/microorganisms13081864





