Variabilities in N2 and E Gene Concentrations in a SARS-CoV-2 Wastewater Multiplex Assay
Abstract
1. Introduction
2. Materials and Methods
2.1. Wastewater Sampling and Water Quality Analysis
2.2. Quantification of N2 and E Gene Regions of SARS-CoV-2
2.3. Data Analysis
3. Results
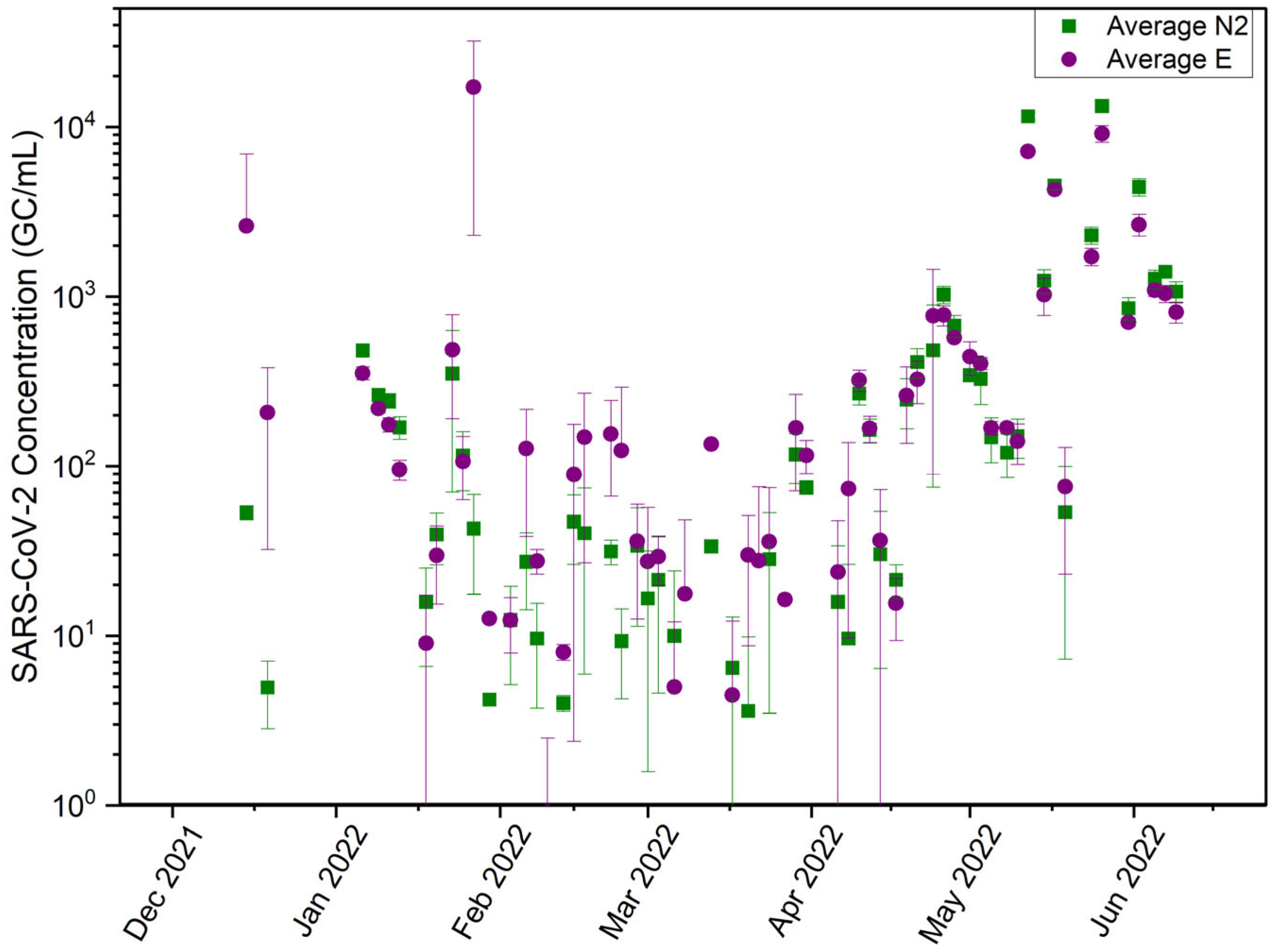
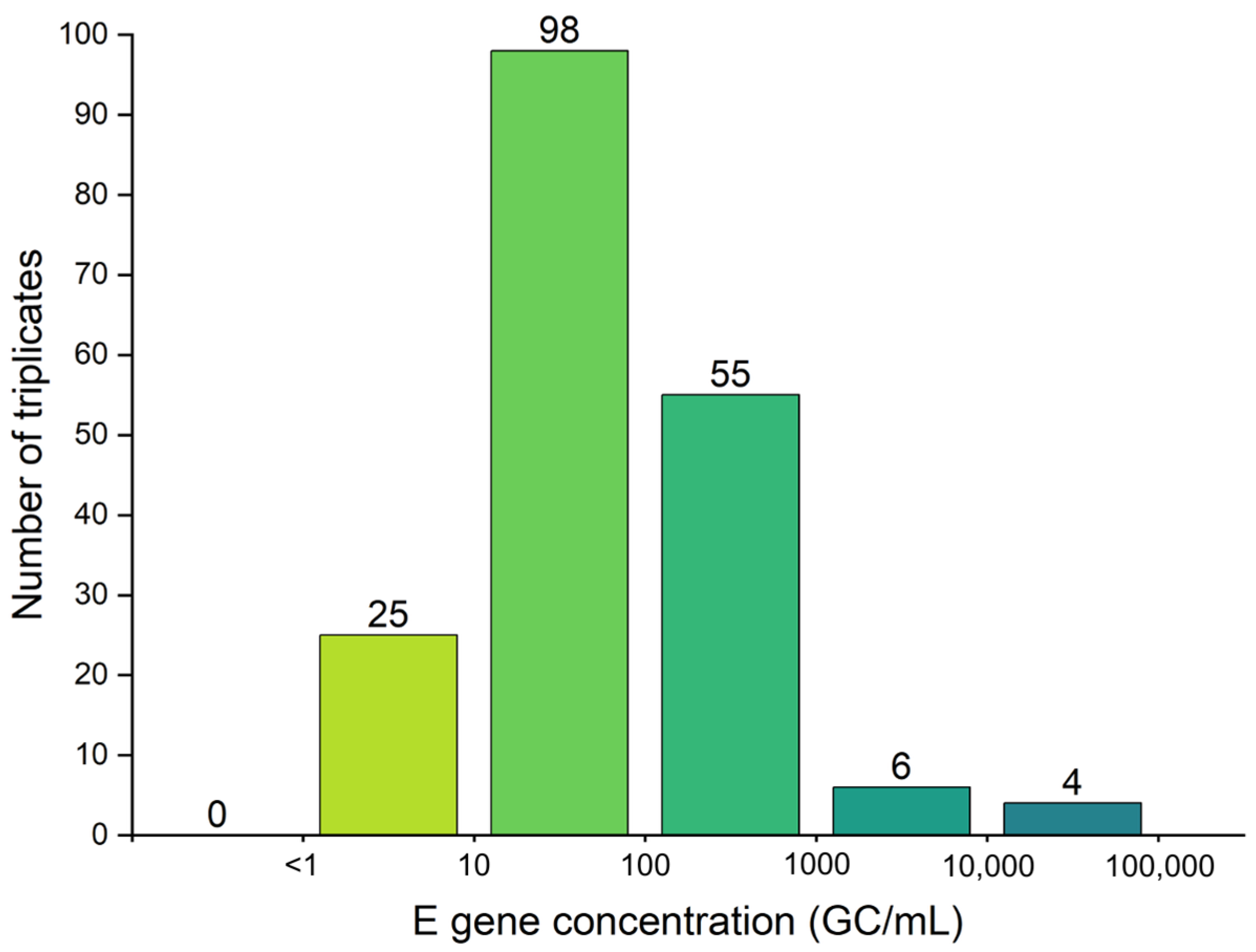
3.1. N2 Gene Underestimates SARS-CoV-2 Concentration in Wastewater
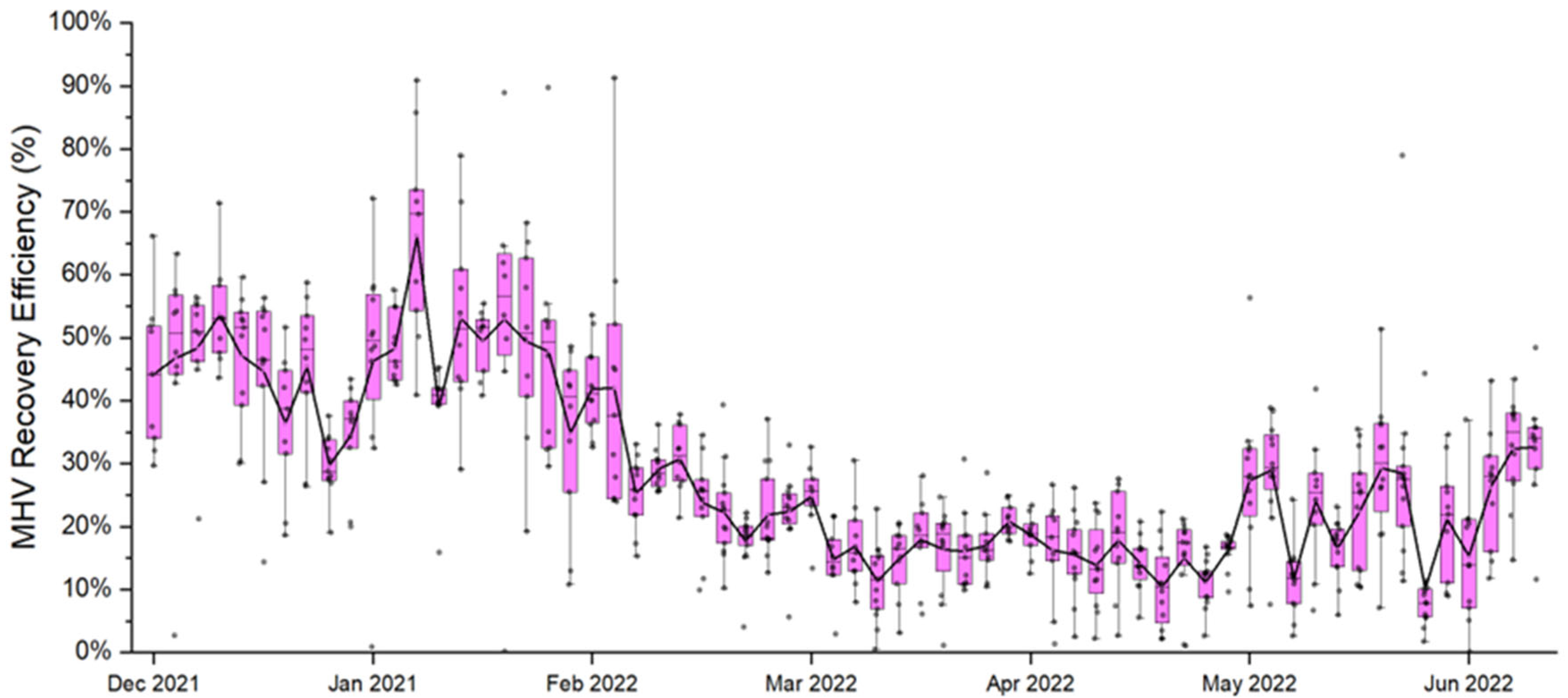
3.2. Wastewater Matrix Effects on Target Gene Amplification
4. Discussion
5. Conclusions
- The N2 and E gene targets both have pros and cons when it comes to their specificity and sensitivity to PCR amplification. Results have shown that the E gene had a higher efficiency of amplification during particularly low viral activity periods.
- PCR inhibition is an important challenge in WBS studies. Matrix recovery controls, such as MHV, can be a useful indicator of inhibition in wastewater monitoring studies. MHV recovery efficiency in this study clearly indicated inhibition in samples with higher TSS and COD, even with careful removal of debris in wastewater samples and purification of RNA.
- WBS programs require reproducibility and cohesion for success and deployment of sustainable, passive surveillance networks. More in-depth methodological studies should be conducted to help standardize the approach to wastewater sample collection, pathogen detection and/or quantification, and data sharing.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carmo dos Santos, M.; Cerqueira Silva, A.C.; dos Reis Teixeira, C.; Pinheiro Macedo Prazeres, F.; Fernandes dos Santos, R.; de Araújo Rolo, C.; de Souza Santos, E.; Santos da Fonseca, M.; Oliveira Valente, C.; Saraiva Hodel, K.V.; et al. Wastewater Surveillance for Viral Pathogens: A Tool for Public Health. Heliyon 2024, 10, e33873. [Google Scholar] [CrossRef]
- Grassly, N.C.; Shaw, A.G.; Owusu, M. Global Wastewater Surveillance for Pathogens with Pandemic Potential: Opportunities and Challenges. Lancet Microbe 2024, 6, 100939. [Google Scholar] [CrossRef]
- Foladori, P.; Cutrupi, F.; Segata, N.; Manara, S.; Pinto, F.; Malpei, F.; Bruni, L.; La Rosa, G. SARS-CoV-2 from Faeces to Wastewater Treatment: What Do We Know? A Review. Sci. Total Environ. 2020, 743, 140444. [Google Scholar] [CrossRef]
- Bertels, X.; Demeyer, P.; Van den Bogaert, S.; Boogaerts, T.; van Nuijs, A.L.N.; Delputte, P.; Lahousse, L. Factors Influencing SARS-CoV-2 RNA Concentrations in Wastewater up to the Sampling Stage: A Systematic Review. Sci. Total Environ. 2022, 820, 153290. [Google Scholar] [CrossRef] [PubMed]
- Kallem, P.; Hegab, H.; Alsafar, H.; Hasan, S.W.; Banat, F. SARS-CoV-2 Detection and Inactivation in Water and Wastewater: Review on Analytical Methods, Limitations and Future Research Recommendations. Emerg. Microbes Infect. 2023, 12, 2222850. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Huang, Z.; Luo, J.; Zhang, X.; Sha, S. Primary Concentration—The Critical Step in Implementing the Wastewater Based Epidemiology for the COVID-19 Pandemic: A Mini-Review. Sci. Total Environ. 2020, 747, 141245. [Google Scholar] [CrossRef] [PubMed]
- Parkins, M.D.; Lee, B.E.; Acosta, N.; Bautista, M.; Hubert, C.R.J.; Hrudey, S.E.; Frankowski, K.; Pang, X.-L. Wastewater-Based Surveillance as a Tool for Public Health Action: SARS-CoV-2 and Beyond. Clin. Microbiol. Rev. 2023, 37, e0010322. [Google Scholar] [CrossRef]
- Nyaruaba, R.; Li, C.; Mwaliko, C.; Mwau, M.; Odiwuor, N.; Muturi, E.; Muema, C.; Xiong, J.; Li, J.; Yu, J.; et al. Developing Multiplex DdPCR Assays for SARS-CoV-2 Detection Based on Probe Mix and Amplitude Based Multiplexing. Expert. Rev. Mol. Diagn. 2021, 21, 119–129. [Google Scholar] [CrossRef]
- Lodder, W.; de Roda Husman, A.M. SARS-CoV-2 in Wastewater: Potential Health Risk, but Also Data Source. Lancet Gastroenterol. Hepatol. 2020, 5, 533–534. [Google Scholar] [CrossRef]
- Rojanaworarit, C. Principles of Public Health Surveillance: A Revisit to Fundamental Concepts. J. Public Health Dev. 2015, 13, 29–46. [Google Scholar] [CrossRef]
- CDC. Building COVID-19 Wastewater Surveillance with the National Wastewater Surveillance System. 7 March 2022. Available online: https://www.cdc.gov/nwss/pdf/328288_National_Wastewater_Surveillance_System_508.pdf (accessed on 27 June 2025).
- Wu, F.; Zhang, J.; Xiao, A.; Gu, X.; Lee, W.L.; Armas, F.; Kauffman, K.; Hanage, W.; Matus, M.; Ghaeli, N.; et al. SARS-CoV-2 Titers in Wastewater Are Higher than Expected from Clinically Confirmed Cases. Msystems 2020, 5, 10-1128. [Google Scholar] [CrossRef] [PubMed]
- Flood, M.T.; D’Souza, N.; Rose, J.B.; Aw, T.G. Methods Evaluation for Rapid Concentration and Quantification of SARS-CoV-2 in Raw Wastewater Using Droplet Digital and Quantitative RT-PCR. Food Environ. Virol. 2021, 13, 303–315. [Google Scholar] [CrossRef]
- LaTurner, Z.W.; Zong, D.M.; Kalvapalle, P.; Gamas, K.R.; Terwilliger, A.; Crosby, T.; Ali, P.; Avadhanula, V.; Santos, H.H.; Weesner, K.; et al. Evaluating Recovery, Cost, and Throughput of Different Concentration Methods for SARS-CoV-2 Wastewater-Based Epidemiology. Water Res. 2021, 197, 117043. [Google Scholar] [CrossRef] [PubMed]
- Philo, S.E.; Keim, E.K.; Swanstrom, R.; Ong, A.Q.W.; Burnor, E.A.; Kossik, A.L.; Harrison, J.C.; Demeke, B.A.; Zhou, N.A.; Beck, N.K.; et al. A Comparison of SARS-CoV-2 Wastewater Concentration Methods for Environmental Surveillance. Sci. Total Environ. 2021, 760, 144215. [Google Scholar] [CrossRef]
- Pérez-Cataluña, A.; Cuevas-Ferrando, E.; Randazzo, W.; Falcó, I.; Allende, A.; Sánchez, G. Comparing Analytical Methods to Detect SARS-CoV-2 in Wastewater. Sci. Total Environ. 2021, 758, 143870. [Google Scholar] [CrossRef]
- Zhou, N.A.; Tharpe, C.; Meschke, J.S.; Ferguson, C.M. Survey of Rapid Development of Environmental Surveillance Methods for SARS-CoV-2 Detection in Wastewater. Sci. Total Environ. 2021, 769, 144852. [Google Scholar] [CrossRef]
- Pecson, B.M.; Darby, E.; Haas, C.N.; Amha, Y.M.; Bartolo, M.; Danielson, R.; Dearborn, Y.; Di Giovanni, G.; Ferguson, C.; Fevig, S.; et al. Reproducibility and Sensitivity of 36 Methods to Quantify the SARS-CoV-2 Genetic Signal in Raw Wastewater: Findings from an Interlaboratory Methods Evaluation in the U.S. Environ. Sci. (Camb.) 2021, 7, 504–520. [Google Scholar] [CrossRef]
- Yang, H.; Rao, Z. Structural Biology of SARS-CoV-2 and Implications for Therapeutic Development. Nat. Rev. Microbiol. 2021, 19, 685–700. [Google Scholar] [CrossRef] [PubMed]
- Yadav, R.; Chaudhary, J.K.; Jain, N.; Chaudhary, P.K.; Khanra, S.; Dhamija, P.; Sharma, A.; Kumar, A.; Handu, S. Role of Structural and Non-Structural Proteins and Therapeutic Targets of SARS-CoV-2 for COVID-19. Cells 2021, 10, 821. [Google Scholar] [CrossRef]
- Navarro, A.; Gómez, L.; Sanseverino, I.; Niegowska, M.; Roka, E.; Pedraccini, R.; Vargha, M.; Lettieri, T. SARS-CoV-2 Detection in Wastewater Using Multiplex Quantitative PCR. Sci. Total Environ. 2021, 797, 148890. [Google Scholar] [CrossRef]
- Boogaerts, T.; Jacobs, L.; De Roeck, N.; Van den Bogaert, S.; Aertgeerts, B.; Lahousse, L.; van Nuijs, A.L.N.; Delputte, P. An Alternative Approach for Bioanalytical Assay Optimization for Wastewater-Based Epidemiology of SARS-CoV-2. Sci. Total Environ. 2021, 789, 148043. [Google Scholar] [CrossRef]
- Moreno, J.L.; Zúñiga, S.; Enjuanes, L.; Sola, I. Identification of a Coronavirus Transcription Enhancer. J. Virol. 2008, 82, 3882–3893. [Google Scholar] [CrossRef]
- Corman, V.M.; Landt, O.; Kaiser, M.; Molenkamp, R.; Meijer, A.; Chu, D.K.W.; Bleicker, T.; Brünink, S.; Schneider, J.; Schmidt, M.L.; et al. Detection of 2019 Novel Coronavirus (2019-NCoV) by Real-Time RT-PCR. Eurosurveillance 2020, 25, 2000045. [Google Scholar] [CrossRef]
- Pappu, A.R.; Green, A.; Oakes, M.; Jiang, S. Wastewater-Based Surveillance Data to Determine the COVID-19 Trends in Communities with Low Population. Data Brief 2025, 61, 111756. [Google Scholar] [CrossRef]
- Kolarević, S.; Micsinai, A.; Szántó-Egész, R.; Lukács, A.; Kračun-Kolarević, M.; Djordjevic, A.; Vojnović-Milutinović, D.; Marić, J.J.; Kirschner, A.K.T.; Farnleitner, A.A.H.; et al. Wastewater-Based Epidemiology in Countries with Poor Wastewater Treatment—Epidemiological Indicator Function of SARS-CoV-2 RNA in Surface Waters. Sci. Total Environ. 2022, 843, 156964. [Google Scholar] [CrossRef]
- Lu, E.; Ai, Y.; Davis, A.; Straathof, J.; Halloran, K.; Hull, N.; Winston, R.; Weir, M.H.; Soller, J.; Bohrerova, Z.; et al. Wastewater Surveillance of SARS-CoV-2 in Dormitories as a Part of Comprehensive University Campus COVID-19 Monitoring. Environ. Res. 2022, 212, 113580. [Google Scholar] [CrossRef]
- Maal-Bared, R.; Qiu, Y.; Li, Q.; Gao, T.; Hrudey, S.E.; Bhavanam, S.; Ruecker, N.J.; Ellehoj, E.; Lee, B.E.; Pang, X. Does Normalization of SARS-CoV-2 Concentrations by Pepper Mild Mottle Virus Improve Correlations and Lead Time between Wastewater Surveillance and Clinical Data in Alberta (Canada): Comparing Twelve SARS-CoV-2 Normalization Approaches. Sci. Total Environ. 2023, 856, 158964. [Google Scholar] [CrossRef]
- Tavazzi, S.; Cacciatori, C.; Comero, S.; Fatta-Kassinos, D.; Karaolia, P.; Iakovides, I.C.; Loutsiou, P.; Gutierrez-Aguirre, I.; Lengar, Z.; Bajde, I.; et al. Short-Term Stability of Wastewater Samples for Storage and Shipment in the Context of the EU Sewage Sentinel System for SARS-CoV-2. J. Environ. Chem. Eng. 2023, 11, 109623. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Chang, Y.-H.; Chen, C.-H.S.; Chang, S.-Y.; Chan, C.-C.; Chen, P.-C.; Su, T.-C. Wastewater SARS-CoV-2 Monitoring in a University Hospital Forecasts Multilevel Epidemic Curves in Taipei City, Taiwan. Ecotoxicol. Environ. Saf. 2025, 299, 118299. [Google Scholar] [CrossRef] [PubMed]
- Chai, X.; Liu, S.; Liu, C.; Bai, J.; Meng, J.; Tian, H.; Han, X.; Han, G.; Xu, X.; Li, Q. Surveillance of SARS-CoV-2 in Wastewater by Quantitative PCR and Digital PCR: A Case Study in Shijiazhuang City, Hebei Province, China. Emerg. Microbes. Infect. 2024, 13, 2324502. [Google Scholar] [CrossRef] [PubMed]
- Tandukar, S.; Thakali, O.; Baral, R.; Tiwari, A.; Haramoto, E.; Tuladhar, R.; Joshi, D.R.; Sherchan, S.P. Application of Wastewater-Based Epidemiology for Monitoring COVID-19 in Hospital and Housing Wastewaters. Sci. Total Environ. 2024, 931, 171877. [Google Scholar] [CrossRef]
- Pappu, A.R.; Green, A.; Oakes, M.; Jiang, S. Tracking COVID-19 Trends in Communities with Low Population by Wastewater-Based Surveillance. Sci. Total Environ. 2025, 970, 179007. [Google Scholar] [CrossRef]
- Ye, Y.; Ellenberg, R.M.; Graham, K.E.; Wigginton, K.R. Survivability, Partitioning, and Recovery of Enveloped Viruses in Untreated Municipal Wastewater. Environ. Sci. Technol. 2016, 50, 5077–5085. [Google Scholar] [CrossRef]
- Larsen, D.A.; Wigginton, K.R. Tracking COVID-19 with Wastewater. Nat. Biotechnol. 2020, 38, 1151–1153. [Google Scholar] [CrossRef] [PubMed]
- Graham, K.E.; Loeb, S.K.; Wolfe, M.K.; Catoe, D.; Sinnott-Armstrong, N.; Kim, S.; Yamahara, K.M.; Sassoubre, L.M.; Mendoza Grijalva, L.M.; Roldan-Hernandez, L.; et al. SARS-CoV-2 RNA in Wastewater Settled Solids Is Associated with COVID-19 Cases in a Large Urban Sewershed. Environ. Sci. Technol. 2021, 55, 488–498. [Google Scholar] [CrossRef]
- Wolfe, M.K.; Topol, A.; Knudson, A.; Simpson, A.; White, B.; Vugia, D.J.; Yu, A.T.; Li, L.; Balliet, M.; Stoddard, P.; et al. High-Frequency, High-Throughput Quantification of SARS-CoV-2 RNA in Wastewater Settled Solids at Eight Publicly Owned Treatment Works in Northern California Shows Strong Association with COVID-19 Incidence. mSystems 2021, 6, e0082921. [Google Scholar] [CrossRef] [PubMed]
- ASTM D5907-18; Standard Test Methods for Filterable and Non-Filterable Matter in Water. ASTM International: West Conshohocken, PA, USA, 2018.
- Ahmed, W.; Bertsch, P.M.; Bivins, A.; Bibby, K.; Farkas, K.; Gathercole, A.; Haramoto, E.; Gyawali, P.; Korajkic, A.; McMinn, B.R.; et al. Comparison of Virus Concentration Methods for the RT-QPCR-Based Recovery of Murine Hepatitis Virus, a Surrogate for SARS-CoV-2 from Untreated Wastewater. Sci. Total Environ. 2020, 739, 139960. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.; Cornwell, D. Introduction to Environmental Engineering, 5th ed.; McGraw-Hill: New York, NY, USA, 2012. [Google Scholar]
- CDC. Wastewater Surveillance Testing Methods. Available online: https://archive.cdc.gov/www_cdc_gov/nwss/testing.html (accessed on 27 June 2025).
- WastewaterSCAN WastwaterSCAN Dashboard. Available online: https://data.wastewaterscan.org/ (accessed on 27 June 2025).
- Besselsen, D.G.; Wagner, A.M.; Loganbill, J.K. Detection of Rodent Coronaviruses by Use of Fluorogenic Reverse Tran-scriptase-Polymerase Chain Reaction Analysis. Comp. Med. 2002, 52, 111–116. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Green, A.; Pappu, A.R.; Oakes, M.; Sandmeyer, S.; Hileman, M.; Jiang, S. Variabilities in N2 and E Gene Concentrations in a SARS-CoV-2 Wastewater Multiplex Assay. Microorganisms 2025, 13, 1862. https://doi.org/10.3390/microorganisms13081862
Green A, Pappu AR, Oakes M, Sandmeyer S, Hileman M, Jiang S. Variabilities in N2 and E Gene Concentrations in a SARS-CoV-2 Wastewater Multiplex Assay. Microorganisms. 2025; 13(8):1862. https://doi.org/10.3390/microorganisms13081862
Chicago/Turabian StyleGreen, Ashley, Aiswarya Rani Pappu, Melanie Oakes, Suzanne Sandmeyer, Matthew Hileman, and Sunny Jiang. 2025. "Variabilities in N2 and E Gene Concentrations in a SARS-CoV-2 Wastewater Multiplex Assay" Microorganisms 13, no. 8: 1862. https://doi.org/10.3390/microorganisms13081862
APA StyleGreen, A., Pappu, A. R., Oakes, M., Sandmeyer, S., Hileman, M., & Jiang, S. (2025). Variabilities in N2 and E Gene Concentrations in a SARS-CoV-2 Wastewater Multiplex Assay. Microorganisms, 13(8), 1862. https://doi.org/10.3390/microorganisms13081862





