Environmental Factors Drive the Changes of Bacterial Structure and Functional Diversity in Rhizosphere Soil of Hippophae rhamnoides subsp. sinensis Rousi in Arid Regions of Northwest China
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Site Description of Chinese Seabuckthorn
2.2. Rhizosphere Soil Sampling of Chinese Seabuckthorn
2.3. Soil Bacterial DNA Extraction, PCR Amplification, and High-Throughput Sequencing for Chinese Seabuckthorn
2.4. Data Analysis
3. Results and Analysis
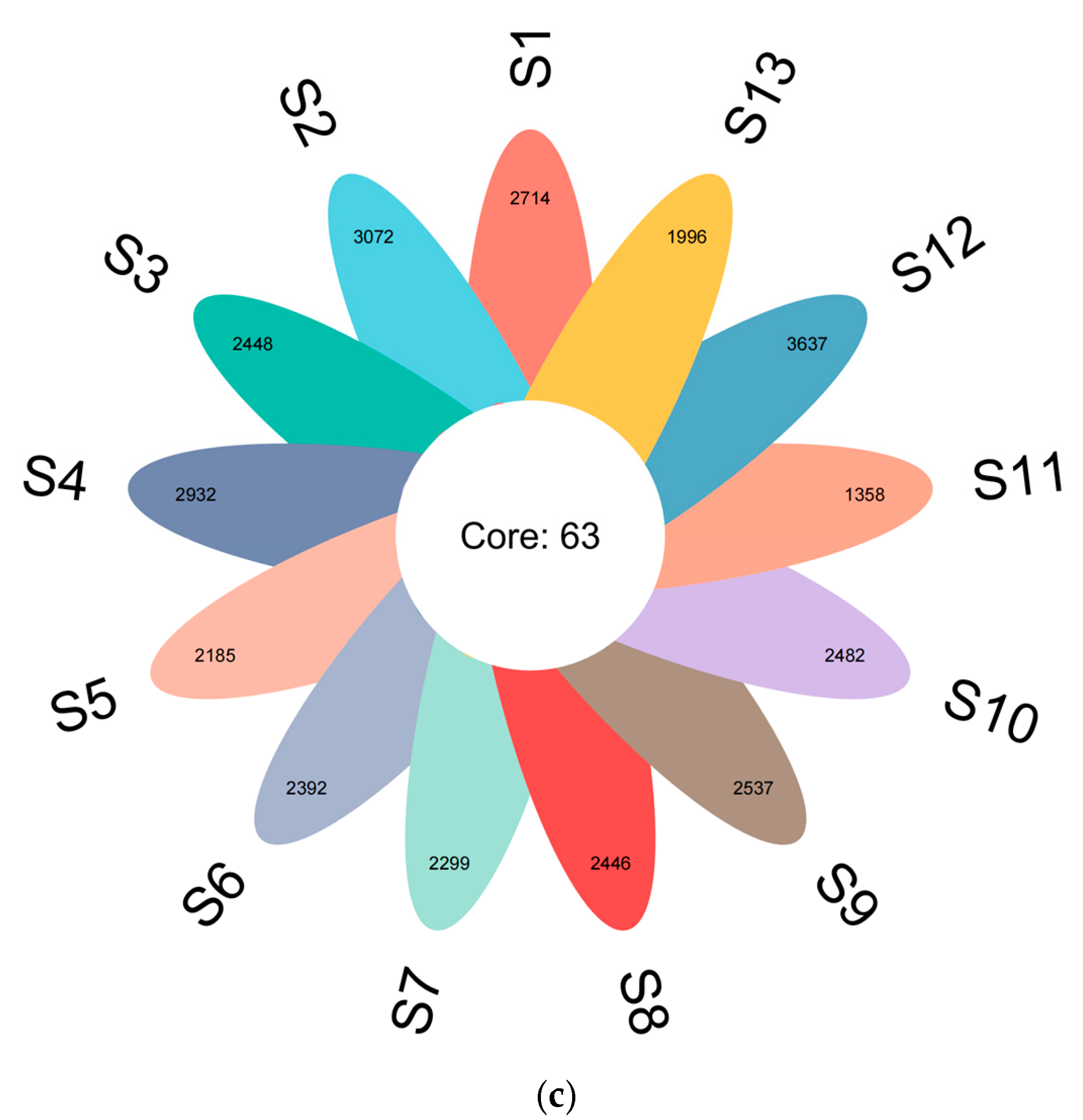
3.1. Changes in the Quality of 16s Sequencing Results and the Number of ASV in Bacterial Communities
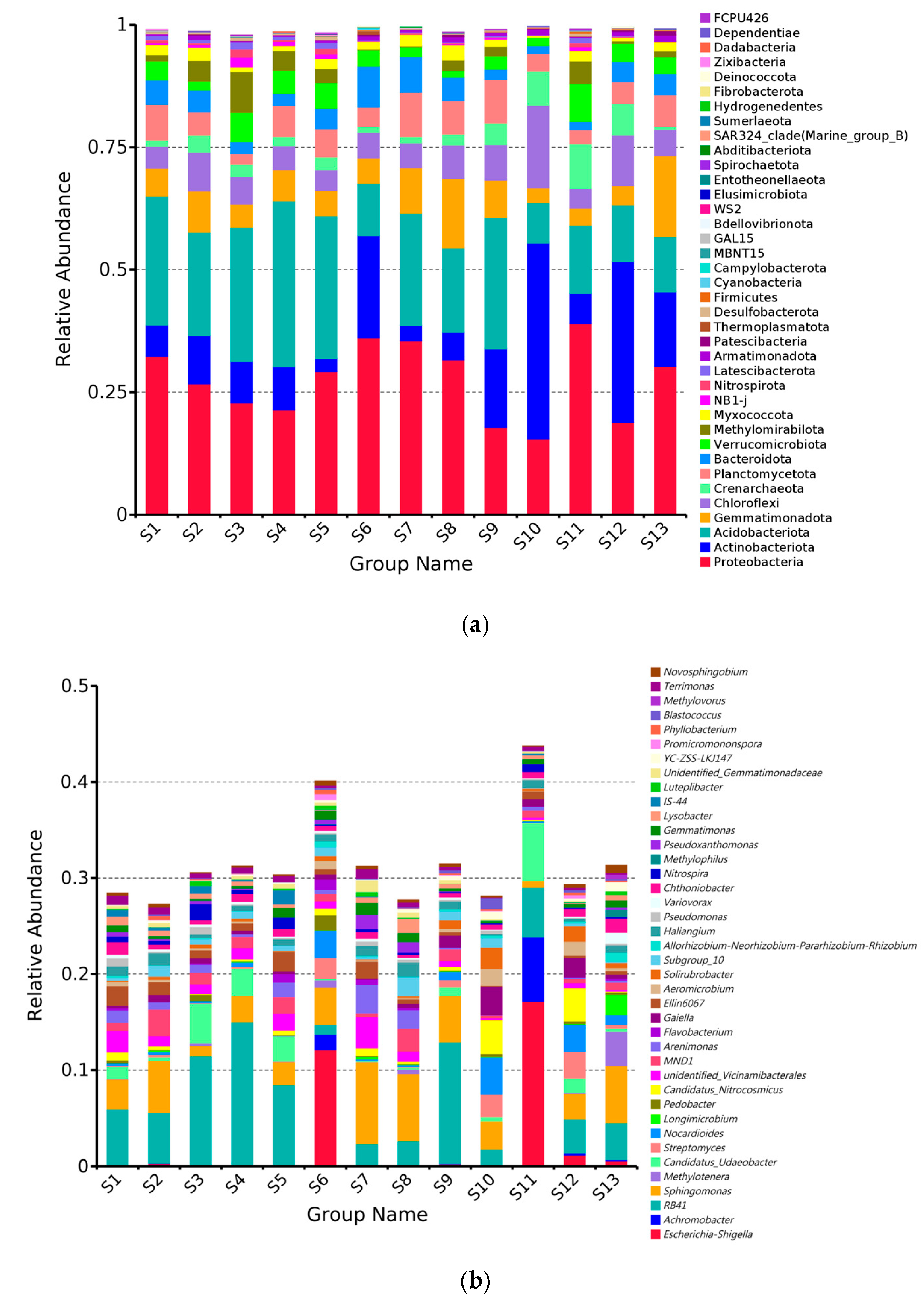
3.2. Changes in Species Composition and Relative Abundance of Soil Bacterial Communities
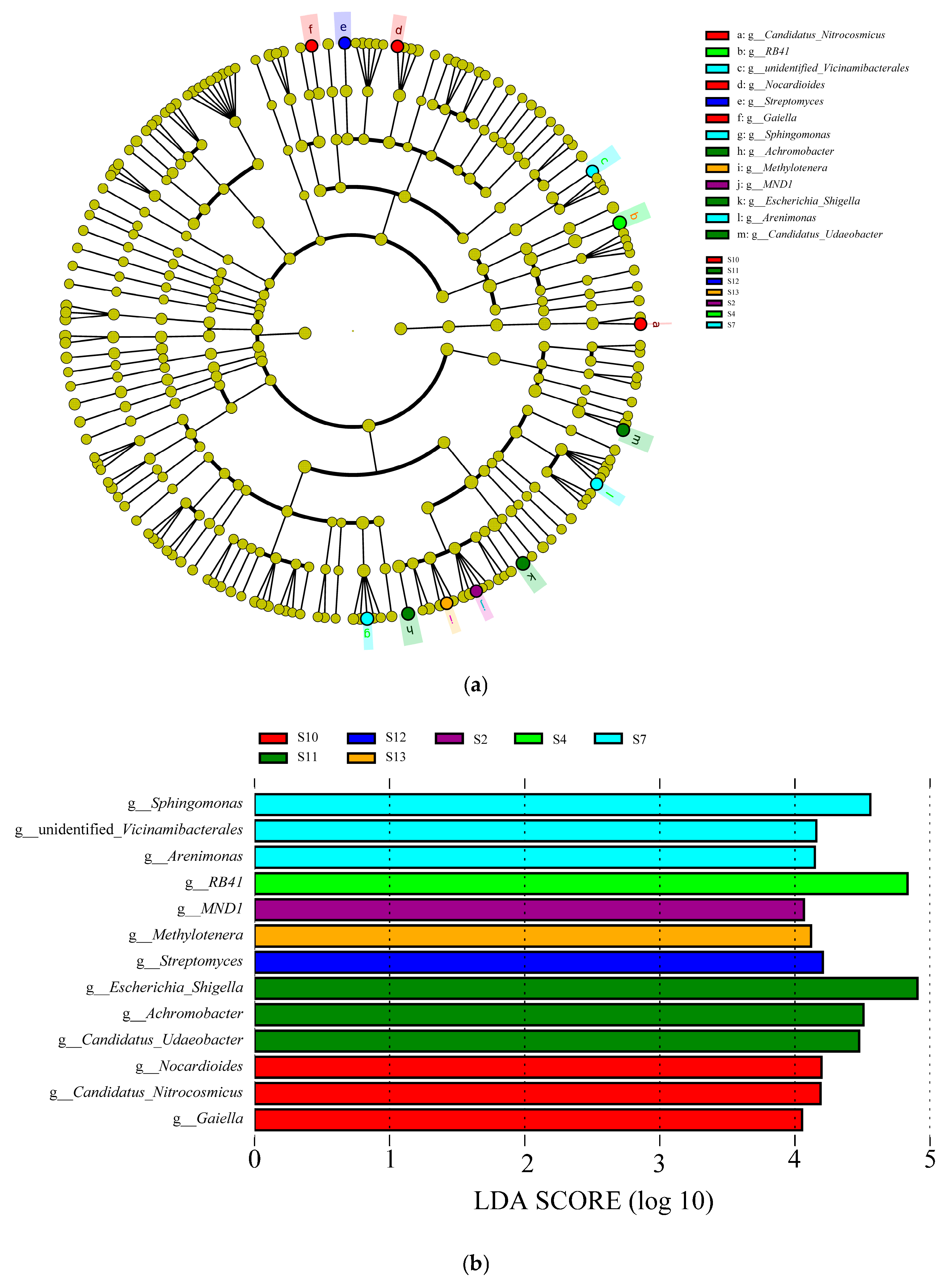
3.3. LEfSe Analysis of Soil Bacterial Community
3.4. Analysis of Soil Bacterial Community Diversity
3.4.1. Analysis of α-Diversity of Soil Rhizosphere Bacterial Community
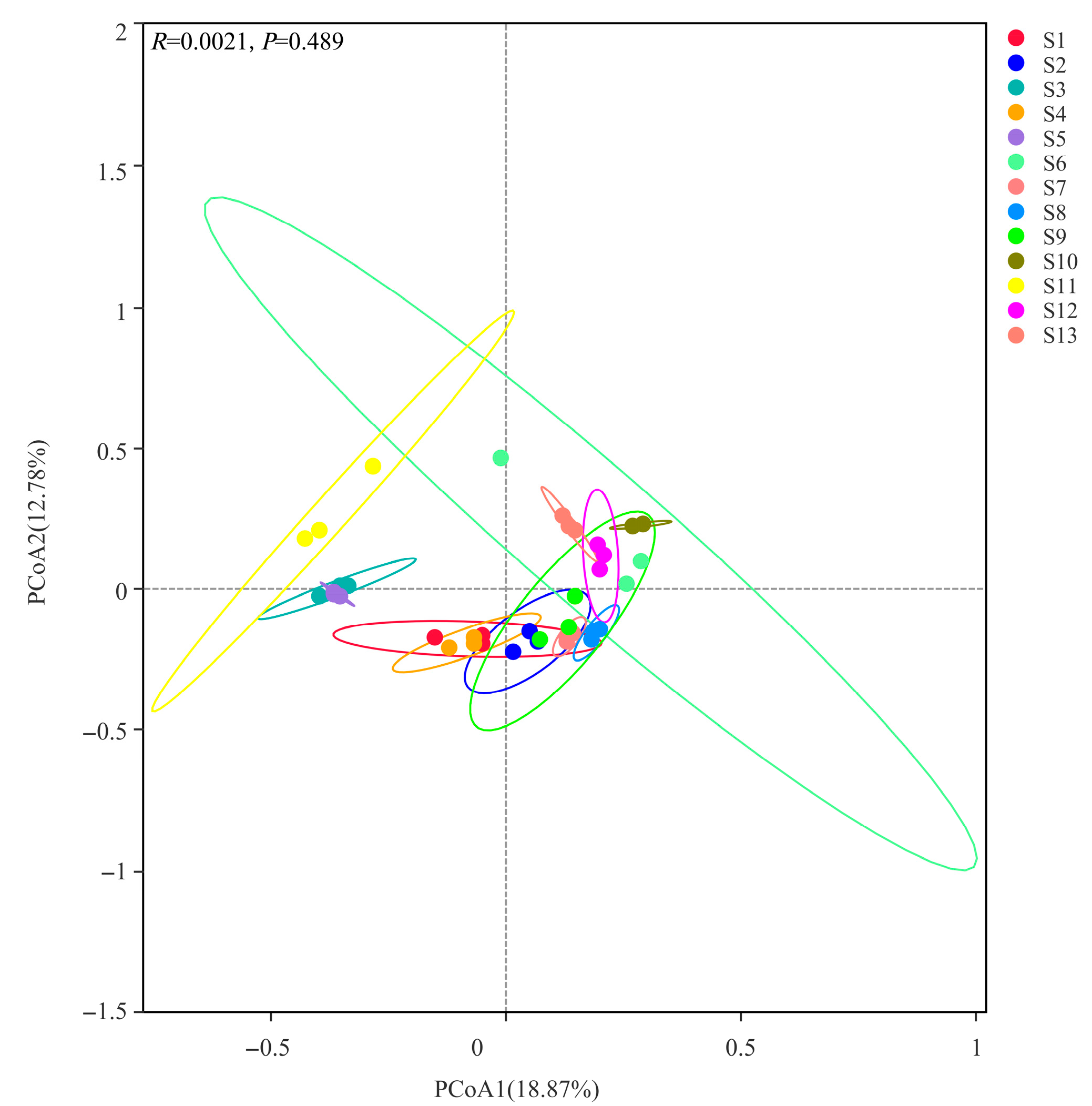
3.4.2. Analysis of Soil Bacterial Community Beta Diversity (PCoA)
3.5. Co-Occurrence of Soil Bacteria Molecular Network Changes
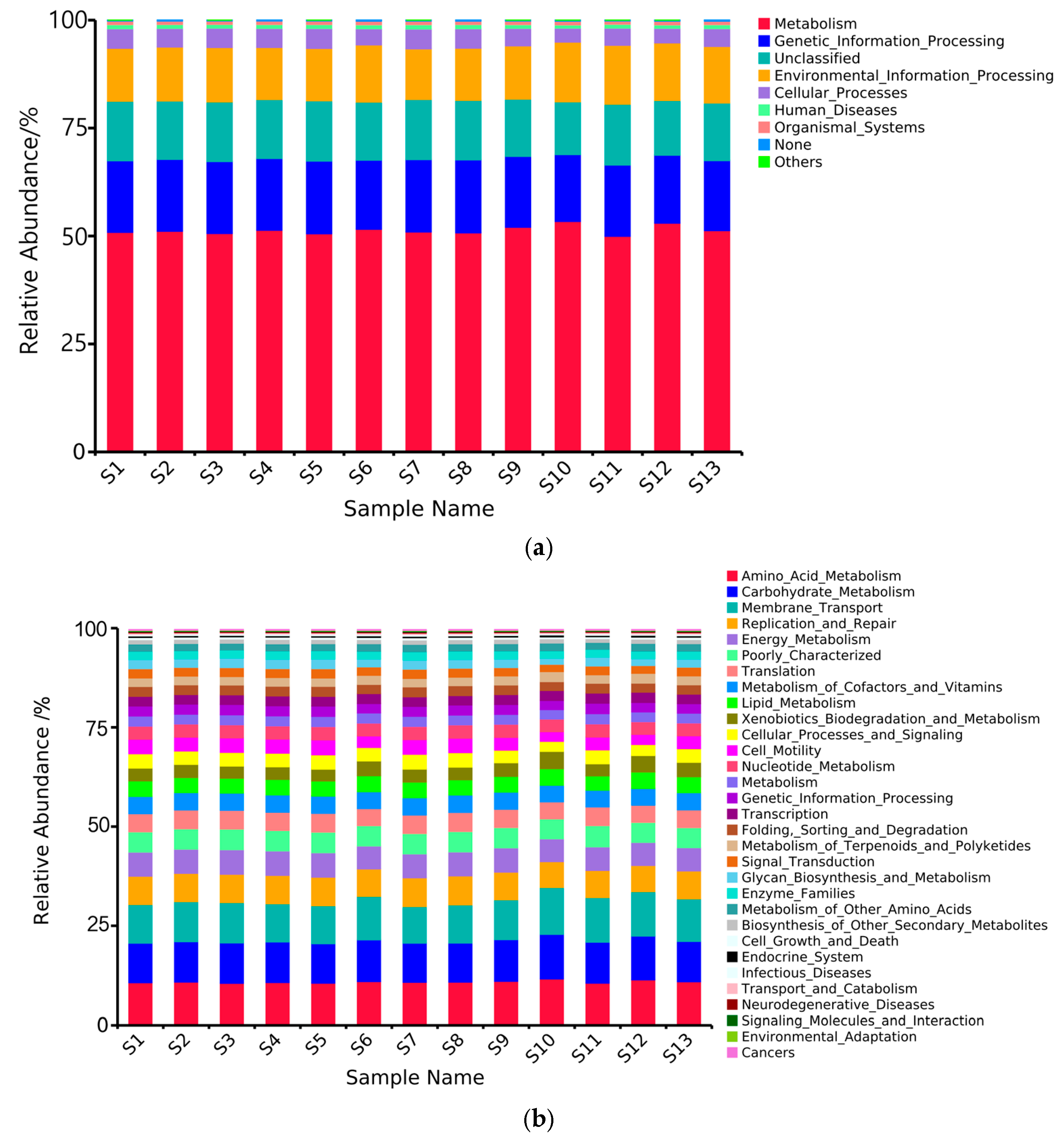
3.6. Prediction of Soil Bacterial Community Function
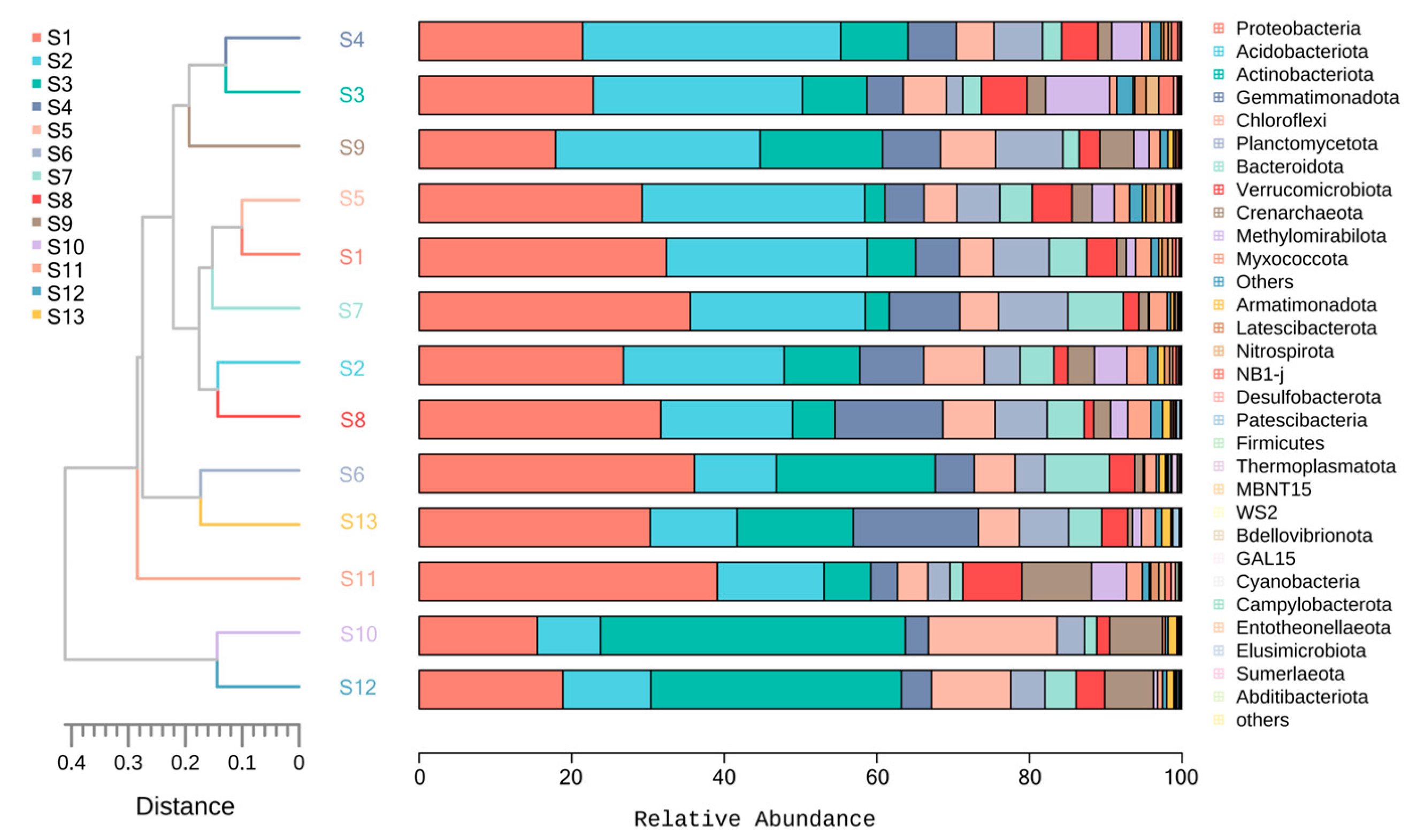
3.7. Cluster Analysis of Rhizosphere Soil Bacterial Community of Chinese Seabuckthorn in Different Distribution Regions
3.8. The Coupling Relationship Between Climate Characteristics, Soil Physicochemical Characteristics Factors, and the Diversity of Rhizosphere Soil Bacterial Community of Chinese Seabuckthorn
3.8.1. Mantel Test Analysis of Climate Characteristics, Soil Physicochemical Characteristics Factors, and Soil Bacterial Community
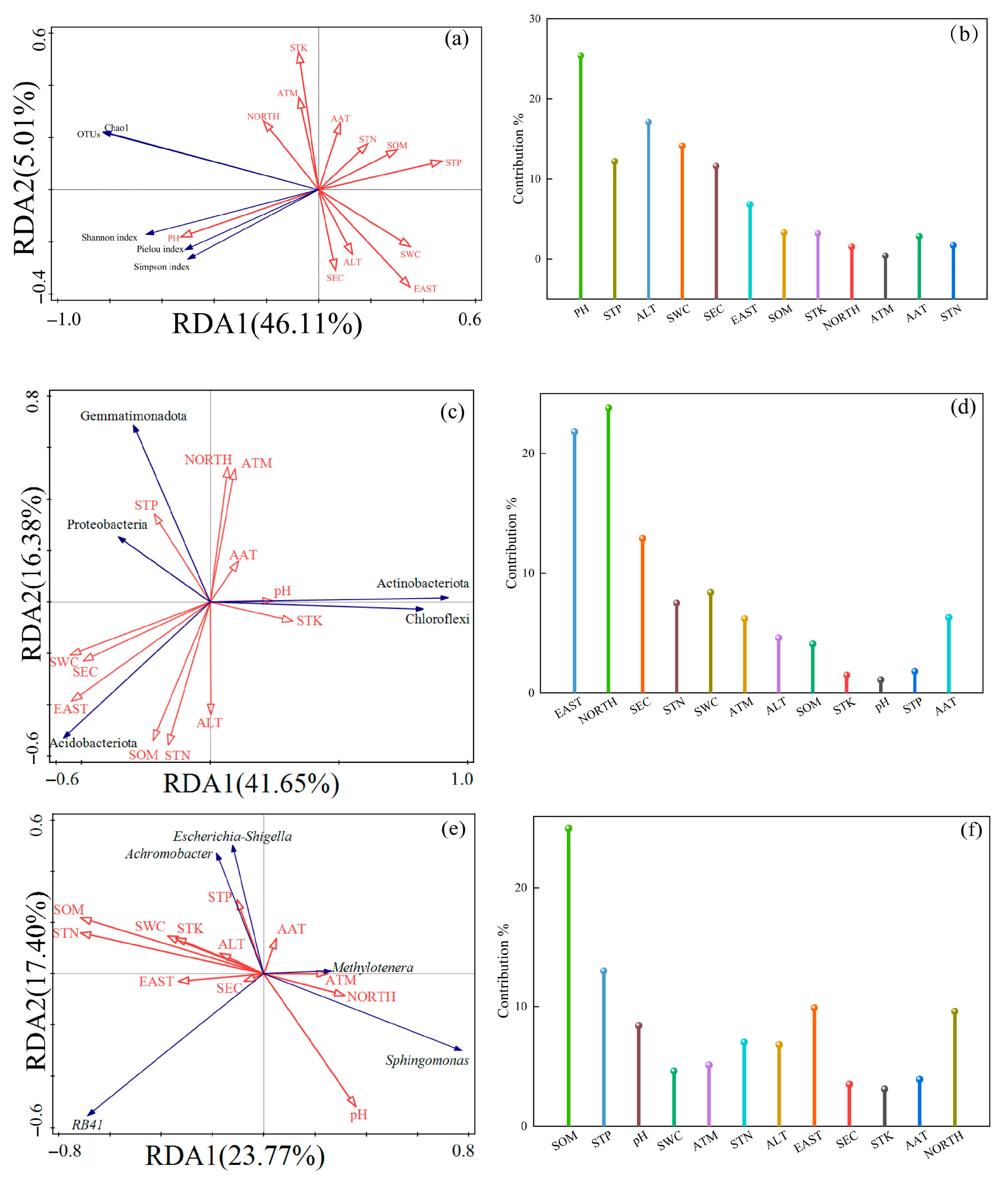
3.8.2. RDA Analysis of Climate Characteristics, Soil Physicochemical Characteristics Factors, and Soil Bacterial Community
4. Discussion
4.1. Changes of Bacterial Community Structure and Function in Rhizosphere Soil of Chinese Seabuckthorn Under 13 Distribution Regions
4.2. Correlation Analysis of Bacterial Community Diversity in Rhizosphere Soil of Chinese Seabuckthorn with Soil Physicochemical Characteristics and Climate Characteristics
5. Conclusions
- (1)
- Among the rhizosphere soil bacterial communities of Chinese seabuckthorn in 13 distribution regions in the northwest arid region of China, the number of bacteria ASV in S2 and S12 distribution regions is the highest, with 3072 and 3637, respectively, while that in S11 distribution area is the least, with only 1358; there are significant differences in α diversity indexes among 13 distribution regions: the number of OTU, Shannon index, Ace index, and Chao1 index in S12 distribution area are the highest, which are significantly higher than those in S11 distribution area.
- (2)
- The phyla Proteobacteria, Actinobacteriota, Acidobacteriota, Gemmatimonadota, and Chloroflexi were identified as the core bacterial taxa in the rhizosphere soil of Chinese seabuckthorn.
- (3)
- Metabolic functions and genetic information processing functions dominated across all distribution regions, with significantly higher abundance of these functions in the S10 compared to the S11 region. Bray-Curtis distance-based clustering grouped the bacterial communities into two distinct clusters: S10 and S12 formed Group 1, while the remaining regions constituted Group 2.
- (4)
- Redundancy analysis (RDA) indicated that soil pH was the primary environmental factor shaping the microbial community α-diversity in the rhizosphere of Chinese seabuckthorn, followed by altitude (ALT) and soil water content (SWC) as secondary influencing factors.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, X.-w.; Sun, K.; Li, Y.-h. Variation in leaf nitrogen and phosphorus stoichiometry in the nitrogen-fixing Chinese sea-buckthorn (Hippophae rhamnoides L. subsp. sinensis Rousi) across northern China. Ecol. Res. 2014, 29, 723–731. [Google Scholar] [CrossRef]
- Liu, Q.-q.; Ye, G.-s.; Ma, Y.-h. Development and application of microsatellite markers in Hippophae rhamnoides subsp. sinensis Rousi (Hippophae rhamnoides L.) based on transcriptome sequencing. Front. Genet. 2024, 15, 151373028. [Google Scholar] [CrossRef]
- Wang, H.-f.; Han, L.; Yang, M.-g.; Bao, L.; Ge, J.-p. Phylogeographic study of Chinese seabuckthorn (Hippophae rhamnoides subsp. sinensis Rousi) reveals two distinct haplotype groups and multiple microrefugia on the Qinghai-Tibet Plateau. Ecol. Evol. 2014, 4, 4370–4379. [Google Scholar] [PubMed]
- Cheng, Z.-y.; Chen, Y.-f.; Yuan, H.-j. Molecular mechanisms of seed germination in Hippophae rhamnoides L. based on transcriptomics. Res. Cold Arid. Reg. 2024, 16, 310–322. [Google Scholar] [CrossRef]
- Su, Y.; Li, S.-x.; Jiang, H.; Duan, B.-l.; Liu, M.-y.; Zhang, Y.-b. Sex-specific physiological and growth responses to elevated temperature and CO2 concentration in Chinese seabuckthorn (Hippophae rhamnoides subsp. sinensis Rousi). Acta Physiol. Plant. 2023, 45, 53. [Google Scholar] [CrossRef]
- Kumar, A.; Guleria, S.; Mehta, P.; Walia, A.; Chauhan, A.; Shirkot, C.-k. Plant growth-promoting traits of phosphate solubilizing bacteria isolated from Hippophae rhamnoides L. (Sea-buckthorn) growing in cold desert Trans-Himalayan Lahul and Spiti regions of India. Acta Physiol. Plant. 2015, 37, 48. [Google Scholar] [CrossRef]
- Liu, Y. Study on Multi-Dimensional Identification Method of Multi-Base Original National Medicine Hippophae rhamnoides; Chengdu University of Traditional Chinese Medicine: Chengdu, China, 2016. [Google Scholar]
- Zhu, P.-y.; Ren, Y.-m.; Qing, C.; Luo, J.-j.; Duan, W.; Ye, X.-q.; Tian, J.-h. Compounds from sea buckthorn and their application in food: A review. Food Chem. 2025, 476, 143428. [Google Scholar] [CrossRef]
- Qudsia, S.; Wang, X.-k.; Ullah, F.-h.; Jiříe, K.; Zahid, M.-m.; Jiri, H.; Adnan, M. Rhizosphere bacteria in plant growth promotion, biocontrol, and bioremediation of contaminated sites: A somprehensive review of effects and mechanisms. Int. J. Mol. Sci. 2021, 22, 10529. [Google Scholar]
- Philippot, L.; Raaijmakers, J.-m.; Lemanceau, P.; Van Der Putten, W.H. Going back to the roots: The microbial ecology of the rhizosphere. Nat. Rev. Microbiol. 2013, 11, 789–799. [Google Scholar] [CrossRef]
- Wojciech, B.; Artur, T.; Agnieszka, K.-b.; Bartłomiej, W.; Marcin, P.; Agnieszka, J.; Edyta, S. Effects of plant functional group and reclamation treatments on microbial networks and nutrient limitation in initial soil developed on spoil heaps after hard coal mining. Appl. Soil. Ecol. 2025, 208, 106002. [Google Scholar]
- Yuan, C.-y.; Li, F.-y.; Yuan, Z.-q.; Li, G.-y.; Liang, X.-q. Response of bacterial communities to mining activity in the alpine area of the Tianshan Mountain region, China. Environ. Sci. Pollut. Res. 2020, 28, 15806–15818. [Google Scholar] [CrossRef]
- Deng, J.-j.; Bai, X.-j.; Zhou, Y.-b.; Zhu, W.-x.; Yin, Y. Variations of soil microbial communities accompanied by different vegetation restoration in an open-cut iron mining area. Sci. Total Environ. 2020, 704, 135243. [Google Scholar] [CrossRef]
- Ali, S.; Xie, L. Plant growth promoting and stress mitigating abilities of soil born microorganisms. Recent Pat. Food Nutr. Agric. 2020, 11, 96–104. [Google Scholar] [CrossRef]
- Liu, H.; Ni, B.-b.; Duan, A.-g.; He, C.-y.; Zhang, J.-g. High Frankia abundance and low diversity of microbial community are associated with nodulation specificity and stability of sea buckthorn root nodule. Front. Plant Sci. 2024, 15, 1301447. [Google Scholar] [CrossRef]
- Dong, Z.-y.; Rao, M.-p.-n.; Liao, T.-J.; Li, L.; Liu, Y.-h.; Xiao, M.; Li, W.-J. Diversity and function of rhizosphere microorganisms between wild and cultivated medicinal plant Glycyrrhiza uralensis Fisch under different soil conditions. Arch. Microbiol. 2021, 203, 3657–3665. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xin, Z.; Huang, Y.; Yu, J. Climate suitability assessment on the Qinghai-Tibet Plateau. Sci. Total Environ. 2022, 816, 151653. [Google Scholar] [CrossRef] [PubMed]
- Prage, N.; Pauli, H.; Ilimer, P. Microbial Diversity in Bulk and Rhizosphere Soil of Ranunculus glacialis Along a High-Alpine Altitudinal Gradient. Front. Microbiol. 2019, 10, 1429. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Li, Z.; Meng, S.; Wu, F. Soil properties and microbial community in the rhizosphere of Populus alba var. pyramidalis along a chronosequence. Microbiol. Res. 2021, 250, 126812. [Google Scholar] [CrossRef]
- Imam, N.; Belda, I.; GarcíaJiménez, B.; Duehl, A.-j.; Doroghazi, J.-r.; Almonacid, D.-e.; Acedo, A. Local Network properties of soil and rhizosphere microbial communities in potato plantations treated with a biological product are important predictors of crop yield. mSphere 2021, 6, e0013021. [Google Scholar] [CrossRef]
- Guo, S.-y.; Ye, G.-s.; Liu, W.-j.; Liu, R.-q.; Ma, Y.-h. Environmental factors drive the biogeographic pattern of Hippophae rhamnoides root endophytic fungal diversity in the arid regions of northwest China. J. Fungi 2024, 10, 679. [Google Scholar] [CrossRef]
- Wang, W.-q.; Yang, B.; Li, X.-w.; Liang, Y.-l.; Li, J.-y. Impact of climate change on the potential geographical distribution of Hippophae rhamnoides subsp. Sinensis 2024, 35, 2813–2821. [Google Scholar]
- Riley, D.; Barber, S.-a. Bicarbonate accumulation and pH changes at the soybean (Glycine max (L.) Merr.) root-soil interface. Soil. Sci. Soc. Am. J. 1969, 33, 905–908. [Google Scholar] [CrossRef]
- Lyu, L.-y.; Gao, P.; Cai, Z.-c.; Li, F.-y.; Shi, J.-j. Topographic habitat drive the change of soil fungal community and vegetation soil characteristics in the rhizosphere of Kengyilia thoroldiana in the Sanjiangyuan Region. J. Fungi 2025, 11, 531. [Google Scholar] [CrossRef]
- Bao, S.-d. Soil Agrochemical Analysis, 3rd ed.; China Agricultural Press: Beijing, China, 2000; pp. 25–100. [Google Scholar]
- Zhao, Z.-f.; Qiu, X.-y.; Yin, J.; Zhao, R.-z.; Yang, Q.-q. Effect of fluoride pollution on the bacterial community in the water-soil system of theQingshui River Basin in Ningxia. J. Lake Sci. 2025, 38, 1–15. [Google Scholar] [CrossRef]
- Wang, R.; Liu, H.-l.; Zhu, A.-m.; Wang, Y.-x.; Ren, Z.-h.; Korea, B.-d. Effects of stocking rates on soil bacteria and carbon and nitrogen content of Artemisia frigida at different rhizosphere distances. Acta Agrestia Sin. 2024, 32, 386–395. [Google Scholar]
- Hariharan, J.; Sengupta, A.; Grewal, P.; Dick, W.-A. Functional predictions of microbial communities in soil as affected by long-term tillage practices. Agric. Environ. Lett. 2017, 2, 170031. [Google Scholar] [CrossRef]
- van de Zande, E.M.; Wantulla, M.; van Loon, J.J.; Dicke, M. Soil amendment with insect frass and exuviae affects rhizosphere bacterial community, shoot growth and carbon/nitrogen ratio of a brassicaceous plant. Plant Soil 2023, 495, 631–648. [Google Scholar] [CrossRef]
- Miao, Y.; Dan, Y.; Xuan, Y. Soil microbial communities and enzyme activities in sea-buckthorn (Hippophae rhamnoides) plantation at different ages. PLoS ONE 2018, 13, e0190959. [Google Scholar]
- Li, S.-j.; Fan, X.-y.; Cui, E.-p.; Gao, F.; Wu, H.-q.; Li, S.-s.; Cui, B.-j.; Hu, C. Effects of dripping rate with reclaimed water on typical microbial community structure in the root zone soil of Tomato. J. Irrig. Drain. 2021, 40, 26–35. [Google Scholar]
- Lu, B.; Ding, L.; Guo, F.; Chen, F.; Zhou, H.-p.; Wang, X.-s.; Dong, X.-l.; Xiang, F.-y. Effects of compound microbial fertilizer on soil nutrients and rhizosphere bacterial community in cotton fields. Crops J. 2024, 4, 209–215. [Google Scholar]
- Ilham, Z.; Mohamed, F.; Khawla, B.; Khalid, D.; Saad, I.-k.; Mohamed, I.; Guido, L.; Naïma, E.-g. Comparative assessment of physicochemical properties, functional diversity and enzymatic activities in three Opuntia ficus-indica soils across diverse climatic regions in Morocco. Sci. Afr. 2025, 28, e02663. [Google Scholar] [CrossRef]
- Guo, L.-l.; Zhang, C.-j.; Wang, F.; Shen, J.-j.; Zhang, K.-y.; He, L.-x.; Guo, Q.; Hou, X.-g. Analysis on the characteristics of rhizosphere soil bacterial community of peony wild species. J. Nanjing For. Univ. (Nat. Sci. Ed.) 2023, 47, 45–55. [Google Scholar]
- Meng, T.-t.; Shi, J.-j.; Zhang, X.-q.; Zhao, X.-q.; Zhang, D.-j.; Chen, L.-y.; Lu, Z.-y.; Cheng, Y.-c.; Hao, Y.-h.; Zhao, X.-y.; et al. Slow-release nitrogen fertilizer application regulated rhizosphere microbial diversity to increase maize yield. Front. Plant Sci. 2024, 15, 1481465. [Google Scholar] [CrossRef] [PubMed]
- Anita, B.; Malone, J.G.; Sanu, A. Diversity, detection and exploitation: Linking soil fungi and plant disease. Curr. Opin. Microbiol. 2022, 70, 102199. [Google Scholar] [CrossRef] [PubMed]
- Branco, S.; Schauster, A.; Liao, H.-l.; Ruytinx, J. Mechanisms of stress tolerance and their effects on the ecology and evolution of mycorrhizal fungi. New Phytol. 2022, 235, 2158–2175. [Google Scholar] [CrossRef]
- Sheng, M.; Chen, X.-d.; Zhang, X.-l.; Hamel, C.; Cui, X.-w.; Chen, J.; Chen, H.; Tang, M. Changes in arbuscular mycorrhizal fungal attributes along a chronosequence of black locust (Robinia pseudoacacia) plantations can be attributed to the plantation-induced variation in soil properties. Sci. Total Environ. 2017, 599–600, 273–283. [Google Scholar] [CrossRef]
- Huang, R. Diversity of Endophytic Bacteria and Allelopathic Potential of Their Metabolites in Differently Aged Casuarina Equisetifolia Roots; Hainan Normal University: Haikou, China, 2019. [Google Scholar]
- Kielak, A.-m.; Barreto, C.-c.; Kowalchuk, G.-a.; Van, V.-j.-a.; Kuramae, E.-e. The ecology of acidobacteria: Moving beyond genes and genomes. Front. Microbiol. 2016, 7, 744. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Z.-y.; Niu, J.-f.; Dang, K.-k.; Zhang, S.-k.; Wang, S.-q.; Wang, Z.-z. Changes in physicochemical properties, enzymatic activities, and the microbial community of soil significantly influence the continuous cropping of Panax quinquefolius L. (American ginseng). Plant Soil. 2021, 463, 427–446. [Google Scholar] [CrossRef]
- Li, X.-w.; Li, X.-l.; Shi, Y.; Zhao, S.-j.; Liu, J.-l.; Lin, Y.-y.; Li, C.; Zhang, C.-h. Effects of microtopography on soil microbial communities in alpine meadows on the Qinghai-Tibetan Plateau. Catena 2024, 239, 107945. [Google Scholar] [CrossRef]
- Kim, H.-s.; Lee, S.-h.; Jo, H.-y.; Finneran, K.-t.; Kwon, M.-j. Diversity and composition of soil Acidobacteria and Proteobacteria communities as a bacterial indicator of past land-use change from forest to farmland. Sci. Total Environ. 2021, 797, 148944. [Google Scholar] [CrossRef]
- Fierer, N.; Leff, J.-w.; Adams, B.-j.; Nielsen, U.-n.; Bates, S.-t.; Lauber, C.-l.; Owens, S.; Gilbert, J.-a.; Wall, D.-h.; Caporaso, J.-g. Cross-biome metagenomic analyses of soil microbial communities and their functional attributes. Proc. Natl. Acad. Sci. USA 2012, 109, 21390–21395. [Google Scholar] [CrossRef] [PubMed]
- Lauber, C.-l.; Hamady, M.; Knight, R.; Fierer, N. Pyrosequencing-based assessment of soil pH as a predictor of soil bacterial community structure at the continental scale. Appl. Environ. Microbiol. 2009, 75, 5111–5120. [Google Scholar] [CrossRef] [PubMed]
- Dao, R.-n.; Zhang, Y.; Li, X.-l.; Li, Q.; Ma, L.-x; Tie, X.-l. Impact of alpine wetland succession to soil bacterial diversity. Environ. Sci. 2025, 46, 1897–1904. [Google Scholar]
- Siciliano, S.-d.; Palmer, A.-s.; Winsley, T.; Lamb, E.; Bissett, A.; Brown, M.-v.; Dorst, J.-v.; Ji, M.-k.; Ferrari, B.-c.; Grogan, P.; et al. Soil fertility is associated with fungal and bacterial richness, whereas pH is associated with community composition in polar soil microbial communities. Soil. Biol. Biochem. 2014, 78, 10–20. [Google Scholar] [CrossRef]
- Liu, J.-j.; Sui, Y.-y.; Yu, Z.-h.; Shi, Y.; Chu, H.-y.; Jin, j.; Liu, X.-b.; Wang, G.-h. High throughput sequencing analysis of biogeographical distribution of bacterial communities in the black soils of northeast China. Soil. Biol. Biochem. 2014, 70, 113–122. [Google Scholar] [CrossRef]
- Wu, Y.-p.; Ma, B.; Zhou, L.; Wang, H.-z.; Xu, J.-m.; Kemmitt, S.; Brookes, P.-c. Changes in the soil microbial community structure with latitude in eastern China, based on phospholipid fatty acid analysis. Appl. Soil. Ecol. 2009, 43, 234–240. [Google Scholar] [CrossRef]
- Judy, S.; Michael, D.; Rodica, P.; Arthur, G.; Heinz, R. Nitrogen nutrition of beech forests in a changing climate: Importance of plant-soil-microbe water, carbon, and nitrogen interactions. Plant Soil. 2017, 418, 89–114. [Google Scholar]
- Rousk, J.; Bååth, E.; Brookes, P.-c.; Lauber, C.-l.; Lozupone, c.; Caporaso, J.-g.; Knight, R.; Fierer, N. Soil bacterial and fungal communities across a pH gradient in an arable soil. ISME J. 2010, 4, 1340–1351. [Google Scholar] [CrossRef]
- Han, Y.-f.; Dong, C.-b.; Ge, W.; Zou, X.; Liang, J.-d.; Chen, W.-h.; Hu, H.-y.; Liang, Z.-q. Research progress on microbial composition, assembly and microbial interaction of symbiotic functional bodies. J. Mt. Agric. Biol. 2022, 41, 1–12. [Google Scholar]
- Tripathi, B.-m.; Kim, M.; Singh, D.; Lee, C.-l.; Lai, H.-a.; Ainuddin, A.-n.; Go, R.; Rahim, R.; Husni, M.-h.-a.; Chun, J.; et al. Tropical soil bacterial communities in Malaysia: pH dominates in the equatorial tropics too. Microb. Ecol. 2012, 64, 474–484. [Google Scholar] [CrossRef]
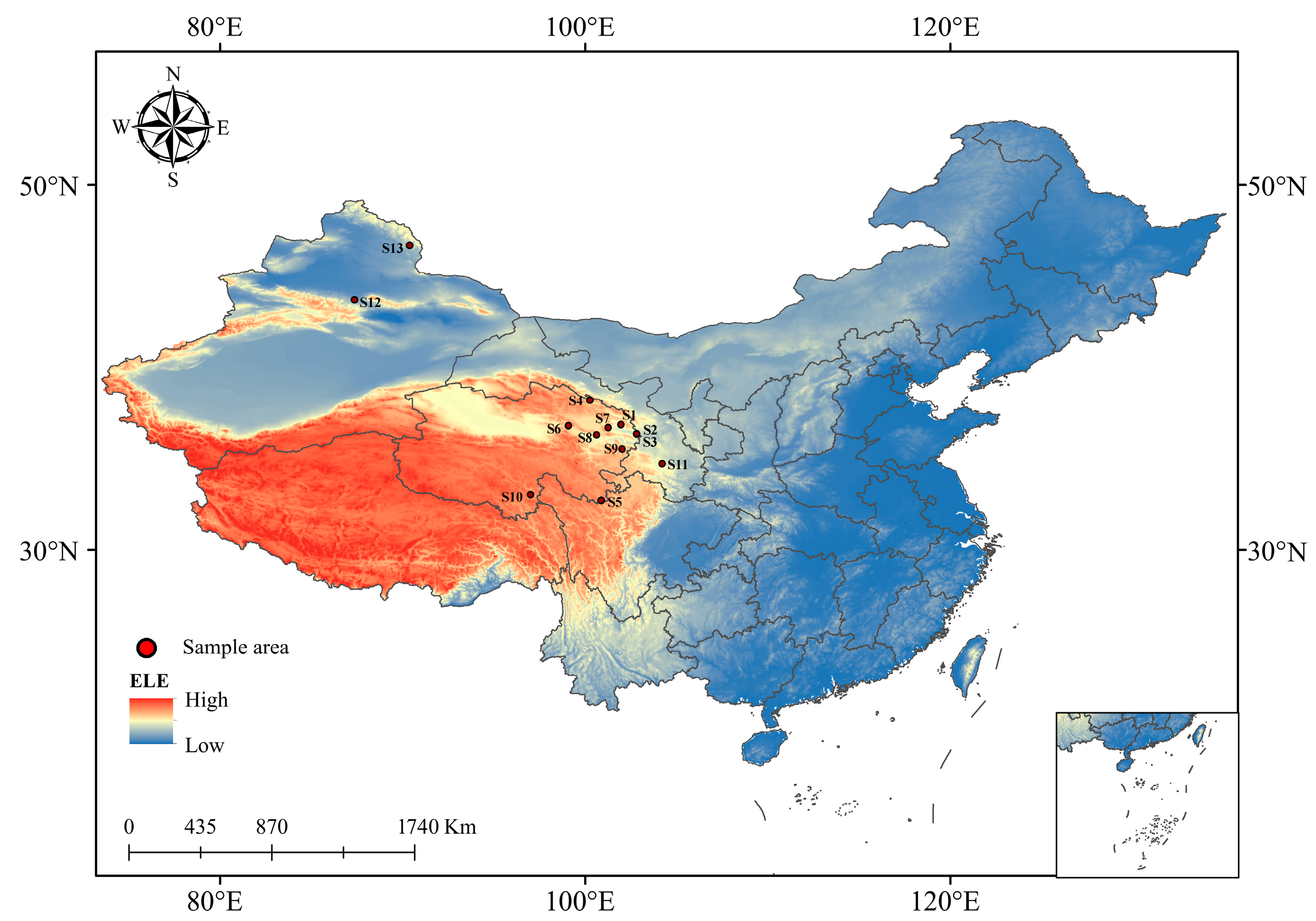





| Number | Sampling Location | East/° | North/° | Altitude (ALT)/m | Air Pressure (ATM)/KPa | Average Annual Temperature (AAT)/℃ | Average Annual Rainfall (AAR)/mm |
|---|---|---|---|---|---|---|---|
| S1 | Huzhu Tuzu Autonomous County | 101.96 | 36.84 | 2696.31 | 71.39 | 1.72 | 126.09 |
| S2 | Minhe Huizu and Tuzu Autonomous County | 102.83 | 36.32 | 2644.83 | 73.94 | 5.46 | 101.83 |
| S3 | Ledu District | 102.83 | 36.33 | 2753.57 | 72.66 | 1.72 | 126.09 |
| S4 | Qilian County | 100.25 | 38.18 | 2920.81 | 71.50 | −0.02 | 124.21 |
| S5 | Banma County | 100.87 | 32.71 | 3441.00 | 61.22 | 1.93 | 158.83 |
| S6 | Wulan County | 99.08 | 36.79 | 3159.06 | 70.10 | 1.36 | 76.41 |
| S7 | Huangyuan County | 101.26 | 36.68 | 2923.11 | 71.71 | 1.76 | 122.61 |
| S8 | Gonghe County | 100.62 | 36.28 | 2947.60 | 71.39 | 3.47 | 102.03 |
| S9 | Tongren City | 102.02 | 35.52 | 2387.93 | 74.40 | 5.53 | 114.66 |
| S10 | Yushu City | 97.01 | 33.01 | 3665.00 | 65.80 | 1.93 | 132.35 |
| S11 | Zhang County | 104.22 | 34.72 | 2725.20 | 73.26 | 4.51 | 124.83 |
| S12 | Urumqi County | 87.36 | 43.69 | 1562.50 | 82.73 | 3.72 | 63.72 |
| S13 | Qinghe County | 90.38 | 46.68 | 1230.00 | 86.43 | 2.21 | 29.04 |
| Number | SOM/(g/kg) | STN/(g/kg) | STP/(g/kg) | STK/(g/kg) | SAN/(mg/kg) | SAP/(mg/kg) | SAK/(mg/kg) | SWC/(%) | pH | SEC/(ms/cm) |
|---|---|---|---|---|---|---|---|---|---|---|
| S1 | 56.77 | 3.05 | 0.75 | 15.49 | 225.55 | 6.74 | 39.07 | 14.52 | 7.60 | 0.580 |
| S2 | 26.70 | 1.79 | 0.64 | 19.05 | 106.05 | 2.10 | 67.98 | 11.09 | 8.07 | 0.547 |
| S3 | 95.35 | 5.97 | 0.99 | 20.60 | 413.27 | 2.85 | 157.39 | 32.74 | 7.53 | 0.623 |
| S4 | 41.77 | 2.55 | 0.63 | 18.34 | 181.03 | 1.20 | 47.38 | 7.21 | 7.99 | 0.563 |
| S5 | 42.65 | 2.61 | 0.59 | 17.95 | 198.99 | 3.07 | 21.16 | 23.88 | 7.65 | 0.683 |
| S6 | 9.04 | 0.90 | 0.56 | 18.33 | 46.43 | 3.70 | 87.48 | 4.22 | 7.92 | 0.603 |
| S7 | 38.03 | 2.14 | 0.75 | 16.08 | 157.39 | 7.98 | 85.09 | 16.96 | 8.39 | 0.663 |
| S8 | 22.73 | 1.65 | 0.59 | 17.97 | 98.34 | 1.25 | 215.70 | 17.63 | 6.85 | 0.613 |
| S9 | 40.60 | 2.22 | 0.68 | 17.21 | 124.07 | 2.70 | 139.98 | 7.44 | 8.05 | 0.603 |
| S10 | 27.46 | 1.83 | 0.53 | 18.27 | 127.15 | 6.11 | 90.20 | 3.72 | 8.08 | 0.513 |
| S11 | 98.58 | 5.00 | 1.17 | 18.55 | 397.37 | 3.39 | 42.63 | 28.40 | 6.46 | 0.603 |
| S12 | 49.16 | 3.30 | 0.79 | 19.76 | 232.10 | 7.16 | 153.40 | 7.22 | 7.89 | 0.617 |
| S13 | 9.45 | 0.77 | 1.25 | 18.39 | 29.04 | 5.73 | 144.20 | 8.25 | 8.31 | 0.537 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, P.; Ye, G.; Guo, S.; Ma, Y.; Zhang, Y.; Sun, S.; Guo, L.; San, H.; Liu, W.; Ren, Q.; et al. Environmental Factors Drive the Changes of Bacterial Structure and Functional Diversity in Rhizosphere Soil of Hippophae rhamnoides subsp. sinensis Rousi in Arid Regions of Northwest China. Microorganisms 2025, 13, 1860. https://doi.org/10.3390/microorganisms13081860
Gao P, Ye G, Guo S, Ma Y, Zhang Y, Sun S, Guo L, San H, Liu W, Ren Q, et al. Environmental Factors Drive the Changes of Bacterial Structure and Functional Diversity in Rhizosphere Soil of Hippophae rhamnoides subsp. sinensis Rousi in Arid Regions of Northwest China. Microorganisms. 2025; 13(8):1860. https://doi.org/10.3390/microorganisms13081860
Chicago/Turabian StyleGao, Pei, Guisheng Ye, Siyu Guo, Yuhua Ma, Yongyi Zhang, Sixuan Sun, Lin Guo, Hongyuan San, Wenjie Liu, Qingcuo Ren, and et al. 2025. "Environmental Factors Drive the Changes of Bacterial Structure and Functional Diversity in Rhizosphere Soil of Hippophae rhamnoides subsp. sinensis Rousi in Arid Regions of Northwest China" Microorganisms 13, no. 8: 1860. https://doi.org/10.3390/microorganisms13081860
APA StyleGao, P., Ye, G., Guo, S., Ma, Y., Zhang, Y., Sun, S., Guo, L., San, H., Liu, W., Ren, Q., Wang, S., & Peng, R. (2025). Environmental Factors Drive the Changes of Bacterial Structure and Functional Diversity in Rhizosphere Soil of Hippophae rhamnoides subsp. sinensis Rousi in Arid Regions of Northwest China. Microorganisms, 13(8), 1860. https://doi.org/10.3390/microorganisms13081860






