Multi-Strain Probiotic Regulates the Intestinal Mucosal Immunity and Enhances the Protection of Piglets Against Porcine Epidemic Diarrhea Virus Challenge
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells, Bacteria, and Virus
2.2. Growth Curves and Acid Production Analysis
2.3. Validation of Synergistic Effects in Multi-Strain Probiotic
2.4. Acid and Bile Salt Tolerance Assay
2.5. Simulated Gastrointestinal Fluid Tolerance Assay
2.6. Antimicrobial Activity Assay
2.7. Electron Microscopic Examination of Lactic-Acid-Bacteria–Pathogen Interactions
2.8. In Vitro Bactericidal Activity Assay
2.9. Anti-Proliferative Effect of Multi-Strain Probiotic Against PEDV
2.10. Animals and Experimental Design
2.11. Detection Indicators and Methods
2.11.1. RNA Extraction and Quantitative PCR Analysis
2.11.2. Histopathological Analysis
2.11.3. AB-PAS Staining of the Small Intestine
2.11.4. Electron Microscopic Examination of Small Intestinal Tissues
2.12. Statistical Analysis
3. Results
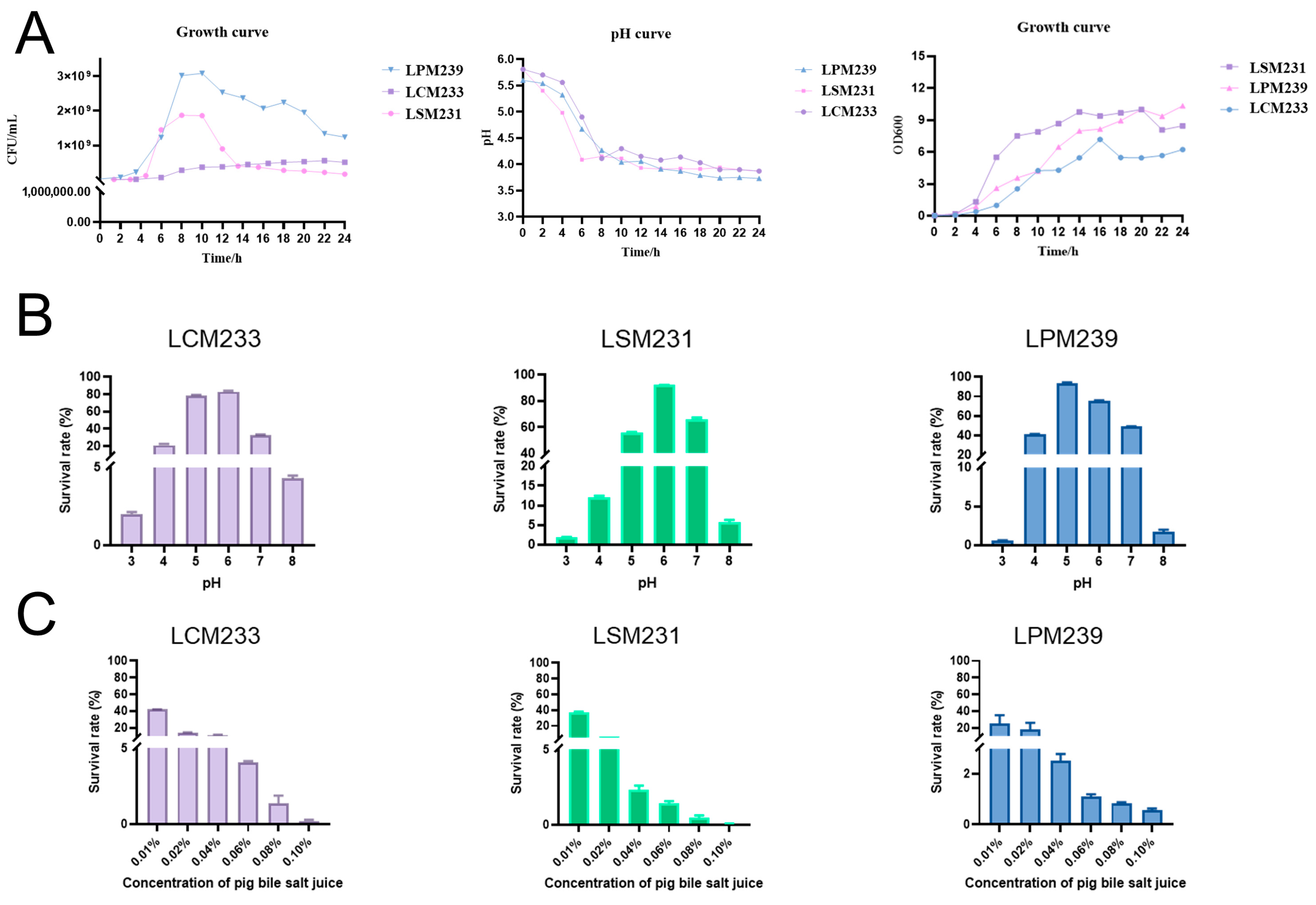
3.1. Biological Characterization of Probiotics Isolated from Min Pigs
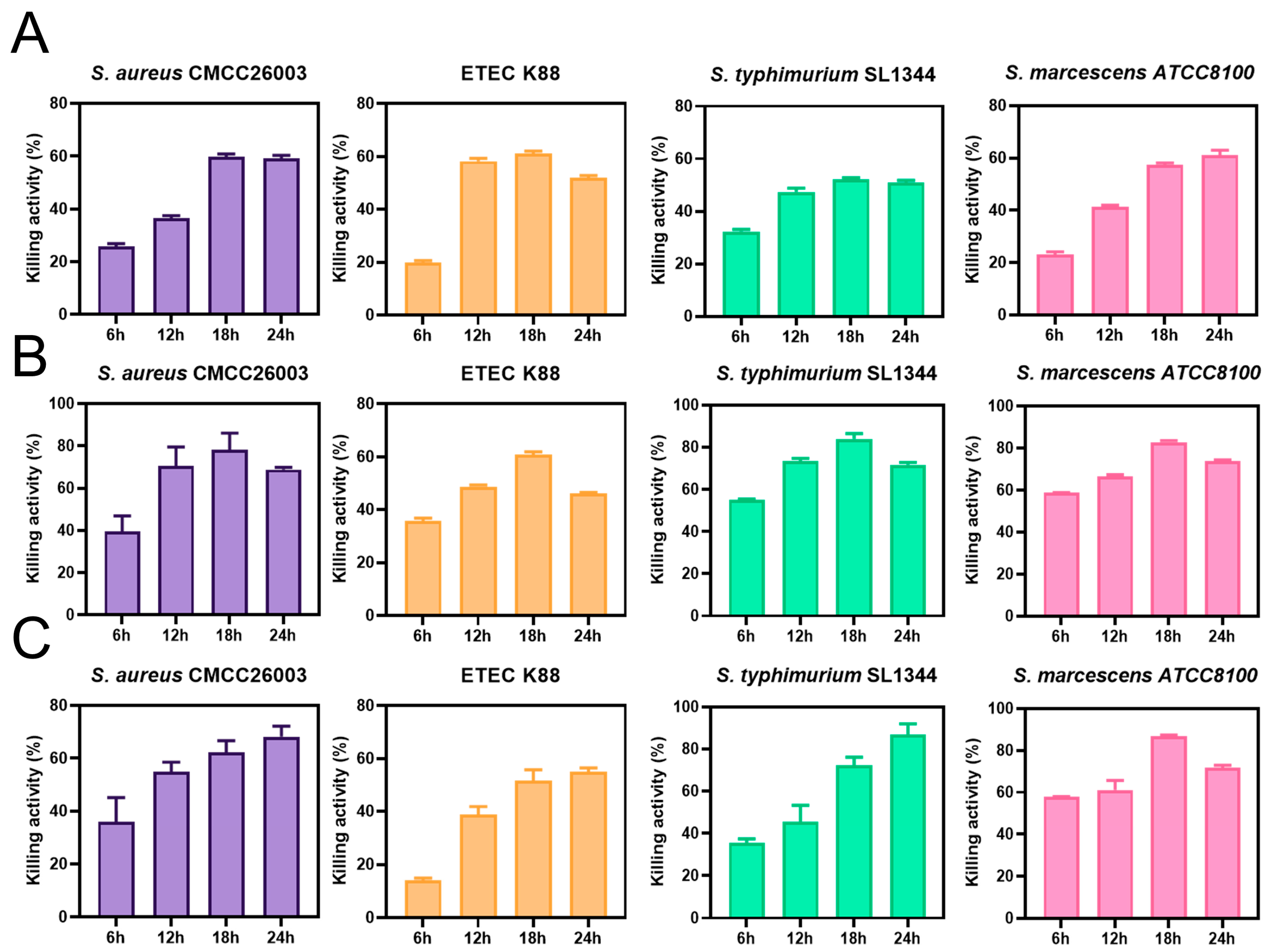
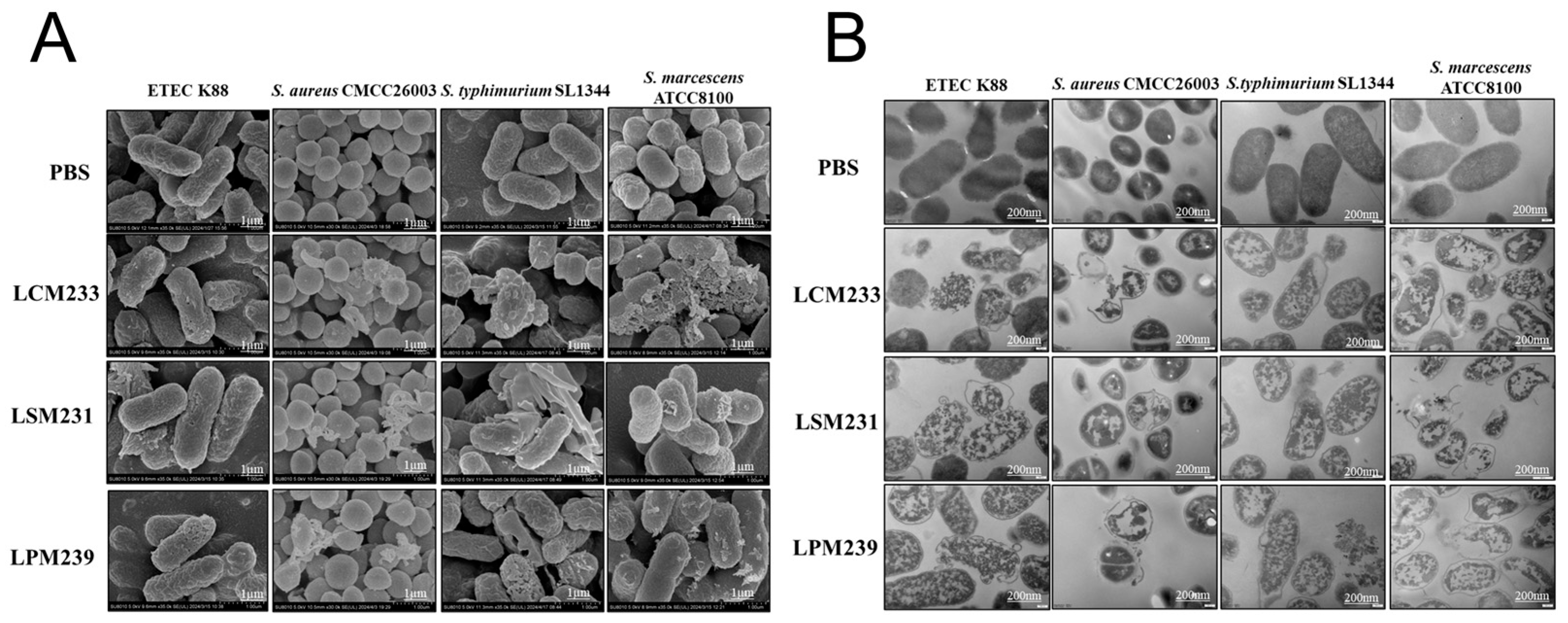
3.2. Antibacterial Activity of LSM231, LCM233, and LPM239
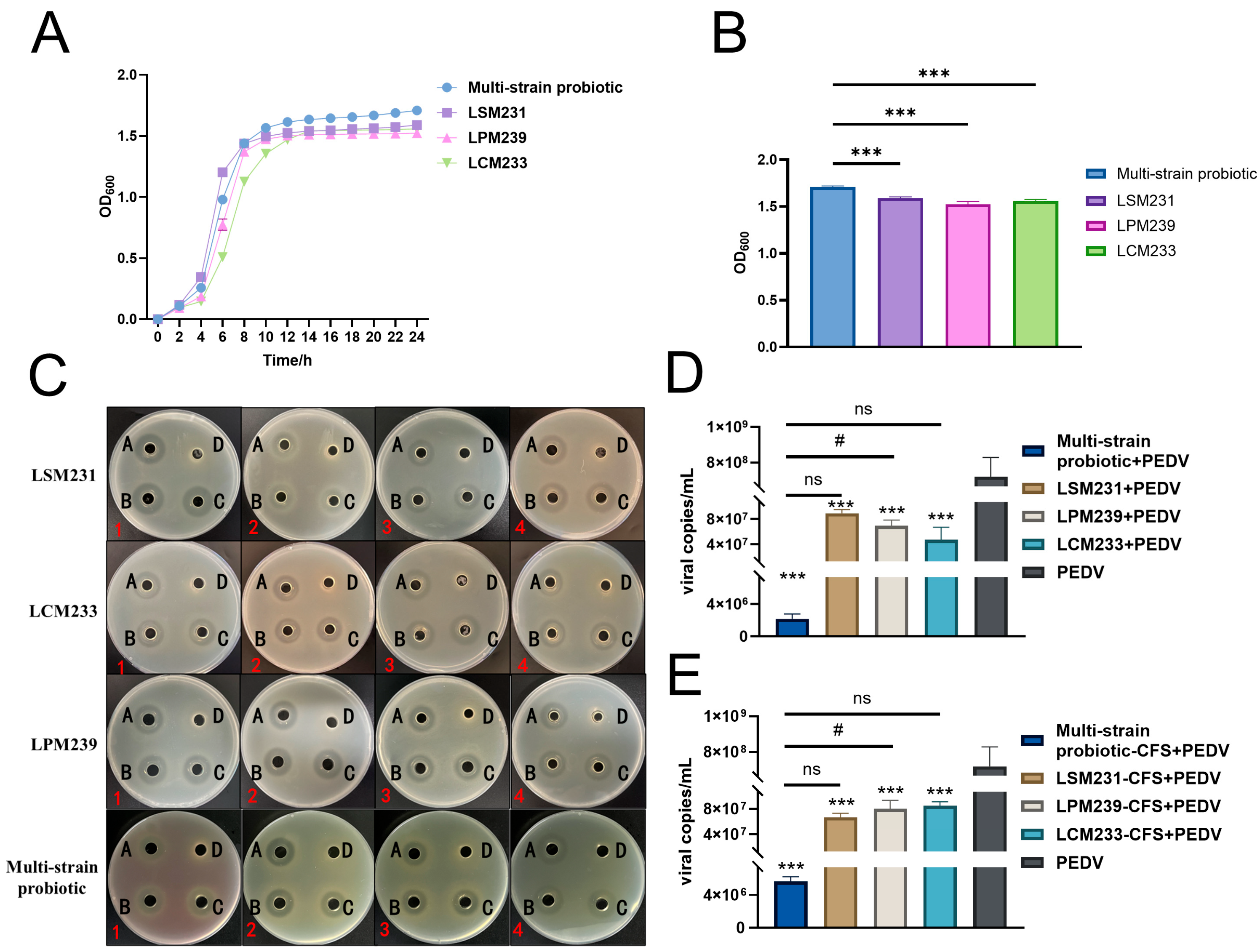
3.3. Synergistic Antimicrobial and Antiviral Activities of Multi-Strain Probiotic
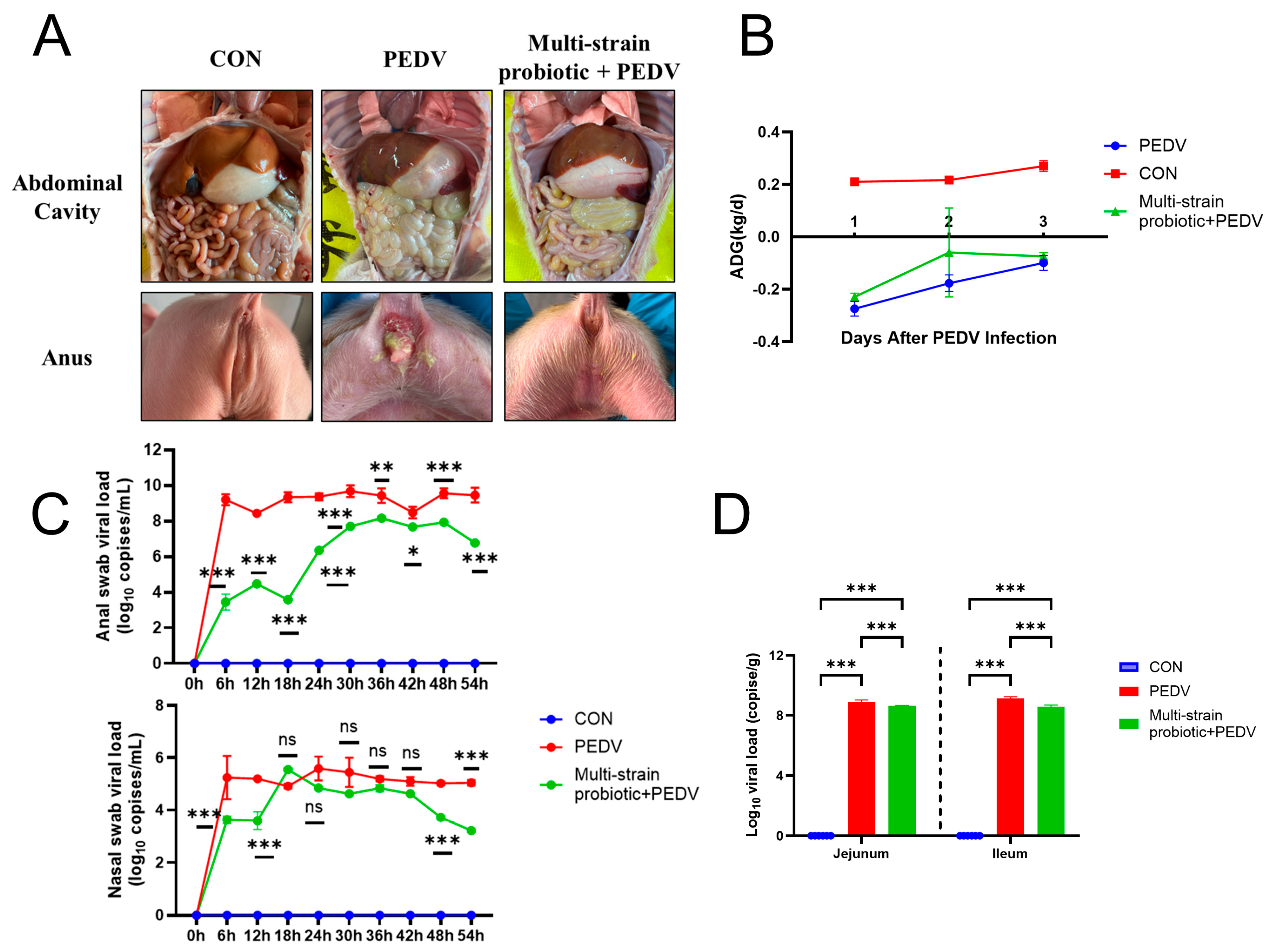
3.4. Multi-Strain Probiotic Provided Protection to Piglets Against PEDV Infection
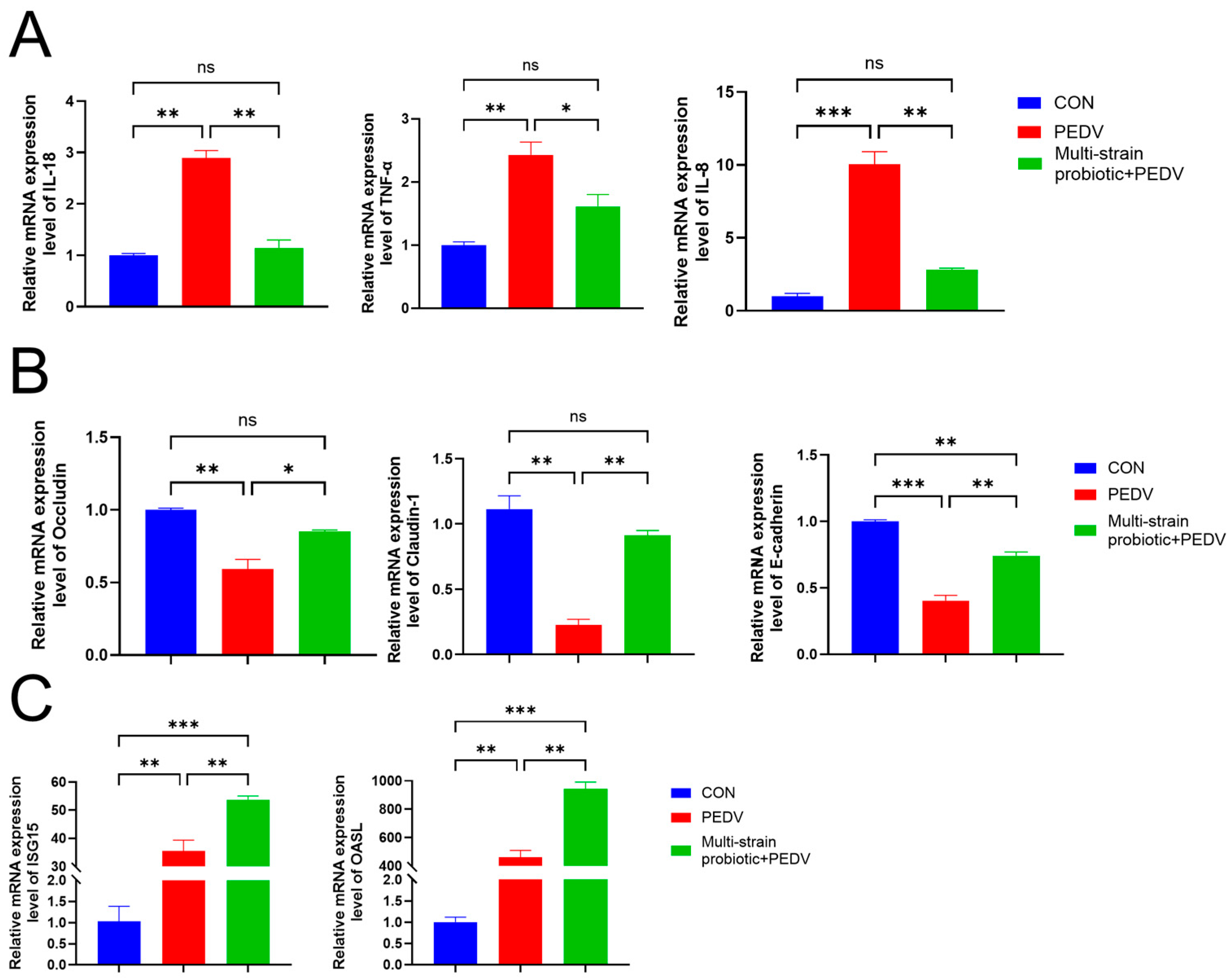
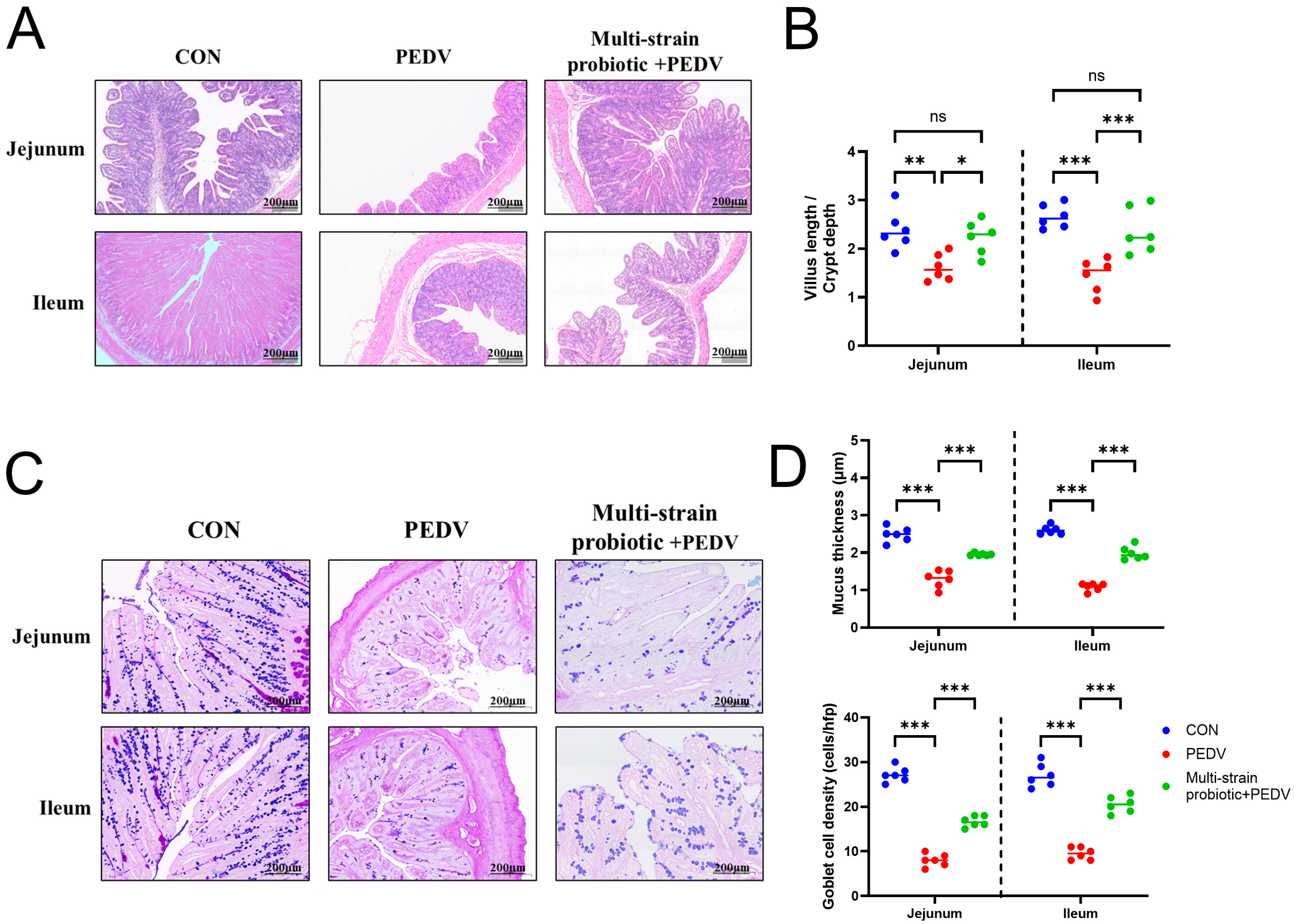
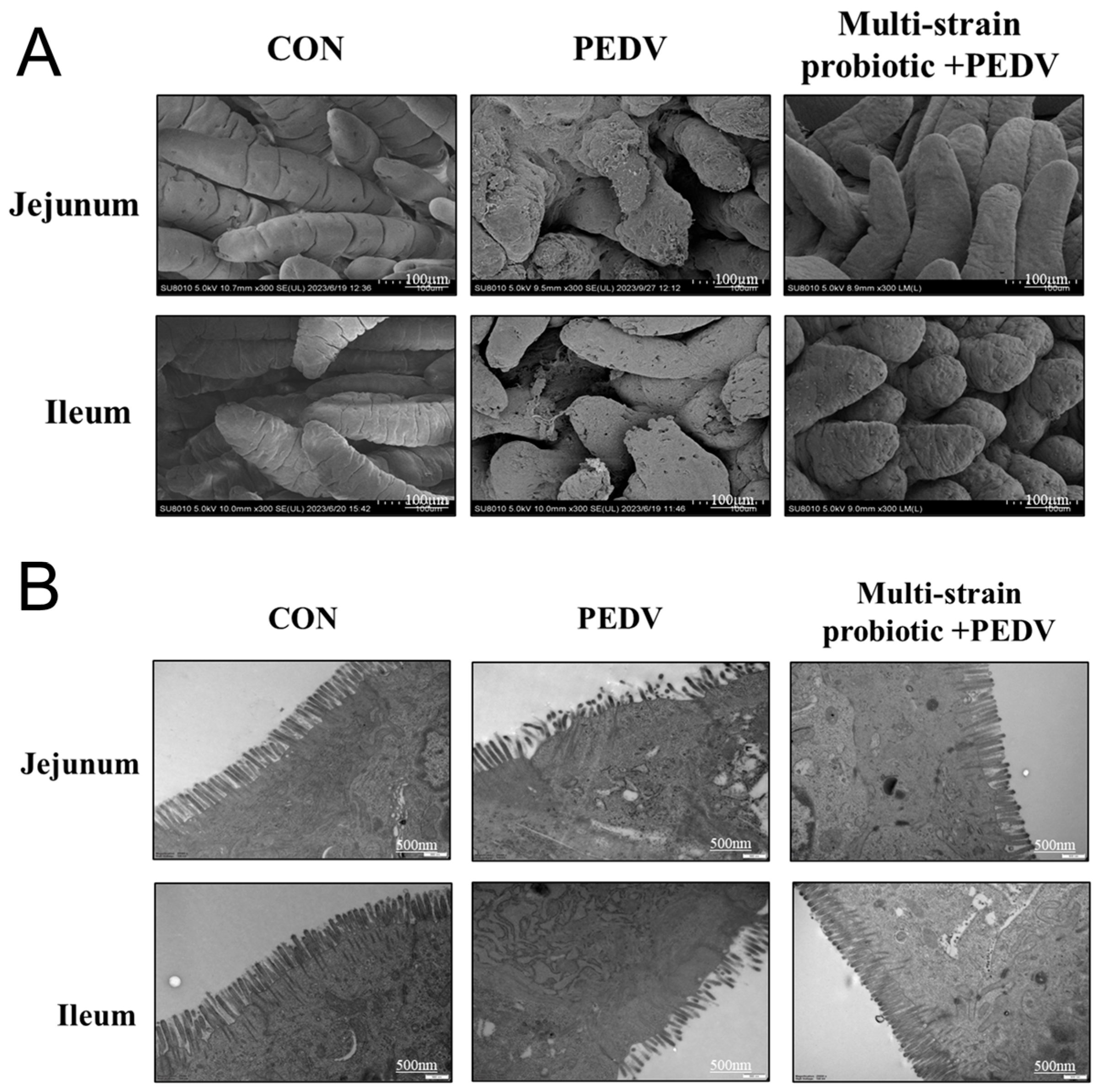
3.5. Multi-Strain Probiotic Protected from Intestinal Damage During PEDV Infection
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jung, K.; Saif, L.J.; Wang, Q. Porcine epidemic diarrhea virus (PEDV): An update on etiology, transmission, pathogenesis, and prevention and control. Virus Res. 2020, 286, 198045. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Fan, B.; Song, X.; Gao, J.; Guo, R.; Yi, C.; He, Z.; Hu, H.; Jiang, J.; Zhao, L.; et al. PEDV-spike-protein-expressing mRNA vaccine protects piglets against PEDV challenge. mBio 2024, 15, e0295823. [Google Scholar] [CrossRef] [PubMed]
- Gallien, S.; Le Poder, S.; Rose, N.; Grasland, B. Porcine epidemic diarrhea: The return of an old disease. Virologie 2016, 20, 335–351. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Fan, X.; Shen, H.; Gou, H.; Zhang, C.; Liu, Z.; Zhang, B.; Wuri, N.; Zhang, J.; Liao, M.; et al. Butyrate limits the replication of porcine epidemic diarrhea virus in intestine epithelial cells by enhancing GPR43-mediated IFN-III production. Front. Microbiol. 2023, 14, 1091807. [Google Scholar] [CrossRef] [PubMed]
- Park, J.E. Porcine Epidemic Diarrhea: Insights and Progress on Vaccines. Vaccines 2024, 12, 212. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, J.; Tian, W.; You, J.; Chi, X.; Wang, X. Phospho-eIF4E stimulation regulates coronavirus entry by selective expression of cell membrane-residential factors. J. Virol. 2024, 98, e0194823. [Google Scholar] [CrossRef] [PubMed]
- Zaccaria, E.; Klaassen, T.; Alleleyn, A.; Boekhorst, J.; Smokvina, T.; Kleerebezem, M.; Troost, F.J. Endogenous small intestinal microbiome determinants of transient colonisation efficiency by bacteria from fermented dairy products: A randomised controlled trial. Microbiome 2023, 11, 43. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Bugenyi, A.W.; Lee, M.R.; Choi, Y.J.; Song, K.D.; Lee, H.K.; Son, Y.O.; Lee, D.S.; Lee, S.C.; Son, Y.J.; et al. High-quality metagenome-assembled genomes from proximal colonic microbiomes of synbiotic-treated korean native black pigs reveal changes in functional capacity. Sci. Rep. 2022, 12, 14595. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Chen, Z.; Xie, L.; Yang, S.; Li, Y.; Wu, J.; Wu, Y.; Li, S.; Zhang, X.; Ma, Y.; et al. Lactobacillus plantarum ST-III culture supernatant protects against acute alcohol-induced liver and intestinal injury. Aging 2023, 16, 2077–2089. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Ding, C.; Chi, C.; Liu, S.; Gao, Z.; Sun, W.; Zhao, H.; Song, S. Lactobacillus crispatus 7-4 Mitigates Salmonella typhimurium-Induced Enteritis via the gamma-Glutamylcysteine-Mediated Nrf2 Pathway. Probiotics Antimicrob. Proteins 2024. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Zhang, Q.; Wu, M.; Zhang, Y.; Li, Z.; Li, H.; Yu, C.; Zhang, X.; Zhao, D.; Wang, L.; et al. Lactobacillus rhamnosus GG powder supplementation alleviates intestinal injury in piglets challenged by porcine epidemic diarrhea virus. Front. Cell Infect. Microbiol. 2024, 14, 1371916. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Yu, Q.; Xie, L.; Ran, L.; Wang, K.; Yang, Y.; Gan, L.; Song, Z. Inhibitory effects of Lactobacillus plantarum metabolites on porcine epidemic diarrhea virus replication. Res. Vet. Sci. 2021, 139, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.M.; Limaye, A.; Chang, H.W.; Liu, J.R. Screening of Lactic Acid Bacterial Strains with Antiviral Activity Against Porcine Epidemic Diarrhea. Probiotics Antimicrob. Proteins 2022, 14, 546–559. [Google Scholar] [CrossRef] [PubMed]
- Maniya, H.; Modasiya, I.; Chauhan, M.; Mori, P.; Kumar, V. Developing Robust Probiotic Consortia: A Methodological Optimization Approach. Curr. Microbiol. 2024, 81, 407. [Google Scholar] [CrossRef] [PubMed]
- Kwoji, I.D.; Okpeku, M.; Aiyegoro, O.A.; Adeleke, M.A. Metabolic interactions of LimosiLactobacillus reuteri ZJ625 and LigiLactobacillus salivarius ZJ614 in co-culture: Implications for multi-strain probiotics. J. Appl. Microbiol. 2024, 135, lxae264. [Google Scholar] [CrossRef] [PubMed]
- Boranbayeva, G.; Tekebayeva, Z.; Temirkhanov, A.; Temirbekova, A.; Yevneyeva, D.; Abilkhadirov, A.; Mkilima, T.; Abzhalelov, A. Probiotic consortium from poultry strains for supporting gut immunity against pathogens. Microb. Pathog. 2025, 204, 107584. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; You, Y.; Cha, S.; Kim, T.R.; Sohn, M.; Park, J. Multi-Species Probiotic Strain Mixture Enhances Intestinal Barrier Function by Regulating Inflammation and Tight Junctions in Lipopolysaccharides Stimulated Caco-2 Cells. Microorganisms 2023, 11, 656. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.H.; Niu, T.M.; Zou, B.S.; Yang, G.L.; Shi, C.W.; Yan, Q.S.; Sun, M.J.; Yu, T.; Zhang, S.M.; Feng, X.Z.; et al. Gut microbiota-derived LCA mediates the protective effect of PEDV infection in piglets. Microbiome 2024, 12, 20. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Ngo, H.H.; Guo, W.; Lee, D.; Nghiem, D.L.; Zhang, J.; Liang, S.; Varjani, S.; Wang, J. Performance of microbial fuel cell for treating swine wastewater containing sulfonamide antibiotics. Bioresour. Technol. 2020, 311, 123588. [Google Scholar] [CrossRef] [PubMed]
- Niu, D.; Feng, N.; Xi, S.; Xu, J.; Su, Y. Genomics-based analysis of four porcine-derived lactic acid bacteria strains and their evaluation as potential probiotics. Mol. Genet. Genom. 2024, 299, 24. [Google Scholar] [CrossRef] [PubMed]
- Mo, J.; Lu, Y.; Jiang, S.; Yan, G.; Xing, T.; Xu, D.; He, Y.; Xie, B.; Lan, G.; Chen, B.; et al. Effects of the Probiotic, Lactobacillus delbrueckii subsp. bulgaricus, as a Substitute for Antibiotics on the Gastrointestinal Tract Microbiota and Metabolomics Profile of Female Growing-Finishing Pigs. Animals 2022, 12, 1778. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Sun, C.; Li, F.; Shan, A.; Shi, B. Characteristics of faecal bacterial flora and volatile fatty acids in Min pig, Landrace pig, and Yorkshire pig. Electron. J. Biotechn. 2021, 53, 33–43. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, X.; Yu, W.; Lin, W.; Wei, W.; Zhang, L.; Liu, D.; Ma, H.; Chen, J. Differential expression of ADRB1 causes different responses to norepinephrine in adipocytes of Duroc-Landrace-Yorkshire pigs and min pigs. J. Therm. Biol. 2024, 123, 103906. [Google Scholar] [CrossRef] [PubMed]
- Giordano, I.; Mauriello, G. Ultrasound Attenuation Improves Some Surface Properties of the Probiotic Strain Lacticaseibacillus casei ATCC 393. Microorganisms 2023, 11, 142. [Google Scholar] [CrossRef] [PubMed]
- Perkovic, L.; Djedovic, E.; Vujovic, T.; Bakovic, M.; Paradzik, T.; Coz-Rakovac, R. Biotechnological Enhancement of Probiotics through Co-Cultivation with Algae: Future or a Trend? Mar. Drugs 2022, 20, 142. [Google Scholar] [CrossRef] [PubMed]
- Abramov, V.M.; Kosarev, I.V.; Priputnevich, T.V.; Machulin, A.V.; Khlebnikov, V.S.; Pchelintsev, S.Y.; Vasilenko, R.N.; Sakulin, V.K.; Suzina, N.E.; Chikileva, I.O.; et al. S-layer protein 2 of Lactobacillus crispatus 2029, its structural and immunomodulatory characteristics and roles in protective potential of the whole bacteria against foodborne pathogens. Int. J. Biol. Macromol. 2020, 150, 400–412. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Li, Z.; Gu, X.; Zhao, J.; Guo, T.; Kong, J. Probiotic Bacillus subtilis LF11 Protects Intestinal Epithelium Against Salmonella Infection. Front. Cell Infect. Microbiol. 2022, 12, 837886. [Google Scholar] [CrossRef] [PubMed]
- Piewngam, P.; Zheng, Y.; Nguyen, T.H.; Dickey, S.W.; Joo, H.S.; Villaruz, A.E.; Glose, K.A.; Fisher, E.L.; Hunt, R.L.; Li, B.; et al. Pathogen elimination by probiotic Bacillus via signalling interference. Nature 2018, 562, 532–537. [Google Scholar] [CrossRef] [PubMed]
- Amaretti, A.; di Nunzio, M.; Pompei, A.; Raimondi, S.; Rossi, M.; Bordoni, A. Antioxidant properties of potentially probiotic bacteria: In vitro and in vivo activities. Appl. Microbiol. Biotechnol. 2013, 97, 809–817. [Google Scholar] [CrossRef] [PubMed]
- Zampieri, G.; Efthimiou, G.; Angione, C. Multi-dimensional experimental and computational exploration of metabolism pinpoints complex probiotic interactions. Metab. Eng. 2023, 76, 120–132. [Google Scholar] [CrossRef] [PubMed]
- Phaengphairee, P.; Boontiam, W.; Wealleans, A.; Hong, J.; Kim, Y.Y. Dietary supplementation with full-fat Hermetia illucens larvae and multi-probiotics, as a substitute for antibiotics, improves the growth performance, gut health, and antioxidative capacity of weaned pigs. BMC Vet. Res. 2023, 19, 7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jiang, Z.; Zhou, Z.; Sun, J.; Yan, S.; Gao, W.; Shao, Y.; Bai, Y.; Wu, Y.; Yan, Z.; et al. A TaqMan Probe-Based Multiplex Real-Time PCR for Simultaneous Detection of Porcine Epidemic Diarrhea Virus Subtypes G1 and G2, and Porcine Rotavirus Groups A and C. Viruses 2022, 14, 1819. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Li, Y.; Wang, C.; Zhao, Y.; Yang, S.; Guo, R.; Hu, M.; Sun, M.; Zhang, G.; Li, Y.; et al. Efficacy evaluation of a bivalent subunit vaccine against epidemic PEDV heterologous strains with low cross-protection. J. Virol. 2024, 98, e0130924. [Google Scholar] [CrossRef] [PubMed]
- Azad, M.; Sarker, M.; Wan, D. Immunomodulatory Effects of Probiotics on Cytokine Profiles. Biomed. Res. Int. 2018, 2018, 8063647. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Dong, J.; Wang, Y.; Zhang, P.; Liu, Y.; Zhang, L.; Liang, P.; Wang, L.; Song, C. Porcine epidemic diarrhea virus nsp4 induces pro-inflammatory cytokine and chemokine expression inhibiting viral replication in vitro. Arch. Virol. 2019, 164, 1147–1157. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Xiao, Y.; Zhang, X.; Zhu, Z.; Zhang, H.; Wei, J.; Zhao, Z.; Li, J.; Chen, T. Probiotics suppress LL37 generated rosacea-like skin inflammation by modulating the TLR2/MyD88/NF-kappaB signaling pathway. Food Funct. 2024, 15, 8916–8934. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xie, X.; Sun, N.; Liu, X.; Liu, J.; Zhang, W.; Cao, Y. Gut microbiota-derived butyrate improved acute leptospirosis in hamster via promoting macrophage ROS mediated by HDAC3 inhibition. mBio 2024, 15, e0190624. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.J.; Tsai, C.F.; Hsu, C.W.; Chang, H.W.; Liu, J.R. In Vitro and In Vivo Evaluation of Bacillus Strains as Prophylactic Agents Against Porcine Epidemic Diarrhea Virus. Animals 2025, 15, 470. [Google Scholar] [CrossRef] [PubMed]
- Saleem, W.; Ren, X.; Van Den Broeck, W.; Nauwynck, H. Changes in intestinal morphology, number of mucus-producing cells and expression of coronavirus receptors APN, DPP4, ACE2 and TMPRSS2 in pigs with aging. Vet. Res. 2023, 54, 34. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Yang, L.; Chu, H. The Gut Microbiota: A Novel Player in Autoimmune Hepatitis. Front. Cell Infect. Microbiol. 2022, 12, 947382. [Google Scholar] [CrossRef] [PubMed]
- Castro-Mejia, J.L.; O’Ferrall, S.; Krych, L.; O’Mahony, E.; Namusoke, H.; Lanyero, B.; Kot, W.; Nabukeera-Barungi, N.; Michaelsen, K.F.; Molgaard, C.; et al. Restitution of gut microbiota in Ugandan children administered with probiotics (Lactobacillus rhamnosus GG and Bifidobacterium animalis subsp. lactis BB-12) during treatment for severe acute malnutrition. Gut Microbes 2020, 11, 855–867. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Liu, M.; Yue, Z.; Chen, X.; Li, C.; Liu, L.; Li, F. Combined Omics Analysis Further Unveils the Specific Role of Butyrate in Promoting Growth in Early-Weaning Animals. Int. J. Mol. Sci. 2023, 24, 1787. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Chi, K.; Yang, M.; Guo, N. Staphylococcal Enterotoxin A Induces Intestinal Barrier Dysfunction and Activates NLRP3 Inflammasome via NF-kappaB/MAPK Signaling Pathways in Mice. Toxins 2022, 14, 29. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Xiong, K. Dietary Epidermal Growth Factor Supplementation Alleviates Intestinal Injury in Piglets with Intrauterine Growth Retardation via Reducing Oxidative Stress and Enhancing Intestinal Glucose Transport and Barrier Function. Animals 2022, 12, 2245. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Tian, G.; Chen, D.; Zheng, P.; Yu, J.; Mao, X.; He, J.; Luo, Y.; Luo, J.; Huang, Z.; et al. Dietary 25-Hydroxyvitamin D(3) Supplementation Alleviates Porcine Epidemic Diarrhea Virus Infection by Improving Intestinal Structure and Immune Response in Weaned Pigs. Animals 2019, 9, 627. [Google Scholar] [CrossRef] [PubMed]
- Cristofori, F.; Dargenio, V.N.; Dargenio, C.; Miniello, V.L.; Barone, M.; Francavilla, R. Anti-Inflammatory and Immunomodulatory Effects of Probiotics in Gut Inflammation: A Door to the Body. Front. Immunol. 2021, 12, 578386. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Chen, J.; Xiang, Y.; Chen, Y.; Shen, W.; Wang, W.; Li, Y.; Wei, P.; He, X. Differential Modulation of Innate Antiviral Profiles in the Intestinal Lamina Propria Cells of Chickens Infected with Infectious Bursal Disease Viruses of Different Virulence. Viruses 2022, 14, 393. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Liu, H.; Tian, Z.; Kay, M.; Wang, H.; Cheng, L.; Xia, W.; Zhang, J.; Wang, W.; Cao, H.; et al. Porcine epidemic diarrhea virus (PEDV) ORF3 protein inhibits cellular type I interferon signaling through down-regulating proteins expression in RLRs-mediated pathway. Res. Vet. Sci. 2023, 159, 146–159. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Shen, H.; Gou, H.; Wuri, N.; Zhang, C.; Liu, Z.; He, H.; Nie, J.; Qu, Y.; Geri, L.; et al. Isolation of LimosiLactobacillus mucosae G01 with inhibitory effects on porcine epidemic diarrhea virus in vitro from Bama pig gastroenteritis. Front. Microbiol. 2024, 15, 1360098. [Google Scholar] [CrossRef] [PubMed]
- Weiss, G.; Rasmussen, S.; Zeuthen, L.H.; Nielsen, B.N.; Jarmer, H.; Jespersen, L.; Frokiaer, H. Lactobacillus acidophilus induces virus immune defence genes in murine dendritic cells by a Toll-like receptor-2-dependent mechanism. Immunology 2010, 131, 268–281. [Google Scholar] [CrossRef] [PubMed]
| Targets | Primer (5′-3′) | Accession Number |
|---|---|---|
| PEDV-N | F: 5′-GGTATTGGAGAAAATCCTGACAGGCATAAGCAACAGCA-3′ | Gene sequencing was accomplished by our laboratory without reference to databases. |
| R: 5′-GACGCATCAACACCTTTTTCGACAAATTCCGCATC-3′ | ||
| β-actin | F: 5′-CGGGACATCAAGGAGAAGC-3′ | NC_010445.4 |
| R: 5′-ACAGCACCGTGTTGGCGTAGAG-3′ | ||
| IL-8 | F: 5′-CCACACCTTTCCACCCCAAA-3′ | NM_213867.1 |
| R: 5′-GGCTCCGGTTCGACACTTTC-3′ | ||
| TNF-α | F: 5′-CACCACGCTCTTCTGCCTAC-3′ | X57321.1 |
| R: 5′-GGCTTTGACATTGGCTACAACG-3′ | ||
| IL-18 | F: 5′-TCTACTCTCTCCTGTAAGAAC-3′ | NM_213997.1 |
| R: 5′-CTTATCATGTCCAGGAAC-3′ | ||
| IL-6 | F: 5′-GCCTTCAGTCCAGTCGCCTTCT-3′ | NM_214399.1 |
| R: 5′-GTGGCATCACCTTTGGCATCTTC-3′ | ||
| E-cadherin | F: 5′-CCCCAACACTTCTCCCTTCACT-3′ | NM_001163060.1 |
| R: 5′-CTCGAGGGTTTTCTTTGGCTTC-3′ | ||
| ZO-1 | F: 5′-CTCTTGGCTTGCTATTCG-3′ | XM_003353439.2 |
| R: 5′-AGTCTTCCCTGCTCTTGC-3′ | ||
| Occludin | F: 5′-GTAGTCGGGTTCGTTTCC-3′ | NM_001163647.2 |
| R: 5′-GACCTGATTGCCTAGAGTGT-3′ | ||
| Integrin | F: 5′-GCAGTTTCAAGGTCAAGATGG-3′ | NM_214002.1 |
| R: 5′-AGCAGGAGGAAGATGAGCAG-3′ | ||
| Claudin-1 | F: 5′-AGATTTACTCCTACGCTGGTGAC-3′ | NM_001244539.1 |
| R: 5′-GCAAAGTGGTGTTCAGATTCAG-3′ | ||
| OASL | F: 5′-GGCACCCCTGTTTTCCTCT-3′ | NM_001031790.1 |
| R: 5′-AGCACCGCTTTTGGATGG-3′ | ||
| ISG15 | F: 5′-AGCATGGTCCTGTTGATGGTG-3′ | NM_001128469.3 |
| R: 5′-CAGAAATGGTCAGCTTGCACG-3′ |
| Strains | Survival Rate (%) | |
|---|---|---|
| Simulated Gastric Fluid | Simulated Intestinal Fluid | |
| LCM233 | 81.82 ± 12.86 a | 48.64 ± 5.36 a |
| LSM231 | 64.06 ± 4.52 b | 58.4 ± 7.92 b |
| LPM239 | 80.00 ± 4.71 a | 42.19 ± 8.14 c |
| Strains | Inhibition Zone Diameters (mm) | |||
|---|---|---|---|---|
| S. aureus CMCC26003 | ETEC K88 | S. marcescens ATCC8100 | S. Typhimurium SL1344 | |
| LCM233 | 17.27 ± 0.17 a | 17.35 ± 0.22 a | 16.64 ± 0.33 a | 16.67 ± 0.2 a |
| LSM231 | 18.25 ± 0.05 b | 18.93 ± 0.11 b | 18.06 ± 0.5 b | 19 ± 0.51 b |
| LPM239 | 18.16 ± 0.49 b | 18.64 ± 0.41 b | 17.84 ± 0.17 c | 17.48 ± 0.26 c |
| Multi-strain probiotic (1:1:1) | 19.42 ± 0.24 c | 19.47 ± 0.21 c | 18.86 ± 0.29 d | 19.01 ± 0.47 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Zhang, Q.; Wang, W.; Wang, X.; Song, B.; Li, J.; Cui, W.; Jiang, Y.; Xie, W.; Tang, L. Multi-Strain Probiotic Regulates the Intestinal Mucosal Immunity and Enhances the Protection of Piglets Against Porcine Epidemic Diarrhea Virus Challenge. Microorganisms 2025, 13, 1738. https://doi.org/10.3390/microorganisms13081738
Wang X, Zhang Q, Wang W, Wang X, Song B, Li J, Cui W, Jiang Y, Xie W, Tang L. Multi-Strain Probiotic Regulates the Intestinal Mucosal Immunity and Enhances the Protection of Piglets Against Porcine Epidemic Diarrhea Virus Challenge. Microorganisms. 2025; 13(8):1738. https://doi.org/10.3390/microorganisms13081738
Chicago/Turabian StyleWang, Xueying, Qi Zhang, Weijian Wang, Xiaona Wang, Baifen Song, Jiaxuan Li, Wen Cui, Yanping Jiang, Weichun Xie, and Lijie Tang. 2025. "Multi-Strain Probiotic Regulates the Intestinal Mucosal Immunity and Enhances the Protection of Piglets Against Porcine Epidemic Diarrhea Virus Challenge" Microorganisms 13, no. 8: 1738. https://doi.org/10.3390/microorganisms13081738
APA StyleWang, X., Zhang, Q., Wang, W., Wang, X., Song, B., Li, J., Cui, W., Jiang, Y., Xie, W., & Tang, L. (2025). Multi-Strain Probiotic Regulates the Intestinal Mucosal Immunity and Enhances the Protection of Piglets Against Porcine Epidemic Diarrhea Virus Challenge. Microorganisms, 13(8), 1738. https://doi.org/10.3390/microorganisms13081738





