Development and Application of a Multiplex Real-Time TaqMan qPCR Assay for the Simultaneous Detection of African Swine Fever Virus, Classical Swine Fever Virus, Porcine Reproductive and Respiratory Syndrome Virus, Pseudorabies Virus, and Porcine Circovirus Type 2
Abstract
1. Introduction
2. Materials and Methods
2.1. Primer and Probe Design, and Standard Plasmid Construction
2.2. Optimization of Multiplex RT-PCR Conditions
2.3. Construction of Standard Curves
2.4. Specificity, Sensitivity, and Reproducibility Evaluation
2.5. Clinical Sample Testing
3. Results
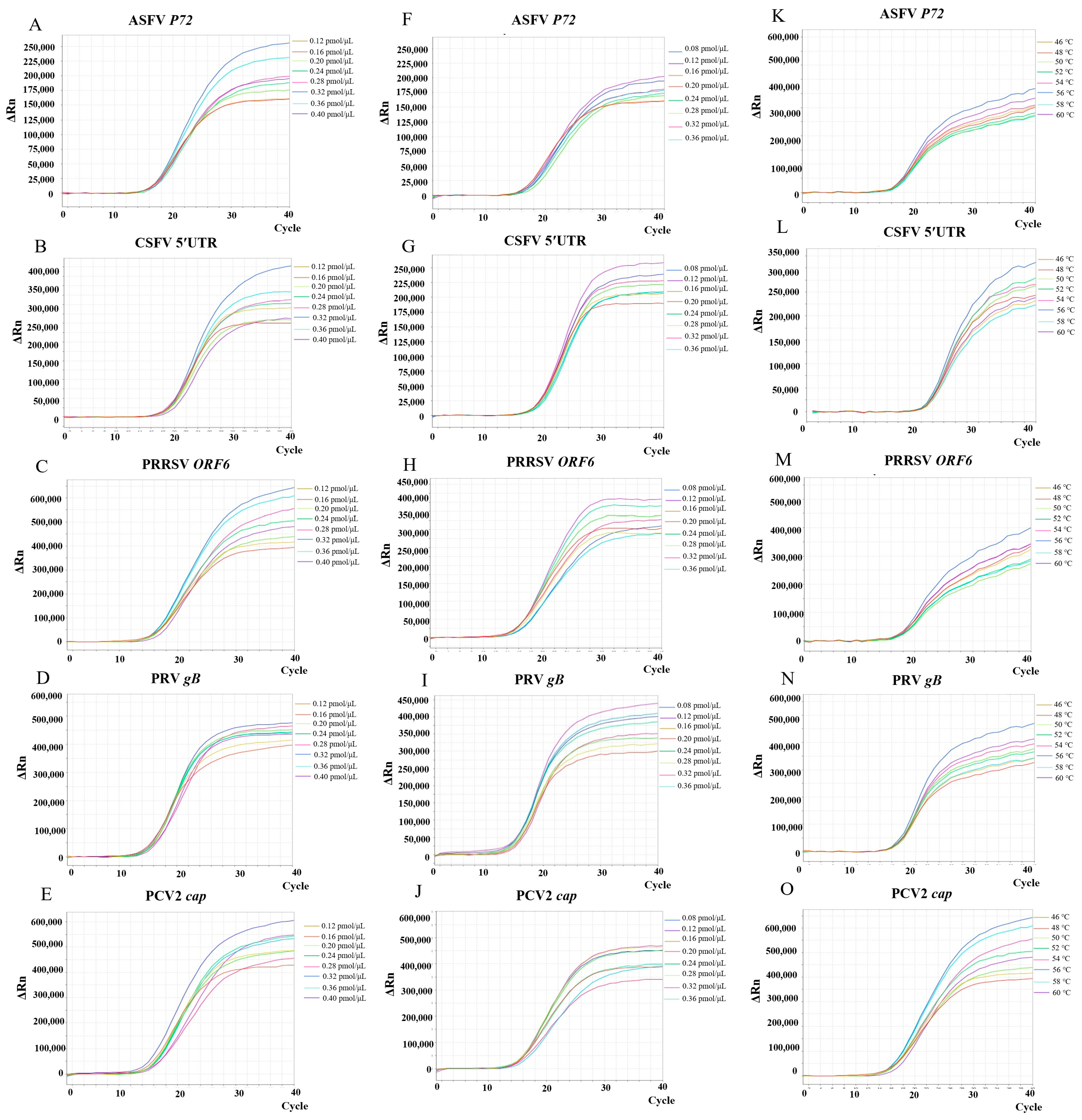
3.1. Optimization of Multiplex RT-PCR Reaction Conditions
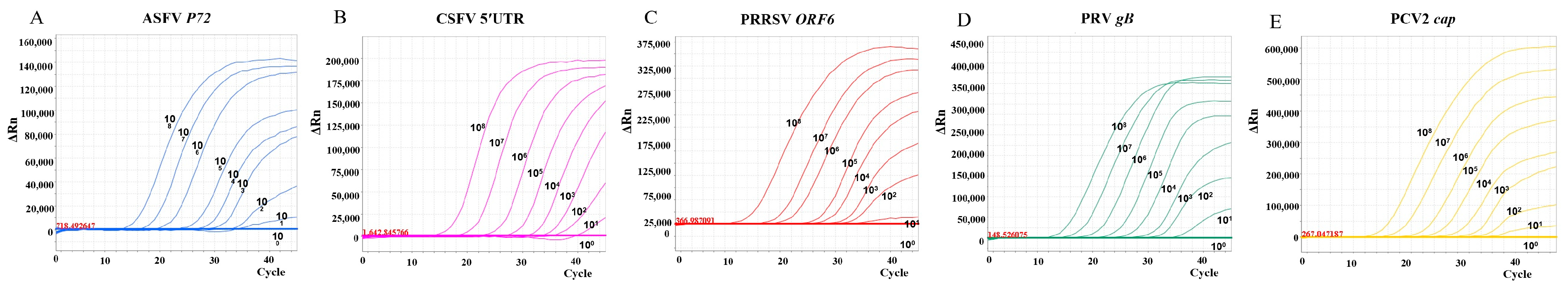
3.2. Establishment of Standard Curves
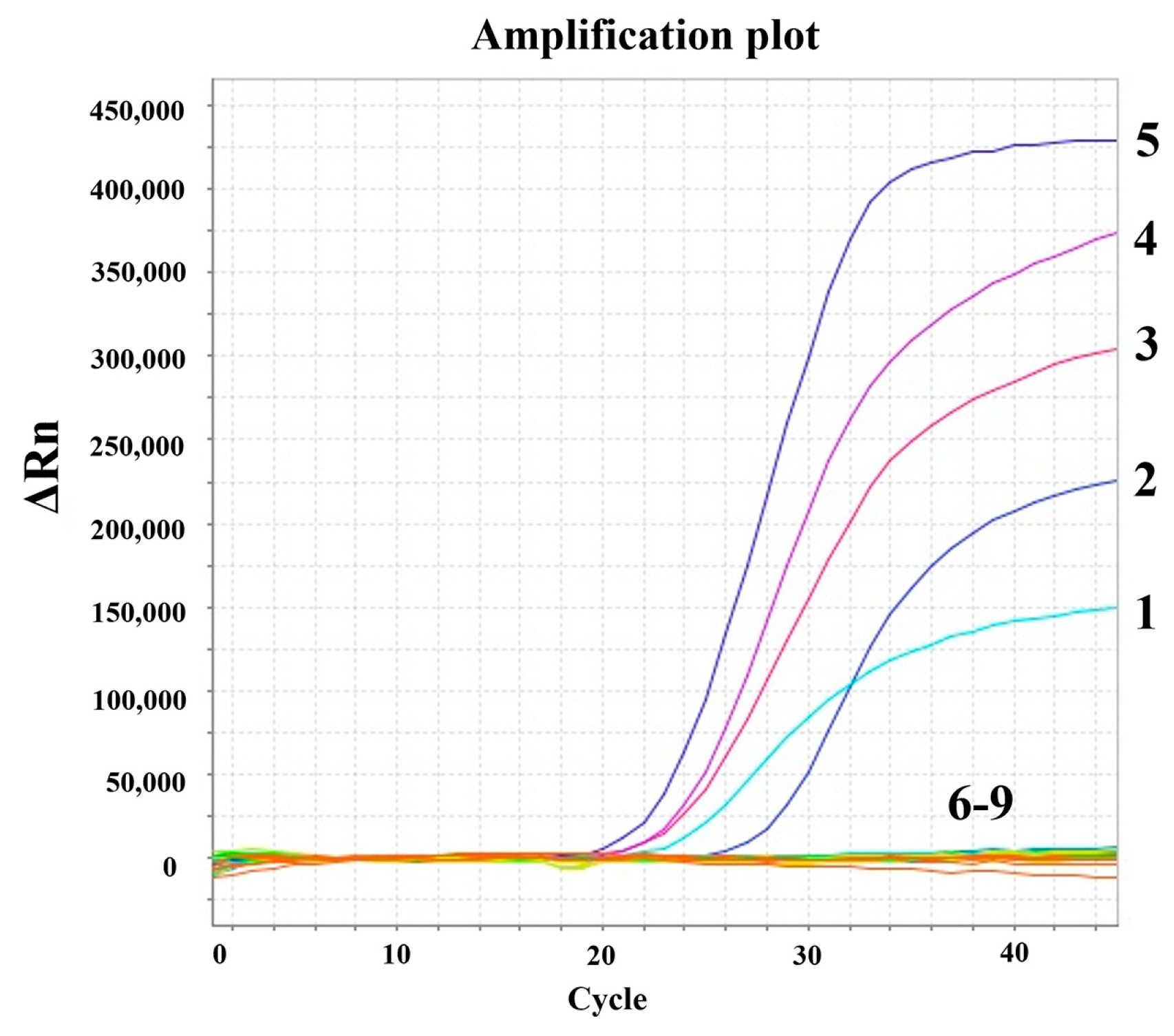
3.3. Specificity of the Multiplex RT-PCR Assay
3.4. Sensitivity of the Multiplex RT-PCR Assay
3.5. Reproducibility of the Multiplex RT-PCR Assay
3.6. Detection of Clinical Samples
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ASF | African swine fever |
| ASFV | African swine fever virus |
| CSFV | Classical swine fever virus |
| PRRSV | Porcine reproductive and respiratory syndrome virus |
| PRV | Pseudorabies virus |
| PCV2 | Porcine circovirus type 2 |
| PCR | Polymerase chain reaction |
| Ct | Cycle threshold |
| R2 | Correlation coefficient |
References
- Dixon, L.K.; Sun, H.; Roberts, H. African swine fever. Antivir. Res. 2019, 165, 34–41. [Google Scholar] [CrossRef]
- Sánchez-Cordón, P.J.; Nunez, A.; Neimanis, A.; Wikström-Lassa, E.; Montoya, M.; Crooke, H.; Gavier-Widén, D. African Swine Fever: Disease Dynamics in Wild Boar Experimentally Infected with ASFV Isolates Belonging to Genotype I and II. Viruses 2019, 11, 852. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Liu, T.; Liu, Y.; Xiao, J.; Wang, H. Transmission of African swine fever in China Through Legal Trade of Live Pigs. Transbound. Emerg. Dis. 2021, 68, 355–360. [Google Scholar] [CrossRef]
- Wu, K.; Liu, J.; Wang, L.; Fan, S.; Li, Z.; Li, Y.; Yi, L.; Ding, H.; Zhao, M.; Chen, J. Current State of Global African Swine Fever Vaccine Development under the Prevalence and Transmission of ASF in China. Vaccines 2020, 8, 531. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Yu, X.; He, D.; Ku, X.; Hong, B.; Zeng, W.; Zhang, H.; He, Q. Investigation and analysis of etiology associated with porcine respiratory disease complex in China from 2017 to 2021. Front. Vet. Sci. 2022, 9, 960033. [Google Scholar] [CrossRef]
- Zheng, H.H.; Fu, P.F.; Chen, H.Y.; Wang, Z.Y. Pseudorabies Virus: From Pathogenesis to Prevention Strategies. Viruses 2022, 14, 1638. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Gong, B.; Sun, Q.; Xu, H.; Zhao, J.; Xiang, L.; Tang, Y.D.; Leng, C.; Li, W.; Guo, Z.; et al. First Detection of NADC34-like PRRSV as a Main Epidemic Strain on a Large Farm in China. Pathogens 2021, 11, 32. [Google Scholar] [CrossRef]
- Martínez-Lobo, F.J.; Díez-Fuertes, F.; Simarro, I.; Castro, J.M.; Prieto, C. The Ability of Porcine Reproductive and Respiratory Syndrome Virus Isolates to Induce Broadly Reactive Neutralizing Antibodies Correlates With In Vivo Protection. Front. Immunol. 2021, 12, 691145. [Google Scholar] [CrossRef]
- Hu, Z.; Lai, R.; Tian, X.; Guan, R.; Li, X. A duplex fluorescent quantitative PCR assay to distinguish the genotype I, II and I/II recombinant strains of African swine fever virus in China. Front. Vet. Sci. 2024, 11, 1422757. [Google Scholar] [CrossRef]
- Chen, Y.; Luo, S.; Tan, J.; Zhang, L.; Qiu, S.; Hao, Z.; Wang, N.; Deng, Z.; Wang, A.; Yang, Q.; et al. Establishment and application of multiplex real-time PCR for simultaneous detection of four viruses associated with porcine reproductive failure. Front. Microbiol. 2023, 14, 1092273. [Google Scholar] [CrossRef]
- Rovira, A.; Balasch, M.; Segalés, J.; García, L.; Plana-Durán, J.; Rosell, C.; Ellerbrok, H.; Mankertz, A.; Domingo, M. Experimental inoculation of conventional pigs with porcine reproductive and respiratory syndrome virus and porcine circovirus 2. J. Virol. 2002, 76, 3232–3239. [Google Scholar] [CrossRef] [PubMed]
- Carmina, G.; Nieto, R.; Arias, M. African Swine Fever Virus (ASFV) Indirect ELISA Test Based on the Use of the Soluble Cytoplasmic Semi- purified Antigen (ASFV CP-Ag). Methods Mol. Biol. 2022, 2503, 133–145. [Google Scholar]
- Caixia, W.; Songyin, Q.; Ying, X.; Haoyang, Y.; Haoxuan, L.; Shaoqiang, W.; Chunyan, F.; Xiangmei, L. Development of a Blocking ELISA Kit for Detection of ASFV Antibody Based on a Monoclonal Antibody Against Full-Length p72. J. AOAC Int. 2022, 105, 1428–1436. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.Z.; Fang, W.H.; Habib, M. First results of detection of PRRSV and CSFV RNA by SYBR Green I-based quantitative PCR. J. Vet. Med. B Infect. Dis. Vet. Public Health 2006, 53, 461–467. [Google Scholar] [CrossRef]
- Lazov, C.M.; Papetti, A.; Belsham, G.J.; Bøtner, A.; Rasmussen, T.B.; Boniotti, M.B. Multiplex Real-Time RT-PCR Assays for Detection and Differentiation of Porcine Enteric Coronaviruses. Pathogens 2023, 12, 1040. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Feng, S.; Shi, K.; Shi, Y.; Yin, Y.; Long, F.; Wei, X.; Li, Z. Development of a quadruplex real-time quantitative RT-PCR for detection and differentiation of PHEV, PRV, CSFV, and JEV. Front. Vet. Sci. 2023, 10, 1276505. [Google Scholar] [CrossRef]
- Wang, G.; Zhu, H.; Zhan, C.; Chen, P.; Wu, B.; Peng, Z.; Qian, P.; Cheng, G. Establishment and Application of a Quadruplex Real-Time Reverse-Transcription Polymerase Chain Reaction Assay for Differentiation of Porcine Reproductive and Respiratory Syndrome Virus, Porcine Circovirus Type 2, Porcine Circovirus Type 3, and Streptococcus suis. Microorganisms 2024, 12, 427. [Google Scholar]
- Mighell, E.; Ward, M.P. African Swine Fever spread across Asia, 2018–2019. Transbound. Emerg. Dis. 2021, 68, 2722–2732. [Google Scholar] [CrossRef]
- Tan, L.; Yao, J.; Yang, Y.; Luo, W.; Yuan, X.; Yang, L.; Wang, A. Current Status and Challenge of Pseudorabies Virus Infection in China. Virol. Sin. 2021, 36, 588–607. [Google Scholar] [CrossRef]
- Horter, D.C.; Pogranichniy, R.M.; Chang, C.C.; Evans, R.B.; Yoon, K.J.; Zimmerman, J.J. Characterization of the carrier state in porcine reproductive and respiratory syndrome virus infection. Vet. Microbiol. 2002, 86, 213–228. [Google Scholar] [CrossRef]
- Liang, C.; Liu, H.; Zhou, J.; Chen, Y.; Ding, P.; Zhu, X.; Wang, M.; Ding, M.; Wang, A. Development of a monoclonal antibody against PRRSV glycoprotein 3 using an immuodominant peptide as immunogen. Int. J. Biol. Macromol. 2021, 187, 683–689. [Google Scholar] [CrossRef]
- Meyer, D.; Fritsche, S.; Luo, Y.; Engemann, C.; Blome, S.; Beyerbach, M.; Chang, C.Y.; Qiu, H.J.; Becher, P.; Postel, A. The double-antigen ELISA concept for early detection of E(rns)-specific classical swine fever virus antibodies and application as an accompanying test for differentiation of infected from marker vaccinated animals. Transbound. Emerg. Dis. 2017, 64, 2013–2022. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Chen, R.; Chen, Q.; Che, Y.; Yan, S.; Zhou, L.; Wang, L. Establishment of an indirect ELISA method for antibody detection of porcine pseudorabies by recombinant gB, gC, and gD proteins. J. Med. Virol. 2023, 95, e28228. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Wang, S.; Fang, Z.; Zhao, M.; Gao, Y.; An, T.; Tu, Y.; Wang, H.; Cai, X. A Sandwich ELISA for Quality Control of PCV2 Virus-like Particles Vaccine. Vaccines 2022, 10, 2175. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Du, N.; Chen, J.; Zhang, K.; Tong, W.; Zheng, H.; Zhao, R.; Tong, G.; Gao, F. Establishment and Application of a Quantitative PCR Method for E248R Gene of African Swine Fever Virus. Vet. Sci. 2022, 9, 417. [Google Scholar] [CrossRef]
- Tu, T.; Pang, M.; Jiang, D.; Zhou, Y.; Wu, X.; Yao, X.; Luo, Y.; Yang, Z.; Ren, M.; Lu, A.; et al. Development of a Real-Time TaqMan RT-PCR Assay for the Detection of NADC34-like Porcine Reproductive and Respiratory Syndrome Virus. Vet. Sci. 2023, 10, 279. [Google Scholar] [CrossRef]
- Ma, Y.; Shi, K.; Chen, Z.; Shi, Y.; Zhou, Q.; Mo, S.; Wei, H.; Hu, L.; Mo, M. Simultaneous Detection of Porcine Respiratory Coronavirus, Porcine Reproductive and Respiratory Syndrome Virus, Swine Influenza Virus, and Pseudorabies Virus via Quadruplex One-Step RT-qPCR. Pathogens 2024, 13, 341. [Google Scholar] [CrossRef]

| Name | Sequence (5′-3′) of Primer/Probe | Product Length (bp) |
|---|---|---|
| ASFV-F | ATCCGATCACATTACCTA | 174 |
| ASFV-R | GCTTCAAAGCAAAGGTAA | |
| ASFV-P | FAM-TTCCGTAACTGCTCATGGTATCAATCT-BHQ1 | |
| CSFV-F | TAGCAAACGGAGGGAC | 87 |
| CSFV-R | CACGTCGAACTACTGAC | |
| CSFV-P | NED-CTCCCTGGGTGGTCTAAGTCCTGA-BHQ2 | |
| PRRSV-F | CGGCAAATGATAACCAC | 155 |
| PRRSV-R | CCGTTGTTATTTGGCATA | |
| PRRSV-P | VIC-CGGCTCCACTACGGTCAACG-BHQ1 | |
| PCV2-F | ATCGGAGGATTACTTCC | 200 |
| PCV2-R | CAGAGAATTTAATCTTAAAGACC | |
| PCV2-P | ROX-AAGAATGCTACAGAACAATCCACGG-BHQ2 | |
| PRV-F | ACACCTACACCAAGATCG | 107 |
| PRV-R | GAAGGAGTCGTAGGGGTA | |
| PRV-P | CY5-CCTCCACCTCCTCGACGATG-BHQ2 |
| Viruses | Standard Copies/uL | CT (Mean ± SD) | CT (Mean ± SD) | ||
|---|---|---|---|---|---|
| Intra-Assay | CV/% | Inter-Assay | CV/% | ||
| ASFV | 1 × 104 | 26.535 ± 0.278 | 1.047 | 26.389 ± 0.259 | 0.981 |
| 1 × 105 | 22.496 ± 0.417 | 1.854 | 22.627 ± 0.153 | 0.676 | |
| 1 × 106 | 18.766 ± 0.064 | 0.341 | 18.504 ± 0.285 | 1.540 | |
| CSFV | 1 × 104 | 28.744 ± 0.218 | 0.758 | 28.715 ± 0.041 | 0.143 |
| 1 × 105 | 25.715 ± 0.294 | 1.143 | 25.517 ± 0.280 | 1.097 | |
| 1 × 106 | 21.408 ± 0.188 | 0.878 | 21.475 ± 0.094 | 0.438 | |
| PRRSV | 1 × 104 | 25.564 ± 0.233 | 0.911 | 25.451 ± 0.124 | 0.487 |
| 1 × 105 | 21.466 ± 0.278 | 1.295 | 21.319 ± 0.207 | 0.971 | |
| 1 × 106 | 17.535 ± 0.364 | 2.076 | 17.484 ± 0.228 | 1.304 | |
| PRV | 1 × 104 | 24.824 ± 0.133 | 0.536 | 24.639 ± 0.206 | 0.836 |
| 1 × 105 | 21.728 ± 0.194 | 0.893 | 21.757 ± 0.038 | 0.175 | |
| 1 × 106 | 18.820 ± 0.008 | 0.043 | 18.503 ± 0.303 | 1.638 | |
| PCV2 | 1 × 104 | 24.486 ± 0.275 | 1.123 | 24.335 ± 0.313 | 1.286 |
| 1 × 105 | 20.509 ± 0.351 | 1.711 | 20.396 ± 0.269 | 1.319 | |
| 1 × 106 | 17.782 ± 0.160 | 0.900 | 17.649 ± 0.394 | 2.232 | |
| Viruses | Multiplex Real-Time PCR Methods | Commercial Singleplex qPCR Kits | Matrix Distribution (n) | ||
|---|---|---|---|---|---|
| Positive Samples | Positive Detection Rates (%) | Positive Samples | Positive Detection Rates (%) | ||
| ASFV | 0 | 0 | 0 | 0 | |
| CSFV | 3 | 3.16 | 3 | 3.16 | Blood (2); Spleen (1) |
| PRRSV | 8 | 8.42 | 8 | 8.42 | Lung (5); Blood (3) |
| PRV | 2 | 2.11 | 2 | 2.11 | Brain (2) |
| PCV2 | 12 | 12.63 | 12 | 12.63 | Liver (6); Blood (4); Kidney (2) |
| CSFV + PRRSV | 1 | 1.05 | 1 | 1.05 | Blood (1) |
| PRRSV + PCV2 | 7 | 7.37 | 7 | 7.37 | Lung (4); Blood (3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, D.; Xu, S.; Liu, Y.; Guo, H.; Lan, M.; Yin, L.; Wang, J.; Dai, Y.; Shen, X.; Zhan, K.; et al. Development and Application of a Multiplex Real-Time TaqMan qPCR Assay for the Simultaneous Detection of African Swine Fever Virus, Classical Swine Fever Virus, Porcine Reproductive and Respiratory Syndrome Virus, Pseudorabies Virus, and Porcine Circovirus Type 2. Microorganisms 2025, 13, 1573. https://doi.org/10.3390/microorganisms13071573
Yin D, Xu S, Liu Y, Guo H, Lan M, Yin L, Wang J, Dai Y, Shen X, Zhan K, et al. Development and Application of a Multiplex Real-Time TaqMan qPCR Assay for the Simultaneous Detection of African Swine Fever Virus, Classical Swine Fever Virus, Porcine Reproductive and Respiratory Syndrome Virus, Pseudorabies Virus, and Porcine Circovirus Type 2. Microorganisms. 2025; 13(7):1573. https://doi.org/10.3390/microorganisms13071573
Chicago/Turabian StyleYin, Dongdong, Shuangshuang Xu, Yayun Liu, Hao Guo, Mengdie Lan, Lei Yin, Jieru Wang, Yin Dai, Xuehuai Shen, Kai Zhan, and et al. 2025. "Development and Application of a Multiplex Real-Time TaqMan qPCR Assay for the Simultaneous Detection of African Swine Fever Virus, Classical Swine Fever Virus, Porcine Reproductive and Respiratory Syndrome Virus, Pseudorabies Virus, and Porcine Circovirus Type 2" Microorganisms 13, no. 7: 1573. https://doi.org/10.3390/microorganisms13071573
APA StyleYin, D., Xu, S., Liu, Y., Guo, H., Lan, M., Yin, L., Wang, J., Dai, Y., Shen, X., Zhan, K., & Pan, X. (2025). Development and Application of a Multiplex Real-Time TaqMan qPCR Assay for the Simultaneous Detection of African Swine Fever Virus, Classical Swine Fever Virus, Porcine Reproductive and Respiratory Syndrome Virus, Pseudorabies Virus, and Porcine Circovirus Type 2. Microorganisms, 13(7), 1573. https://doi.org/10.3390/microorganisms13071573






