Genetic and Evolutionary Analysis of Porcine Kobuvirus in Guangxi Province, Southern China, Between 2021 and 2025
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection and Detection of Clinical Samples for PKV
2.2. Amplification and Sequencing
2.3. Sequence Comparison and Phylogenetic Analysis
2.4. Bayesian Dynamic Analysis of the VP1 Gene
2.5. Recombinant Analysis of the VP1, 2B, and 3D Genes
3. Results
3.1. Detection of Clinical Samples
3.2. Identity Analysis of VP1, 2B, and 3D Genes
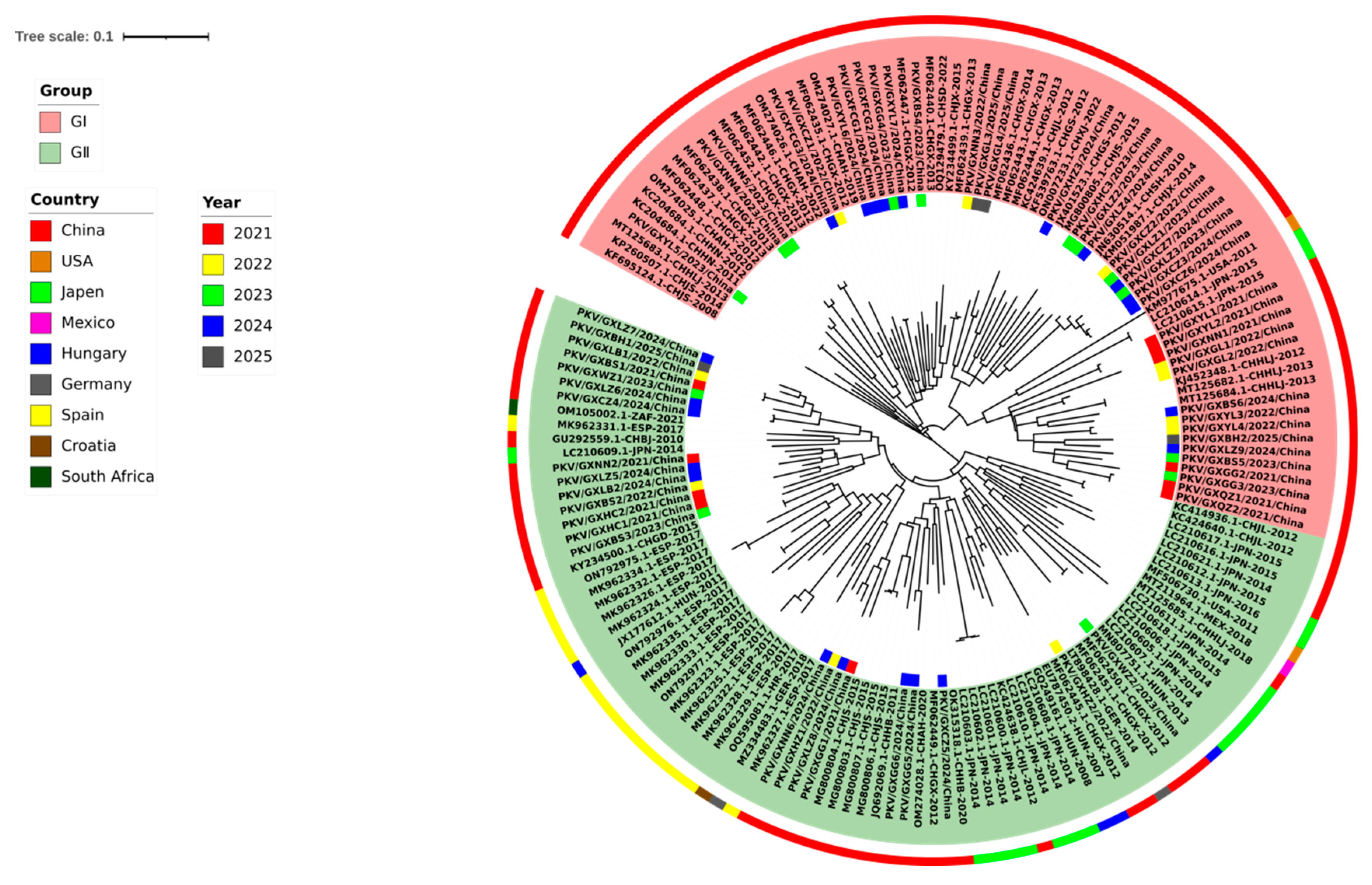
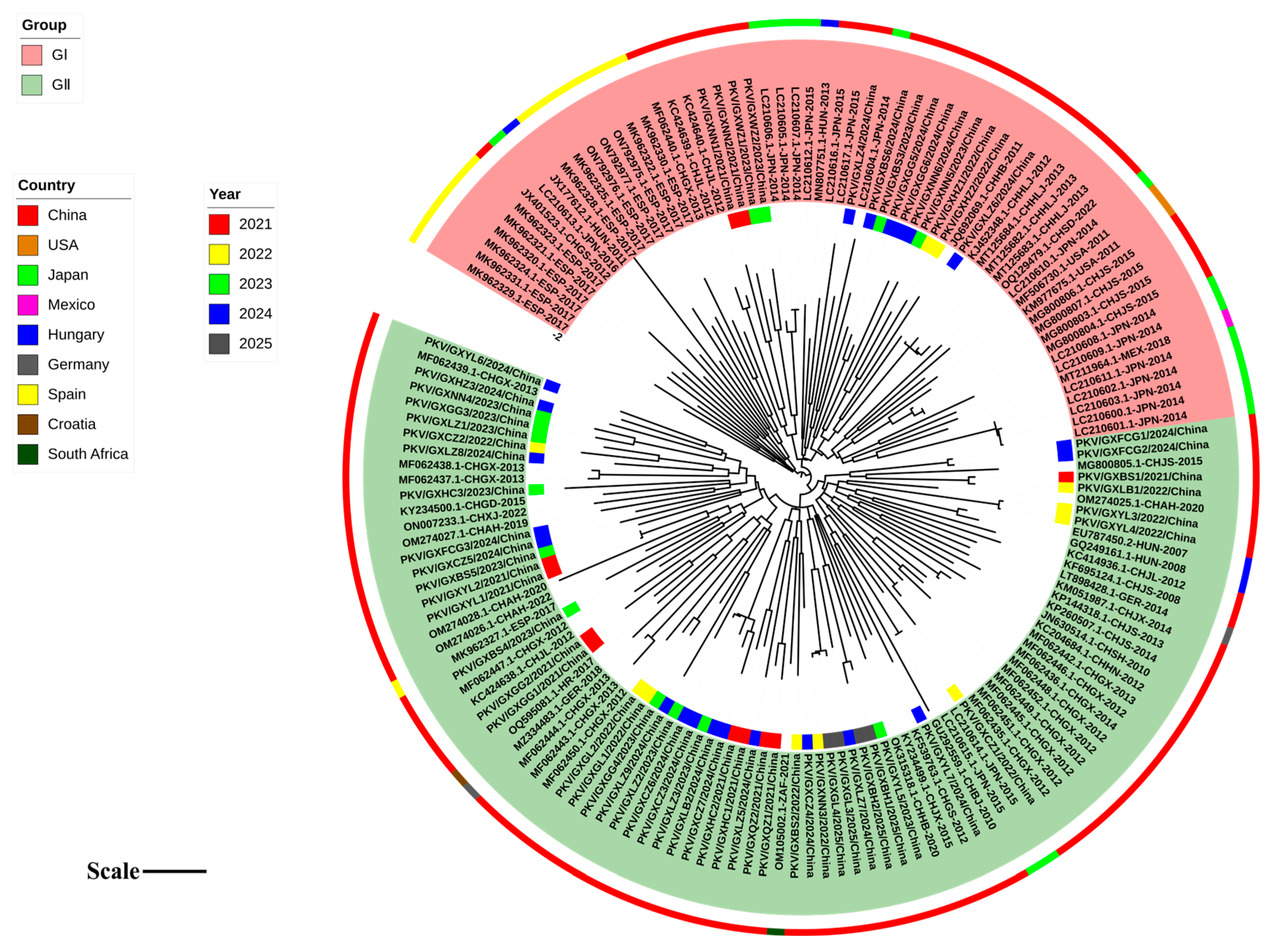
3.3. Phylogenetic Analysis Based on VP1 Gene Sequences
3.4. Phylogenetic Analysis Based on 2B Gene Sequences
3.5. Phylogenetic Analysis Based on 3D Gene Sequences
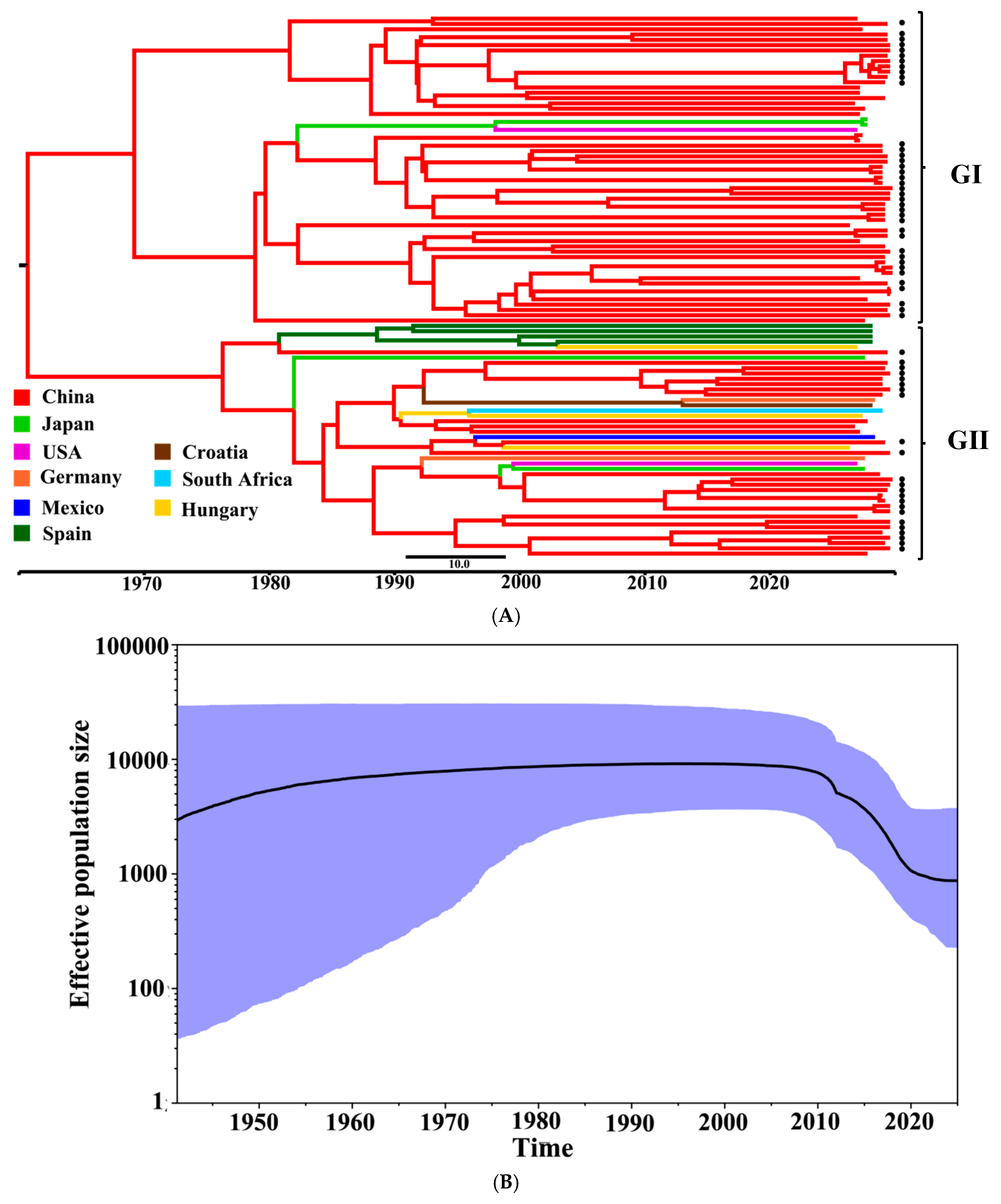
3.6. Bayesian Temporal Dynamic Analysis
3.7. Recombination Analysis of the VP1, 2B, and 3D Genes
3.8. Estimation of Evolution Rates of VP1, 2B, and 3D Genes
3.9. Analysis of VP1 Gene Amino Acid Sequences
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Turlewicz-Podbielska, H.; Pomorska-Mól, M. Porcine Coronaviruses: Overview of the State of the Art. Virol. Sin. 2021, 36, 833–851. [Google Scholar] [CrossRef]
- Meng, L.; Tao, J.; Li, B.; Ma, Y.; Cheng, J.; Zhang, C.; Liu, H. Clinical Detection of Seven Porcine Diarrhea-Associated Viruses and Evolution Analysis of Porcine Kobuvirus. Sheng Wu Gong Cheng Xue Bao 2017, 33, 1292–1303. [Google Scholar]
- Werid, G.M.; Ibrahim, Y.M.; Chen, H.; Fu, L.; Wang, Y. Molecular Detection and Genetic Characterization of Potential Zoonotic Swine Enteric Viruses in Northern China. Pathogens 2022, 11, 417. [Google Scholar] [CrossRef]
- Reuter, G.; Boldizsár, A.; Kiss, I.; Pankovics, P. Candidate New Species of Kobuvirus in Porcine Hosts. Emerg. Infect. Dis. 2008, 14, 1968–1970. [Google Scholar] [CrossRef]
- Eriksen, E.Ø. A Systematic Review: Is Porcine Kobuvirus Causing Gastrointestinal Disease in Young Pigs? Vet. Sci. 2023, 10, 286. [Google Scholar] [CrossRef] [PubMed]
- Zhai, S.L.; Zhang, H.; Lin, T.; Chen, S.N.; Zhou, X.; Chen, Q.L.; Lv, D.H.; Wen, X.H.; Zhou, X.R.; Jia, C.L.; et al. A Novel Porcine Kobuvirus Emerged in Piglets with Severe Diarrhoea in China. Transbound. Emerg. Dis. 2017, 64, 1030–1036. [Google Scholar] [CrossRef] [PubMed]
- Zell, R.; Delwart, E.; Gorbalenya, A.E.; Hovi, T.; King, A.M.Q.; Knowles, N.J.; Lindberg, A.M.; Pallansch, M.A.; Palmenberg, A.C.; Reuter, G.; et al. ICTV Virus Taxonomy Profile: Picornaviridae. J. Gen. Virol. 2017, 98, 2421–2422. [Google Scholar] [CrossRef] [PubMed]
- Reuter, G.; Boldizsár, Á.; Pankovics, P. Complete Nucleotide and Amino Acid Sequences and Genetic Organization of Porcine Kobuvirus, a Member of a New Species in the Genus Kobuvirus, Family Picornaviridae. Arch. Virol. 2009, 154, 101–108. [Google Scholar] [CrossRef]
- Cui, Y.; Li, J.; Guo, J.; Pan, Y.; Tong, X.; Liu, C.; Wang, D.; Xu, W.; Shi, Y.; Ji, Y.; et al. Evolutionary Origin, Genetic Recombination, and Phylogeography of Porcine Kobuvirus. Viruses 2023, 15, 240. [Google Scholar] [CrossRef]
- Khamrin, P.; Maneekarn, N.; Kongkaew, A.; Kongkaew, S.; Okitsu, S.; Ushijima, H. Porcine Kobuvirus in Piglets, Thailand. Emerg. Infect. Dis. 2009, 15, 2075–2076. [Google Scholar] [CrossRef]
- Goecke, N.B.; Hjulsager, C.K.; Kongsted, H.; Boye, M.; Rasmussen, S.; Granberg, F.; Fischer, T.K.; Midgley, S.E.; Rasmussen, L.D.; Angen, Ø.; et al. No Evidence of Enteric Viral Involvement in the New Neonatal Porcine Diarrhoea Syndrome in Danish Pigs. BMC Vet. Res. 2017, 13, 315. [Google Scholar] [CrossRef]
- Khamrin, P.; Maneekarn, N.; Hidaka, S.; Kishikawa, S.; Ushijima, K.; Okitsu, S.; Ushijima, H. Molecular Detection of Kobuvirus Sequences in Stool Samples Collected from Healthy Pigs in Japan. Infect. Genet. Evol. 2010, 10, 950–954. [Google Scholar] [CrossRef] [PubMed]
- An, D.J.; Jeoung, H.Y.; Jeong, W.; Lee, H.S.; Park, J.Y.; Kim, B. Porcine Kobuvirus from Pig Stool in Korea. Virus Genes 2011, 42, 208–211. [Google Scholar] [CrossRef]
- Yu, J.; Jin, M.; Zhang, Q.; Li, H.; Li, D.; Xu, Z.; Li, J.; Cui, S.; Yang, S.; Liu, N.; et al. Candidate Porcine Kobuvirus, China. Emerg. Infect. Dis. 2009, 15, 823–825. [Google Scholar] [CrossRef]
- Capai, L.; Piorkowski, G.; Maestrini, O.; Casabianca, F.; Masse, S.; De Lamballerie, X.; Charrel, R.N.; Falchi, A. Detection of Porcine Enteric Viruses (Kobuvirus, Mamastrovirus and Sapelovirus) in Domestic Pigs in Corsica, France. PLoS ONE 2022, 17, e0260161. [Google Scholar] [CrossRef]
- Van Dung, N.; Anh, P.H.; Van Cuong, N.; Hoa, N.T.; Carrique-Mas, J.; Hien, V.B.; Sharp, C.; Rabaa, M.; Berto, A.; Campbell, J.; et al. Large-Scale Screening and Characterization of Enteroviruses and Kobuviruses Infecting Pigs in Vietnam. J. Gen. Virol. 2016, 97, 378–388. [Google Scholar] [CrossRef] [PubMed]
- Amimo, J.O.; Okoth, E.; Junga, J.O.; Ogara, W.O.; Njahira, M.N.; Wang, Q.; Vlasova, A.N.; Saif, L.J.; Djikeng, A. Molecular Detection and Genetic Characterization of Kobuviruses and Astroviruses in Asymptomatic Local Pigs in East Africa. Arch. Virol. 2014, 159, 1313–1319. [Google Scholar] [CrossRef] [PubMed]
- Barry, A.F.; Ribeiro, J.; Alfieri, A.F.; van der Poel, W.H.; Alfieri, A.A. First Detection of Kobuvirus in Farm Animals in Brazil and the Netherlands. Infect. Genet. Evol. 2011, 11, 1811–1814. [Google Scholar] [CrossRef]
- Verma, H.; Mor, S.K.; Abdel-Glil, M.Y.; Goyal, S.M. Identification and Molecular Characterization of Porcine Kobuvirus in U. S. Swine. Virus Genes 2013, 46, 551–553. [Google Scholar] [CrossRef]
- Milićević, V.; Kureljušić, B.; Maksimović-Zorić, J.; Savić, B.; Spalević, L.; Žutić, J. Molecular Detection and Characterization of Porcine Kobuvirus in Domestic Pigs and Wild Boars in Serbia. Res. Vet. Sci. 2020, 132, 404–406. [Google Scholar] [CrossRef]
- Di Profio, F.; Ceci, C.; Di Felice, E.; Marsilio, F.; Di Martino, B. Molecular Detection of Porcine Kobuviruses in Italian Swine. Res. Vet. Sci. 2013, 95, 782–785. [Google Scholar] [CrossRef] [PubMed]
- García-Hernández, M.E.; Trujillo-Ortega, M.E.; Alcaraz-Estrada, S.L.; Lozano-Aguirre-Beltrán, L.; Sandoval-Jaime, C.; Taboada-Ramírez, B.I.; Sarmiento-Silva, R.E. Molecular Detection and Characterization of Porcine Epidemic Diarrhea Virus and Porcine Aichivirus C Coinfection in México. Viruses 2021, 13, 738. [Google Scholar] [CrossRef] [PubMed]
- Dufkova, L.; Scigalkova, I.; Moutelikova, R.; Malenovska, H.; Prodelalova, J. Genetic Diversity of Porcine Sapoviruses, Kobuviruses, and Astroviruses in Asymptomatic Pigs: An Emerging New Sapovirus GIII Genotype. Arch. Virol. 2013, 158, 549–558. [Google Scholar] [CrossRef]
- Malik, Y.S.; Bhat, S.; Sircar, S.; Verma, A.K.; Barman, N.N.; Deka, P.J.; Ghosh, S.; Reuter, G.; Dhama, K. Kobuvirus Detection in the Critically Endangered Pygmy Hog (Porcula Salvania), India. J. Zoo Wildl. Med. 2021, 52, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Qiu, M.; Li, S.; Xiao, Y.; Li, J.; Zhang, Y.; Li, X.; Feng, B.; Li, C.; Lin, H.; Zhu, J.; et al. Pathogenic and Metagenomic Evaluations Reveal the Correlations of Porcine Epidemic Diarrhea Virus, Porcine Kobuvirus and Porcine Astroviruses with Neonatal Piglet Diarrhea. Microb. Pathog. 2022, 170, 105703. [Google Scholar] [CrossRef]
- Su, M.; Li, C.; Qi, S.; Yang, D.; Jiang, N.; Yin, B.; Guo, D.; Kong, F.; Yuan, D.; Feng, L.; et al. A Molecular Epidemiological Investigation of PEDV in China: Characterization of Co-infection and Genetic Diversity of S1-Based Genes. Transbound. Emerg. Dis. 2020, 67, 1129–1140, Erratum in Transbound. Emerg. Dis. 2021, 68, 2634–2635. [Google Scholar] [CrossRef]
- Jackova, A.; Sliz, I.; Mandelik, R.; Salamunova, S.; Novotny, J.; Kolesarova, M.; Vlasakova, M.; Vilcek, S. Porcine Kobuvirus 1 in Healthy and Diarrheic Pigs: Genetic Detection and Characterization of Virus and Co-Infection with Rotavirus A. Infect. Genet. Evol. 2017, 49, 73–77. [Google Scholar] [CrossRef]
- Zhao, Z.P.; Yang, Z.; Lin, W.D.; Wang, W.Y.; Yang, J.; Jin, W.J.; Qin, A.J. The Rate of Co-Infection for Piglet Diarrhea Viruses in China and the Genetic Characterization of Porcine Epidemic Diarrhea Virus and Porcine Kobuvirus. Acta Virol. 2016, 60, 55–61. [Google Scholar] [CrossRef]
- Wu, S.; Gou, F.; Meng, J.; Jin, X.; Liu, W.; Ding, W.; Xu, W.; Gu, C.; Hu, X.; Cheng, G.; et al. Porcine Kobuvirus Enhances Porcine Epidemic Diarrhea Virus Pathogenicity and Alters the Number of Intestinal Lymphocytes in Piglets. Vet. Microbiol. 2024, 293, 110100. [Google Scholar] [CrossRef]
- Chuchaona, W.; Khamrin, P.; Yodmeeklin, A.; Kongkaew, A.; Vachirachewin, R.; Kumthip, K.; Ushijima, H.; Maneekarn, N. Detection and Molecular Characterization of Porcine Kobuvirus in Piglets in 2009–2013 in Northern Thailand. Trop. Anim. Health Prod. 2017, 49, 1077–1080. [Google Scholar] [CrossRef]
- Lu, L.; Van Dung, N.; Ivens, A.; Bogaardt, C.; O’Toole, A.; Bryant, J.E.; Carrique-Mas, J.; Van Cuong, N.; Anh, P.H.; Rabaa, M.A.; et al. Genetic Diversity and Cross-Species Transmission of Kobuviruses in Vietnam. Virus Evol. 2018, 4, vey002. [Google Scholar] [CrossRef]
- Puente, H.; Arguello, H.; Cortey, M.; Gómez-García, M.; Mencía-Ares, O.; Pérez-Perez, L.; Díaz, I.; Carvajal, A. Detection and Genetic Characterization of Enteric Viruses in Diarrhoea Outbreaks from Swine Farms in Spain. Porc. Health Manag. 2023, 9, 29. [Google Scholar] [CrossRef] [PubMed]
- Cortey, M.; Díaz, I.; Vidal, A.; Martín-Valls, G.; Franzo, G.; Gómez De Nova, P.J.; Darwich, L.; Puente, H.; Carvajal, A.; Martín, M.; et al. High Levels of Unreported Intraspecific Diversity among RNA Viruses in Faeces of Neonatal Piglets with Diarrhoea. BMC Vet. Res. 2019, 15, 441. [Google Scholar] [CrossRef]
- Ding, G.; Fu, Y.; Li, B.; Chen, J.; Wang, J.; Yin, B.; Sha, W.; Liu, G. Development of a Multiplex RT-PCR for the Detection of Major Diarrhoeal Viruses in Pig Herds in China. Transbound. Emerg. Dis. 2020, 67, 678–685. [Google Scholar] [CrossRef]
- Akagami, M.; Ito, M.; Niira, K.; Kuroda, M.; Masuda, T.; Haga, K.; Tsuchiaka, S.; Naoi, Y.; Kishimoto, M.; Sano, K.; et al. Complete Genome Analysis of Porcine Kobuviruses from the Feces of Pigs in Japan. Virus Genes 2017, 53, 593–602. [Google Scholar] [CrossRef]
- Park, S.J.; Kim, H.K.; Moon, H.J.; Song, D.S.; Rho, S.M.; Han, J.Y.; Nguyen, V.G.; Park, B.K. Molecular Detection of Porcine Kobuvirus in Pigs in Korea and Their Association with Diarrhea. Arch. Virol. 2010, 155, 1803–1811. [Google Scholar] [CrossRef]
- Wang, C.; Lan, D.; Hua, X. Porcine Kobuvirus from Pig Stool Specimens in Shanghai, China. Virus Genes 2011, 43, 350–352. [Google Scholar] [CrossRef]
- Zhou, W.; Ullman, K.; Chowdry, V.; Reining, M.; Benyeda, Z.; Baule, C.; Juremalm, M.; Wallgren, P.; Schwarz, L.; Zhou, E.; et al. Molecular Investigation of the Prevalence and Viral Load of Enteric Viruses in Pigs from Five European Countries. Vet. Microbiol. 2016, 182, 75–81. [Google Scholar] [CrossRef]
- Zang, Y.; Feng, B.; Huang, Z.; Zhao, D.; Qi, W.; Qiu, Y.; Qiu, M.; Li, C.; Lin, H.; Zheng, W.; et al. Epidemiologic and Genomic Characterizations of Porcine Kobuviruses in Diarrheic and Healthy Pigs. Animals 2023, 13, 3129. [Google Scholar] [CrossRef]
- Jin, W.J.; Yang, Z.; Zhao, Z.P.; Wang, W.Y.; Yang, J.; Qin, A.J.; Yang, H.C. Genetic Characterization of Porcine Kobuvirus Variants Identified from Healthy Piglets in China. Infect. Genet. Evol. 2015, 35, 89–95. [Google Scholar] [CrossRef]
- Yang, F.; Liu, X.; Zhou, Y.; Lyu, W.; Xu, S.; Xu, Z.; Zhu, L. Histopathology of Porcine Kobuvirus in Chinese Piglets. Virol. Sin. 2015, 30, 396–399. [Google Scholar] [CrossRef]
- Reuter, G.; Boros, A.; Pankovics, P. Kobuviruses—A Comprehensive Review. Rev. Med. Virol. 2011, 21, 32–41. [Google Scholar] [CrossRef]
- Pham, N.T.K.; Trinh, Q.D.; Khamrin, P.; Nguyen, T.A.; Dey, S.K.; Phan, T.G.; Hoang, L.P.; Maneekarn, N.; Okitsu, S.; Mizuguchi, M.; et al. Sequence Analysis of the Capsid Gene of Aichi Viruses Detected from Japan, Bangladesh, Thailand, and Vietnam. J. Med. Virol. 2008, 80, 1222–1227. [Google Scholar] [CrossRef]
- Yamashita, T.; Ito, M.; Kabashima, Y.; Tsuzuki, H.; Fujiura, A.; Sakae, K. Isolation and Characterization of a New Species of Kobuvirus Associated with Cattle. J. Gen. Virol. 2003, 84, 3069–3077. [Google Scholar] [CrossRef]
- Liu, X.; Oka, T.; Wang, Q. Genomic Characterization of a US Porcine Kobuvirus Strain. Arch. Microbiol. 2015, 197, 1033–1040. [Google Scholar] [CrossRef]
- Shi, D.; Zhang, S.; Chen, J.; Shi, H.; Zhang, X.; Feng, L. Molecular Characterization of a Porcine Kobuvirus Variant Strain in China. Arch. Virol. 2013, 158, 2379–2383. [Google Scholar] [CrossRef]
- Yu, J.; Xu, Z.; Li, B.; Zhang, Q.; Cui, S.; Jin, M.; Duan, Z. Analysis and Characterization of the Complete Genome of a Member of a New Species of Kobuvirus Associated with Swine. Arch. Virol. 2011, 156, 747–751. [Google Scholar] [CrossRef]
- Tu, J.; Lin, Z.; Sun, E.; Yu, T.; Zhang, W.; Sun, Y.; Zhu, H.; Qian, P.; Cheng, G. Establishment and Application of a Triplex Real-Time Reverse-Transcription Polymerase Chain Reaction Assay for Differentiation of PEDV, TGEV and PKV. Vet. Sci. 2024, 11, 413. [Google Scholar] [CrossRef]
- Li, B.; Shi, K.; Shi, Y.; Feng, S.; Yin, Y.; Lu, W.; Long, F.; Wei, Z.; Wei, Y. A Quadruplex RT-qPCR for the Detection of Porcine Sapelovirus, Porcine Kobuvirus, Porcine Teschovirus, and Porcine Enterovirus G. Animals 2025, 15, 1008. [Google Scholar] [CrossRef]
- Fan, S.; Sun, H.; Ying, Y.; Gao, X.; Wang, Z.; Yu, Y.; Li, Y.; Wang, T.; Yu, Z.; Yang, S.; et al. Identification and Characterization of Porcine Kobuvirus Variant Isolated from Suckling Piglet in Gansu Province, China. Viruses 2013, 5, 2548–2560. [Google Scholar] [CrossRef]
- Yang, Z.; Jin, W.; Zhao, Z.; Lin, W.; Zhang, D.; Yu, E.; Qin, A.; Yang, H. Genetic Characterization of Porcine Kobuvirus and Detection of Coinfecting Pathogens in Diarrheic Pigs in Jiangsu Province, China. Arch. Virol. 2014, 159, 3407–3412. [Google Scholar] [CrossRef] [PubMed]
- Okitsu, S.; Khamrin, P.; Thongprachum, A.; Hidaka, S.; Kongkaew, S.; Kongkaew, A.; Maneekarn, N.; Mizuguchi, M.; Hayakawa, S.; Ushijima, H. Sequence Analysis of Porcine Kobuvirus VP1 Region Detected in Pigs in Japan and Thailand. Virus Genes 2012, 44, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Shang, R.; Cheng, K.; Wang, S.; Yuan, X.; Wu, J.; Yu, Z. Phylogenetic Analysis and Molecular Characterization of the Co-Infection of the New Variant of the Porcine Epidemic Diarrhea Virus and the Novel Porcine Kobuvirus Isolated from Piglets with Diarrhea. Braz. J. Microbiol. 2023, 54, 2527–2534. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Lan, D.; Cui, L.; Yang, Z.; Yuan, C.; Hua, X. Molecular Characterization of a Porcine Kobuvirus Strain in China. Arch. Virol. 2012, 157, 573–578. [Google Scholar] [CrossRef]
- Lu, L.; Van Dung, N.; Bryant, J.E.; Carrique-Mas, J.; Van Cuong, N.; Anh, P.H.; Rabaa, M.A.; Baker, S.; Simmonds, P.; Woolhouse, M.E. Evolution and Phylogeographic Dissemination of Endemic Porcine Picornaviruses in Vietnam. Virus Evol. 2016, 2, vew001. [Google Scholar] [CrossRef]
- Cho, Y.Y.; Lim, S.I.; Kim, Y.K.; Song, J.Y.; Lee, J.B.; An, D.J. Molecular Evolution of Kobuviruses in Cats. Arch. Virol. 2015, 160, 537–541. [Google Scholar] [CrossRef]
- Park, S.J.; Kim, H.K.; Song, D.S.; Moon, H.J.; Park, B.K. Molecular Detection and Genetic Characterization of Kobuviruses in Fecal Samples Collected from Diarrheic Cattle in Korea. Infect. Genet. Evol. 2011, 11, 1178–1182. [Google Scholar] [CrossRef]
- Hasan, M.K.; Mun, H.S.; Ampode, K.M.B.; Lagua, E.B.; Park, H.R.; Kim, Y.H.; Sharifuzzaman, M.; Yang, C.J. Transformation toward Precision Large-Scale Operations for Sustainable Farming: A Review Based on China’s Pig Industry. J. Adv. Vet. Anim. Res. 2024, 11, 1076–1092. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Qi, W.; Yang, Y.; Liu, Z.; An, T.; Wu, X.; Chen, J. Prevention and Control Strategies of African Swine Fever and Progress on Pig Farm Repopulation in China. Viruses 2021, 13, 2552. [Google Scholar] [CrossRef]


| Gene | Primer | Sequence (5′→3′) | Product/bp |
|---|---|---|---|
| VP1 | PKV-VP1-F | GTCACTAACATGGCTAACCAGAA | 1162 |
| PKV-VP1-R | CCATCCAGTGACGTGGTTCTACCTC | ||
| 2B | PKV-2B-F | GCCGTGCAAGCGTCCAAAGG | 654 |
| PKV-2B-R | CGCGTTGACAGCATCATTGTA | ||
| 3D | PKV-3D1-F | TCGAGCAGTTTGCGATTGACCAA | 1048 |
| PKV-3D1-R | GGATGGATGGGCGGATCACACCC | ||
| PKV-3D2-F | GGTGGACTCATCGAGTACATGCA | 899 | |
| PKV-3D2-R | CGGTCTTAGGAAAGCATGAGTCTAT |
| Gene | Mean Evolutionary Rate (s/s/y) | 95% Highest Posterior Density (HPD) |
|---|---|---|
| VP1 | 3.72 × 10−3 | 3.11 × 10−3–4.44 × 10−3 |
| 2B | 2.50 × 10−2 | 1.42 × 10−2–3.93 × 10−2 |
| 3D | 2.88 × 10−4 | 1.38 × 10−4–6.99 × 10−4 |
| Group | Site | Mutation | Site | Mutation |
|---|---|---|---|---|
| I | 2 | D→G | 121 | F→Y |
| 3 | D→N/G | 131 | E→D/N/G | |
| 4 | D→A | 132 | E→Q | |
| 5 | N→D | 134 | P→S | |
| 7 | P→Q/T | 135 | G→S | |
| 8 | P→T | 137 | S→A/T | |
| 12 | S→N | 139 | G→S | |
| 17 | T→S | 141 | F→S | |
| 18 | A→T | 144 | I→V | |
| 19 | T→A | 146 | A→V/T | |
| 20 | T→S | 148 | I→V | |
| 21 | E→Q | 151 | T→A | |
| 24 | L→P | 152 | S→F | |
| 28 | F→L | 154 | A→I/V/T | |
| 29 | S→T | 158 | I→L | |
| 44 | F→S | 160 | V→I | |
| 45 | F→L | 177 | S→T | |
| 52 | F→Y | 178 | D→G | |
| 54 | D→N | 181 | G→S | |
| 56 | E→Q/D/G | 183 | Q→H/R | |
| 57 | D→N | 188 | T→A | |
| 61 | G→S | 197 | V→I/A | |
| 65 | E→G/A | 198 | S→N/T/A | |
| 66 | A→N/D/V | 202 | D→E/G | |
| 69 | T→A | 205 | A→S/I/T | |
| 70 | F→L | 206 | T→S/P/A | |
| 71 | P→Q/H | 208 | M→L/Y | |
| 76 | D→G/N | 210 | T→A | |
| 80 | N→T/I/V/M | 213 | T→A | |
| 82 | G→S | 221 | C→N | |
| 86 | T→A | 231 | L→I | |
| 97 | F→I | 232 | E→G | |
| 99 | A→V | 233 | Q→A/T | |
| 104 | A→T/V | 234 | V/I/S/A/N/L | |
| 105 | I→L | 236 | P→T/S/V | |
| 107 | F→L | 237 | P→A/V/S | |
| 109 | N→T | 238 | L→P/S/A | |
| 112 | A→P | 239 | L→A/V/T/I | |
| 113 | Y→F | 240 | P→A/V/S/T | |
| 114 | A→T | 241 | A→L/V | |
| 115 | A→V | 250 | P→R | |
| 116 | R→C/S | 251 | V→I | |
| 117 | V→I | 252 | V→T | |
| 118 | T→I | 254 | Q→R | |
| 119 | I→V | |||
| II | 5 | D→N | 137 | S→P |
| 7 | Q→E/P | 146 | T→I/V/A | |
| 8 | P→T | 148 | I→V/A | |
| 17 | S→T | 151 | I→T | |
| 19 | T→A | 154 | A→T/V | |
| 38 | D→G | 156 | I→V | |
| 43 | R→C | 158 | I→V | |
| 56 | Q→E/T | 160 | I→V | |
| 66 | A→T | 173 | F→L | |
| 69 | T→I | 183 | H→Q | |
| 70 | F→L/S | 197 | V→A | |
| 71 | P→H | 198 | S→A/V/T | |
| 80 | I→N/V | 200 | Q→L | |
| 86 | S→T | 205 | A→N/T/S | |
| 96 | Y→C | 206 | I→P/A | |
| 99 | A→G | 210 | T→I | |
| 100 | D→G | 223 | Y→F | |
| 102 | R→H | 234 | S→L/F/P | |
| 113 | Y→F | 236 | I→T/A | |
| 115 | A→V | 237 | /→P | |
| 116 | C→F/S | 238 | P→S | |
| 117 | V→I | 239 | A→V/T | |
| 118 | T→I | 240 | A→T | |
| 130 | G→T/N | 241 | T→A | |
| 132 | D→N | 250 | R→P | |
| 135 | T→I |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Y.; Shi, Y.; Shi, K.; Yin, Y.; Feng, S.; Long, F.; Si, H. Genetic and Evolutionary Analysis of Porcine Kobuvirus in Guangxi Province, Southern China, Between 2021 and 2025. Microorganisms 2025, 13, 1921. https://doi.org/10.3390/microorganisms13081921
Tang Y, Shi Y, Shi K, Yin Y, Feng S, Long F, Si H. Genetic and Evolutionary Analysis of Porcine Kobuvirus in Guangxi Province, Southern China, Between 2021 and 2025. Microorganisms. 2025; 13(8):1921. https://doi.org/10.3390/microorganisms13081921
Chicago/Turabian StyleTang, Yang, Yuwen Shi, Kaichuang Shi, Yanwen Yin, Shuping Feng, Feng Long, and Hongbin Si. 2025. "Genetic and Evolutionary Analysis of Porcine Kobuvirus in Guangxi Province, Southern China, Between 2021 and 2025" Microorganisms 13, no. 8: 1921. https://doi.org/10.3390/microorganisms13081921
APA StyleTang, Y., Shi, Y., Shi, K., Yin, Y., Feng, S., Long, F., & Si, H. (2025). Genetic and Evolutionary Analysis of Porcine Kobuvirus in Guangxi Province, Southern China, Between 2021 and 2025. Microorganisms, 13(8), 1921. https://doi.org/10.3390/microorganisms13081921






