Abstract
Winemaking involves a microbial ecosystem where yeast diversity, shaped by terroir and winemaking conditions, determines wine characteristics. Understanding the microbial diversity of vineyards and spontaneous fermentation is crucial for explaining a winery’s typical wine profile. Studying and inoculating indigenous strains make it possible to produce high quality wines, reflecting the production environment. This study analyzes the yeast species involved in 16 spontaneous fermentations (8 in 2022 and 8 in 2023) from grapes of four distinct vineyards under two sets of winemaking conditions. A total of 1100 yeast colonies were identified by MALDI-TOF and DNA sequencing techniques. Saccharomyces (S.) cerevisiae and Hanseniaspora uvarum were the most prevalent species, alongside significant populations of non-Saccharomyces yeasts such as Lachancea thermotolerans and Metchnikowia pulcherrima, which were the most abundant ones. Minor yeast species, including Aureobasidium pullulans, Starmerella bacillaris, Kazachstania servazzi, and other Hanseniaspora spp., were also detected. The results demonstrated that yeast diversity in spontaneous fermentations varied according to vineyard origin and winemaking conditions. Differences between the two vintages studied indicated that annual climatic conditions significantly influenced yeast diversity, especially among non-Saccharomyces species. This substantial diversity represents a valuable source of indigenous yeasts for preserving the typicity of a winery’s wines under controlled conditions.
1. Introduction
Spontaneous fermentation, the most traditional and minimally interventive approach to alcoholic fermentation, yields wines of notable complexity. This complexity arises from the intricate interplay of diverse yeast species, encompassing both Saccharomyces and non-Saccharomyces varieties [1]. However, the inherent variability of indigenous microbiota renders this method unpredictable. Consequently, the modern wine industry has widely adopted inoculation with S. cerevisiae to mitigate these risks. Nonetheless, recent research has demonstrated that monoculture fermentations using highly efficient S. cerevisiae strains fail to replicate the complexity achieved through diverse yeast populations. This diversity, conversely, enhances complexity and allows winemakers to express distinct regional or varietal characteristics, thereby enhancing brand differentiation in a competitive market [2]. Furthermore, non-Saccharomyces yeasts are now recognized not as disruptive agents, but as valuable biotechnological tools for modulating wine attributes [3,4,5,6,7]. Accordingly, commercial non-Saccharomyces strains are increasingly employed in sequential inoculations with S. cerevisiae to refine the physicochemical and sensory profiles of wines [8].
While starter cultures have improved the reproducibility and predictability of wine quality, concerns persist regarding the homogenization of wine styles due to the widespread use of commercial strains. This practice diminishes the diversity of indigenous yeasts, particularly non-Saccharomyces species, thereby compromising regional typicity and distinct producer styles. In response to growing consumer demand for innovative wines and market competition, there is an increasing emphasis on wine typicity linked to specific geographical areas (terroir), wineries, or single vineyards. The concept of microbial terroir, which recognizes the influence of indigenous yeast composition on grape characteristics and final wine attributes, has gained prominence [9,10,11]. The massive use of commercial yeast has reduced the potential contributions of native yeast populations (Saccharomyces and non-Saccharomyces) [2]. In addition, regional dispersion of native yeast cells has been estimated to cover an area not more than 10 km away from their origin [12]. This ensures a restricted limit for microbiota diversity within a few vineyards of a certain microregion, mainly for non-Saccharomyces strains [13]. Thus, inoculation with indigenous yeasts from specific vineyards offers a strategy to produce wines with unique and distinctive styles.
Moreover, the burgeoning interest in organic wines has prompted winemakers to explore additive-free winemaking, notably reducing or eliminating sulfites. This approach alters yeast ecology and fermentation dynamics, potentially impacting wine quality. Harnessing natural biodiversity and implementing biocontrol agents to optimize winemaking management can facilitate the production of typical, high-quality wines [14,15].
Consequently, many wineries are implementing programs to isolate and select indigenous yeasts, both Saccharomyces and non-Saccharomyces, based on their representation of the local terroir. These selections are intended for use as inocula, ensuring the typicity, complexity, and stability of wines. Employing native yeast selections is a crucial strategy for securing quality initial fermentation and mitigating the risks associated with uncontrolled spontaneous fermentation. Prior to selection, ecological and biodiversity studies of yeast populations during spontaneous alcoholic fermentation are essential for understanding the complex interactive networks that govern species dominance. This research aims to investigate the yeast ecology of spontaneous fermentations in a commercial winery over two consecutive vintages. The study focuses on analyzing the diversity of yeast populations in relation to grape vineyard origin, winemaking conditions, and vintage variations.
2. Materials and Methods
2.1. Grapes and Spontaneous Fermentation
In this study, conducted over two consecutive vintages (2022 and 2023), grapes of two red varieties (Tempranillo and Graciano) from four vineyards (1, 2, 3, and 4), located in La Rioja (Spain) and belonging to Bodegas Bilbaínas, were used. These vineyards are located within a radius of less than 10 km of each other. Vineyards 2 and 3 are located in the same plantation, but vineyard 2 is made up of Tempranillo grapes and vineyard 3 of Graciano grapes. These vineyards have yielded grapes for the winery’s premium wines over recent decades. For this reason, they have set out to study the microbiota present in the spontaneous fermentations carried out with these grapes, with the objective of developing proprietary yeast inoculums.
Grapes were manually harvested at optimal ripeness (26 September 2022, and 29 September 2023). For each grape batch, two distinct spontaneous fermentation protocols were implemented: laboratory fermentation (LF), conducted in sterile low-capacity bags (without sulfur dioxide addition or aeration and at a temperature of 23 °C in a refrigerated environment) and winery fermentation (WF), performed under industrial-scale conditions (sulfites addition: 60 mg SO2/kg of grapes, pump-overs, and temperature control at 28 °C). LF involved aseptically processing 2 kg of grapes from each sample at harvest. Grapes were crushed within sterile 3 L bags to initiate spontaneous fermentation, relying solely on the indigenous yeast populations present on the grapes. WF, utilizing the remaining grapes from each vineyard plot, were conducted at the winery’s facilities in 1500 L wine tanks under standard commercial conditions.
2.2. Yeast Isolation and Identification
For LF, yeast isolation samples were collected at specific time points: 48 h and 96 h seven and ten days post-vatting in 2022. In 2023, the initial sampling schedule was modified, with the 48 and 96 h samples replaced by a single 72 h sample. In WF, must samples were collected at 72 h post-vatting and during tumultuous fermentation, defined by a must density of 1.025. All samples were collected in sterile 50 mL Falcon tubes and transported to the laboratory for subsequent analysis.
The must samples were processed according to the method of successive decimal dilutions and sowing in Petri dishes containing the appropriate culture media: GYP (20 g glucose, 20 g agar, 5 g mycological peptone, and 5 g yeast extract in 1 L of water) (Scharlau, Barcelona, Spain) and Agar-Lysine (66 g Lysine medium in 1 L of water with 10 mL of 50% potassium lactate and 1 mL of 2.5% bromocresol green dissolved in absolute ethanol) (Scharlau, Barcelona, Spain). GYP medium facilitated the growth of a broad range of yeast species, while Agar-Lysine medium selectively promoted growth yeasts with the ability to utilize lysine as a nitrogen source. Plates were incubated at 28 °C for 48 h, and colonies were then randomly isolated from plates containing between 30 and 300 colonies (15 colonies from the Agar-Lysine medium and 10 from the GYP medium). A total of 1100 yeast colonies were isolated: 600 from the 2022 vintage and 500 from the 2023 vintage. The isolation medium was not differentiated in the subsequent analysis of these 25-colony sets.
Yeast isolates were identified to the species level using Matrix-Assisted Laser Desorption/Ionization-Time of Flight (MALDI-TOF) mass spectrometry (Bruker Daltonik GmbH, Bremen, Germany). This technique, which has been validated for microorganisms of oenological origin [16], produces a characteristic and reproducible protein spectrum that can be used to differentiate microorganisms at genus and species level. Mass spectra were acquired using a Microflex Mass Spectrometer (Bruker Daltonik GmbH) equipped with a nitrogen laser (λ = 337 nm), controlled by flexControl software (Version 3.4; Bruker Daltonik GmbH, Bremen, Germany). For identification, a fresh yeast culture was placed directly on a target plate using a sterile toothpick. An on-target extraction was then carried out by applying 1 µL of formic acid directly onto the spotted microorganism to increase cell lysis. Once dried, the spotted microorganism was overlaid with a matrix solution (HCCA). Once the matrix was dry, the target plate was loaded into the ionization chamber, where the ionized molecules are accelerated by an electric charge and travel through a vacuum tube to a detector. On their way, the ionized molecules are separated according to their mass-to-charge ratio [17]. The instrument’s mass analyzer measures the time required for each ion to reach the detector and records the TOF (time of fly), generating a mass spectrum. The spectrum was then compared to a reference spectrum database (MALDI Biotyper) to identify the unknown microorganism. A score ≥ 2.0 indicates high confidence identification; scores between 1.70 and 1.99 indicate low confidence identification; and scores ≤ 1.70 indicate that it is not possible to identify the organism.
Isolates that yielded inconclusive MALDI-TOF profiles were subjected to sequencing. DNA was extracted from fresh cultures using the rapid extraction protocol without the Taq polymerase inhibitors described by López [18]. Specifically, 250 µL of lysis buffer (Tris 50 mM, pH 8; β-mercaptoethanol 10 mM) from a pure culture on a plate, grown for 48 h, was collected with an inoculation loop and resuspended in an Eppendorf tube. This mixture was vortexed, allowed to stand for 10 min at room temperature, and then subjected to a 100 °C water bath for 10 min in boiling water. While still hot, it was vortexed again and centrifuged at 13,000 rpm for 3 min. The supernatant, containing the genomic DNA, was collected in a sterile Eppendorf tube and 10 µL of this was used for each via polymerase chain reaction (PCR).
The D1/D2 domain was amplified by PCR using the primers and conditions described by Kurtzman and Robnett [19]. PCR products were purified and sequenced by Macrogen Spain to determine the yeast genus and species. The resulting sequences were used for comparison to the GenBank database using the Basic Local Alignment Search Tool (BLAST) in the NCBI database (https://blast.ncbi.nlm.nih.gov/Blast.cgi accessed on November 2024) to determine the species identity of each isolate.
3. Results and Discussion
3.1. Grape Must Maturity and Climatic Influences
The characteristics of the four batches of grapes used in the two years are shown in Table 1. Within each vintage, variations in probable alcoholic strength were observed across vineyards, despite their proximity (<10 km) and thus similar regional climate. These variations underscore the influence of vineyard-specific attributes (location, orientation, variety, and soil type) on grape composition during ripening. Furthermore, significant inter-annual climatic variations, as reported by regional meteorological services [20], impacted grape maturity. The 2022 vintage was characterized by warmer and drier conditions, including heatwaves and minimal rainfall, compared to the more moderate 2023 vintage with cooler nights and regular precipitation. As anticipated, vineyards 2 and 3 exhibited higher probable alcohol content in 2022. However, vineyard 1 showed slightly lower ripeness in 2022, and vineyard 4 showed minimal inter-annual differences. These discrepancies highlight the differential impact of climatic conditions on vineyards with varying characteristics, consistent with the observation that vineyard adaptation to climate change varies [21,22].

Table 1.
Degree of maturity (PAS—probable alcoholic strength) of the grapes from the 4 vineyards in the two years studied.
3.2. Distribution of Yeast Species in the Different Stages of Fermentation
Figure 1 illustrates the relative abundances of yeast species during laboratory fermentation (LF) and winery fermentation (WF) across four vineyards over 2022 and 2023. Consistent with the expectations presented by Portillo and Mas [23], who showed that the genera Hanseniaspora and Candida were dominant during initial and mid fermentation while the final fermentation was mainly dominated by Candida and Saccharomyces, a temporal shift in microbial populations was observed, with H. uvarum dominating early fermentation stages. However, exceptions were noted in LF from vineyards 2 and 3 (2022), where H. uvarum was minimally detected.
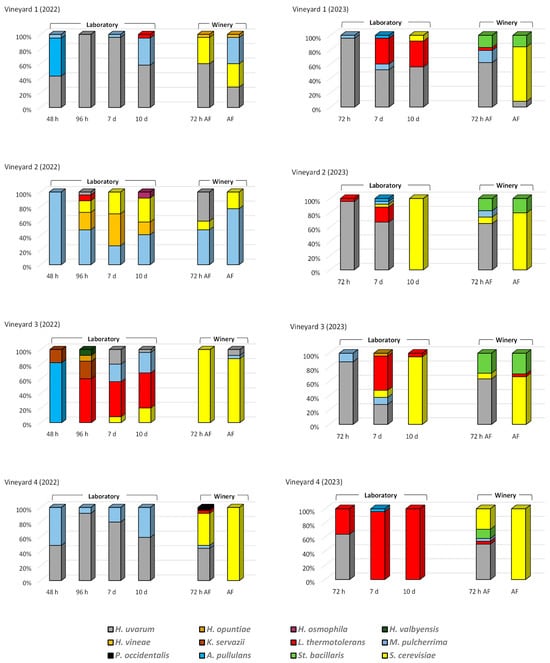
Figure 1.
Yeast species (%) present at different times during fermentations carried out on grapes from 4 vineyards under different conditions (laboratory and winery) in two consecutive years (2022 and 2023). A significant abundance of non-Saccharomyces yeasts was observed during early fermentation, followed by an increase in S. cerevisiae abundance (particularly in WF) as fermentation progressed, as has been described by other authors [24]. However, in LF, S. cerevisiae dominance was less pronounced, with absence in three out of eight fermentations. These observations align with findings by Shimizu et al. [25] in non-sulfited sterile fermentations. The limited S. cerevisiae presence in early LF stages reflects its low abundance on grape surfaces. The prevalence of non-Saccharomyces yeasts in LF is likely attributable to low temperatures and the absence of sulfur dioxide [26]. These results suggest that bioprotection strategies, involving early inoculation with fermentative yeasts, are crucial for stabilizing non-sulfited fermentations [15].
In 2023, the S. cerevisiae species dominated fermentation progress in almost all WFs. However, in 2022, tumultuous fermentation of vineyards 1 and 2 exhibited high abundances of non-Saccharomyces yeasts, particularly Metchnikowia (M.) pulcherrima. This species is recommended in winemaking for its potential to reduce ethanol content, enhance aroma, and provide biocontrol due to its antimicrobial properties against spoilage yeasts [27].
Together with M. pulcherrima, Lanchancea (L.) thermotolerans was another prevalent species that contributes to increased acidity and reduced ethanol production, mitigating climate change impacts while also influencing the aromatic profile, which is one of the most important characteristics determining the wine’s distinctive character [28]. This species has been detected mainly in the LF of vineyard 3 in 2022 and in all LFs in 2023, either occasionally or in all phases of LF.
Among the minor non-Saccharomyces yeasts detected, Starmerella (St.) bacillaris (synonym Candida zemplinina) was isolated in all WF, but only in 2023. Furthermore, the species K. servazzii and several species of Hanseniaspora (H.) (H. opuntiae, H. osmophila, and H. vinae), were detected only in 2022. St. bacillaris is a cryotolerant and osmotolerant species present in oenological environments that used together with S. cerevisiae enhances the analytical composition and aroma profile of wine [29]. Kazachstania (K.) servazzi is a less studied and less common yeast in winemaking. It has been found on grape surfaces or grape must in different countries, but with low frequency. However, it has been shown to have similar fermentative lifestyles to S. cerevisiae and to provide positive aromatic attributes in wine that should be further explored [30].
Although the genus Hanseniaspora has always been associated with spoilage during winemaking, recent studies have re-evaluated the role of various species of this genus in wine production mainly as partners of S. cerevisiae in fermentations. H. uvarum, the most abundant species of this group and traditionally considered detrimental to wine quality, has been described recently as suitable and beneficial for winemaking due to its ability to increase wine complexity [31,32]. Badura et al. [33] observed lactic acid depletion in fermentations with H. osmophila and the increase of certain terpenols with H. opuntiae. One species in particular, H. vineae, has been repeatedly shown to provide several desirable aroma compounds during fermentation, up to 50 orders more than S. cerevisiae [34], which led to the development of a commercially available starter culture.
3.3. Influence of Winemaking Conditions (LF and WF) on Yeast Species Distribution
Important differences in yeast species distribution were observed between LF and WF, indicating a strong influence of winemaking conditions (laboratory and winery). These differences are evident in the eight grape samples studied. In general, WF exhibited higher S. cerevisiae abundances, while LF favored non-Saccharomyces species. In the WF there was a similar distribution in the eight vinifications: majority presence of non-Saccharomyces yeasts at 72 h and of S. cerevisiae in the tumultuous fermentation. The lower competitiveness of non-Saccharomyces yeasts in WF is attributed to their lower stress tolerance [35] so that their participation in WF was lower.
These differences between LF and WF are likely due to variations in fermentation conditions. WF involves larger volumes, sulfite addition to the crushed grapes, temperature control at 28 °C, and daily pump-overs during the winemaking process, whereas LF involved smaller volume, no sulfites, lower temperatures (23 °C), and no aeration. Fermentations conducted at low temperature may show a greater contribution of non-Saccharomyces species [36] and, in addition, these species show higher sensitivity to SO2 [37]. Oxygen availability also impacts yeast survival and dominance [38,39]. All of this resulted in yeasts with little or no fermentative power and that are more sensitive to SO2 being more abundant in the LF. L. thermotolerans and M. pulcherrima, along with H. uvarum, were the predominant isolated non-Saccharomyces species. Within these two species, L. thermotolerans was isolated almost exclusively from LF, while M. pulcherrima was present in both LF and WF. In addition, St. bacillaris was only isolated from all WF in 2023 and was not detected in any of the LF. So, the conditions under which fermentations are carried out influence the different adaptations of the yeasts that drive the fermentations in the cellar.
Inoculation with winery-resident S. cerevisiae strains [40,41,42] may also contribute to differences between LF and WF. S. cerevisiae is rarely found on grape surfaces [43] suggesting that its presence in spontaneous fermentations is primarily due to winery environments. The dominance of non-Saccharomyces during sterile spontaneous fermentation of grapes (LF), even in some cases where they exclusively managed the fermentation during the 10 days, highlights the impact of winemaking conditions. Only in the LF of plots 2 and 3 in 2023, in the sample taken after 10 days, did the S. cerevisiae species dominate, while in the rest of the 2023 and in all the 2022 LF, this phase of fermentation was led by non-Saccharomyces yeasts. Thus, S. cerevisiae represented a small contribution to the overall broad species diversity found in the LFs of this study. However, the presence of S. cerevisiae in some LF samples indicates its potential as a source of vineyard-specific strains. Shimizu et al. [25] also detected this yeast species in laboratory-scale spontaneous winemaking using sterilized labware to avoid winery-resident microbes.
3.4. Influence of Grape Origin and Vintage on Yeast Species Distribution
Significant differences were observed in the yeast species present in the fermentations depending on the origin of the grapes. In LFs, where only grape yeasts were involved, the differences between yeast species present in fermentations with grapes from different vineyards each year were greater than in WFs, which would indicate a clear vineyard effect. Tempère et al. [44] indicated that vineyard management and soil and site characteristics influence the grape microbiota that enter a winery. Thus, in 2022 in LFs of grapes from vineyards 1 and 4 H. uvarum was the majority species with a slight presence of M. pulcherrima, while in the LFs of grapes from vineyards 2 and 3, M. pulcherrima and L. thermotolerans, respectively, were the majority species. In addition, only a small presence of S. cerevisiae was detected in the fermentations of vineyards 2 and 3. However, in 2023 LFs, S. cerevisiae was prevalent in vineyards 2 and 3, while L. thermotolerans dominated in vineyards 1 and 4.
The grape variety in vineyard 3 was Graciano and in the other three vineyards it was Tempranillo. Although in 2022 the differences between the microbiota in LFs of vineyard 3 and the rest were evident, in 2023 the distribution of species was similar, and in this year the greatest differences occurred in LFs of vineyard 4. Therefore, contrary to what was indicated by other authors [9], it seems that the variety alone is not a determining factor in the development of yeasts on the grapes. It should be noted that vineyards 2 and 3 are within the same plantation, and there were differences between them, especially in 2022, which would indicate that the climatic conditions of each year are much more influential on the microbiota than the grape variety.
Furthermore, in LF, great differences in yeast species were detected in the two years (2022 and 2023) carried out with grapes from the same vineyard, which would indicate a vintage effect. Thus, in vineyards 1 and 4, in both 2022 and 2023, the majority species was H. uvarum, and a slight presence of M. pulcherrima was detected in 2022 and of L. thermotolerans in 2023. In vineyards 2 and 3 in 2022 H. uvarum was barely detected and its presence was high in 2023. The main species accompanying H. uvarum in 2023 were L. thermotolerans and S. cerevisiae in both vineyards. In 2022, in fermentations in vineyard 2 the main species were H. vineae, M. pulcherrima and S. cerevisiae, and in vineyard 3 L. thermotolerans, M. pulcherrima and K. servazzii. The differences in the yeasts present in the LF between the two years studied with grapes from the same vineyard were probably due to the different environmental conditions that occurred during the ripening period in the two years of study. Gilbert et al. [9] indicated that vintage can influence microbiological patterns at the level of individual plots, causing noticeable changes in the population. Thus, variations in climatic conditions between years can affect the grape microbiota of a specific vineyard, which can have an impact on the characteristics of the wines obtained.
In the WFs, the distribution of species in the fermentations of vineyards 1 and 4 hardly differed between years 2022 and 2023, while in vineyards 2 and 3 the distribution was very different. Thus, in WF the vineyard origin had a less pronounced effect, with S. cerevisiae dominance in tumultuous fermentation likely due to winemaking conditions and winery ecosystem inoculation.
No specific yeast species associated with the vineyard of origin were detected in either LF or WF. However, they were detected depending on the winemaking conditions and the year of study. Some species such as K. servazzii were only detected in LF in 2022, others such as St. bacillaris only in WF in 2023, and even L. thermotolerans were mainly isolated in LF and their presence was sporadic and in a minority in WF.
There is significant variability in yeast distribution during fermentation, both in grapes from the same vineyards in different years and globally considering all the isolates made each year together (Figure 2). We can see how both S. cerevisiae and H. uvarum are the species most frequently found and at similar levels in both 2022 and 2023. In both years, colonies of M. pulcherrima and L. thermotolerans were detected, with the first being more abundant in 2022 and the second in 2023, which could be due to the different weather in each year. We also detected the presence of minority yeasts that appeared sporadically and were only present in one of the two years of study: Aureobasidium (A.) pullulans, St. bacillaris, and K. servazzi.
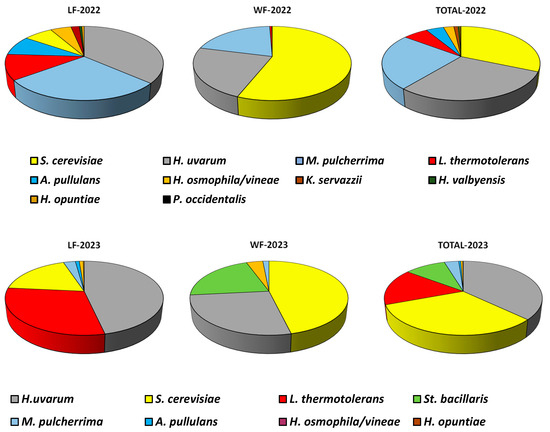
Figure 2.
Overall distribution (%) of all the yeast species isolated each year in the 4 laboratory fermentations (LFs), in the 4 cellar fermentations (WFs), and in the total of the 8 laboratory and cellar fermentations carried out for each vintage (total).
Variations in total yeast populations between 2022 and 2023 are much more evident in LFs (Figure 2), those carried out exclusively with yeasts present on grapes. Considering that in both years vineyard management practices have been similar, these data would indicate that inter-annual variations in total yeast populations were more pronounced in LF, indicating that grape microbiota is susceptible to climatic variations during the ripening period, which would affect both the health of the grapes and the composition of the musts, and thus, to the development of different yeast species during fermentation. These results would concur with those of Schütz and Gafner [45], who consider that the population of yeasts can be considered to be dependent on harvest and vineyard.
4. Conclusions
The use of indigenous starters, carefully chosen from specific environmental microbiota, is essential for producing wines with region-specific characteristics. Unlocking microbial diversity in spontaneous fermentations represents the pivotal first step in selecting native yeasts for winery-specific wines. From the 1100 yeast colonies identified in this work, S. cerevisiae and H. uvarum were the most dominant species. Nevertheless, other non-Saccharomyces yeasts such as L. thermotolerans, M. pulcherrima, St. bacillaris, and H. vineae, all with proven potential as starter cultures, were also detected. The distribution of these species across the 16 fermentations studied was, however, notably heterogeneous. The presence and proportion of yeast species were shaped by numerous factors, with annual climatic conditions, the vineyard’s specific traits, and inoculation from the winery ecosystem exerting greater influence on the microbiota than the grape variety itself.
Therefore, to maximize the recovery of these native yeasts, both non-Saccharomyces and S. cerevisiae, it is advisable to isolate colonies from vinifications conducted under diverse conditions and across several vintages. This strategy would provide a collection of yeasts that, after oenological evaluation and selection, could serve as invaluable, tailor-made starter cultures for a winery. Additionally, exploring the intraspecific diversity, by studying the different clones within each species across vineyards and vintage, would offer important insights, further enriching the yeast repository for optimized winery- specific applications.
Author Contributions
Conceptualization, L.G.-A. and A.R.G.; methodology, A.B.-C. and B.L.; validation, P.S. and A.R.G.; formal analysis, P.S. and A.R.G.; investigation, A.B.-C. and B.L.; data curation, P.S.; writing—original draft preparation, A.B.-C.; writing—review and editing, A.R.G. and P.S.; visualization L.G.-A.; supervision, L.G.-A. and A.R.G.; project administration, A.R.G.; funding acquisition, M.T.C.d.L.B. and A.R.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Ministerio de Ciencia e Innovación, Gobierno de España (Ref.: PID CDTI IDI-20220694).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
Author María Teresa Calvo de La Banda was employed by the company Bodegas Bilbaínas. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Varela, C.; Siebert, T.; Cozzolino, D.; Rose, L.; McLean, H.; Henschke, P.A. Discovering a chemical basis for differentiating wines made by fermentation with wild indigenous and inoculated yeasts: Role of yeasts volatile compounds. Aust. J. Grape Wine Res. 2009, 15, 238–248. [Google Scholar] [CrossRef]
- Carrau, F.; Boido, E.; Ramey, D. Yeasts for low input winemaking: Microbial terroir and flavour differentiation. Adv. Appl. Microbiol. 2020, 111, 89–121. [Google Scholar] [CrossRef] [PubMed]
- Ciani, M.; Comitini, F.; Mannazzu, I.; Domizio, P. Controlled mixed culture fermentation: A new perspective on the use of non-Saccharomyces yeasts in winemaking. FEMS Yeast Res. 2010, 10, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Jolly, N.P.; Varela, C.; Pretorius, I.S. Not your ordinary yeast: Non-Saccharomyces yeasts in wine production uncovered. FEMS Yeast Res. 2014, 14, 215–237. [Google Scholar] [CrossRef] [PubMed]
- Padilla, B.; Zulian, L.; Ferreres, A.; Pastor, R.; Esteve-Zarzoso, B.; Beltran, G.; Mas, A. Sequential inoculation of native non- Sac-charomyces and Saccharomyces cerevisiae strains for winemaking. Front. Microbiol. 2017, 8, 1293. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liu, S.; Yang, Q.; Liu, X.; Xu, Y.; Zhou, Z.; Mao, J. Effects of simultaneous inoculation of non-Saccharomyces yeasts and Saccharomyces cerevisiae on overall quality, flavor compounds, and sensory analysis of huangjiu. Food Biosci. 2023, 53, 102539. [Google Scholar] [CrossRef]
- Villar, N.; Pérez-Nevado, F.; Andrés, A.I.; Garcia-Parra, J.; Ramirez, M.; Valdés, M.E.; Moreno, D. Influence of yeast inoculum (Saccharomyces cerevisiae and Torulaspora delbrueckii) on the production of rosé wines from high hydrostatic pressure-treated musts. Eur. Food Res. Technol. 2025, 251, 467–482. [Google Scholar] [CrossRef]
- García-Luque, E.; González, R.; Cao, R.; Soto, E.; Blanco, P. Sequential fermentation with non-Saccharomyces yeasts improves the chemical and sensory characteristics of Albariño and Lado wines. Fermentation 2025, 11, 73. [Google Scholar] [CrossRef]
- Gilbert, J.A.; van der Lelie, D.; Zarraonaindia, I. Microbial terroir for wine grapes. Proc. Natl. Acad. Sci. USA 2014, 111, 5–6. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhang, P.; Chen, D.; Howell, K.S. From the vineyard to the winery: How microbial ecology drives regional distinctiveness of wine. Front. Microbiol. 2019, 10, 2679. [Google Scholar] [CrossRef] [PubMed]
- Pretorius, I.S. Tasting the terroir of wine of wine yeast innovation. FEMS Yeast Res. 2020, 20, foz084. [Google Scholar] [CrossRef] [PubMed]
- Goddard, M.R.; Anfang, N.; Tang, R.; Gardner, R.C.; Jun, C. A distinct population of Saccharomyces cerevisiae in New Zeeland: Evidence for local dispersal by insects and human-aided global dispersal in oak barrels. Environ. Microbiol. 2010, 12, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Francesca, N.; Canale, D.E.; Settanni, L.; Moschetti, G. Dissemination of wine-related yeasts by migratory birds. Environ. Microbiol. Rep. 2012, 4, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Comitini, F.; Capece, A.; Ciani, M.; Romano, P. New insights on the use of wine yeasts. Curr. Opin. Food Sci. 2017, 13, 44–49. [Google Scholar] [CrossRef]
- Di Ganvito, P.; Englezos, V.; Rantsiou, K.; Cocolin, L. Bioprotection strategies in winemaking. Int. J. Food Microbiol. 2022, 364, 109532. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Courbin, A.; Lucas, M.; Dutilh, L.; Miot-Sertier, C.; Windholtz, S.; Lucas, P.; Masneuf-Pomarede, I.; Maupeu, J. Identificación de las levaduras y bacterias enológicas por espectrometría de masa de tipo MALDI-TOF. IVES Tech. Rev. 2022. [Google Scholar] [CrossRef]
- Bourassa, L.; Butler-Wu, S.M. MALDI-TOF mass spectrometry for microorganism identification. Methods Microbiol. 2015, 42, 37–85. [Google Scholar] [CrossRef]
- López, I. Detección y Control por Técnicas de Biología Molecular de Bacterias Lácticas Autóctonas Responsables de la Fermentación Maloláctica de Vinos Tintos de D.O.Ca Rioja. Master’s Thesis, University of La Rioja, Logroño, Spain, 2004. [Google Scholar]
- Kurtzman, C.P.; Robnett, C.J. Identification and phylogeny of ascomycetous yeasts from analysis of nuclear large subunit (26S) ribosomal DNA partial sequences. Antonie Van Leeuwenhoek 1998, 73, 331–371. [Google Scholar] [CrossRef] [PubMed]
- Agroclimatic Information Service of La Rioja, SIAR. Available online: https://www.larioja.org/agricultura/es/informacion-agroclimatica (accessed on January 2025).
- van Leeuwen, C.; Destrac-Irvine, A.; Dubernet, M.; Duchêne, E.; Gowdy, M.; Marguerit, E.; Pieri, P.; Parker, A.; de Rességuier, L.; Ollat, N. An Update on the Impact of Climate Change in Viticulture and Potential Adaptations. Agronomy 2019, 9, 514. [Google Scholar] [CrossRef]
- Rogiers, S.Y.; Greer, D.H.; Liu, Y.; Baby, T.; Xiao, Z. Impact of climate change on grape berry ripening: An assessment of adaptation strategies for the Australian vineyard. Front. Plant Sci. 2022, 13, 1094633. [Google Scholar] [CrossRef] [PubMed]
- Portillo, M.C.; Mas, A. Analysis of microbial diversity and dynamics during wine fermentation of Grenache grape variety by high throughput barcoding sequencing. LWT Food Sci. Technol. 2016, 72, 317–321. [Google Scholar] [CrossRef]
- Fugelsang, K.C.; Edwards, C.G. Microbial ecology during vinification. In Wine Microbiology; Fugelsang, K.C., Edwards, C.G., Eds.; Springer: New York, NY, USA, 2007; Volume 2, pp. 82–101. Available online: https://link.springer.com/book/10.1007/978-0-387-33349-6#toc (accessed on January 2025).
- Shimizu, H.; Kamada, A.; Koyama, K.; Iwasita, K.; Goto-Yamamoto, N. Yeast diversity during the spontaneous fermentation of wine with only the microbiota on grapes cultivated in Japan. J. Biosci. Bioeng. 2023, 136, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Morgan, S.C.; Tantikachornkiat, M.; Scholl, C.M.; Benson, N.L.; Cliff, M.A.; Durall, D.M. The effect of sulfur dioxide addition at crush on the fungaland bacterial communities and the sensory attributes of Pinot gris wines. Int. J. Food Microbiol. 2019, 290, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Binati, R.L.; Maule, M.; Luzzini, G.; Martelli, F.; Felis, G.E.; Ugliano, M.; Torrian, S. From bioprotective effects to diversification of wine aroma: Expanding the knowledge on Metschnikowia pulcherrima oenological potential. Food Res. Int. 2023, 174, 113550. [Google Scholar] [CrossRef] [PubMed]
- Salopek, D.D.; Vrhovsek, U.; Carlin, S.; Radeka, S.; Lukić, I. In-Depth characterization of the volatile aroma profile and other characteristics of white wine produced by sequential inoculation with a Lachancea thermotolerans starter yeast strain. Fermentation 2024, 10, 515. [Google Scholar] [CrossRef]
- Englezos, V.; Cravero, F.; Torchio, F.; Rantsiou, K.; Ortiz-Julien, A.; Lambri, M.; Gerbi, V.; Rolle, L.; Cocolin, L. Oxygen availability and strain combination modulate yeast growth dynamics in mixed culture fermentations of grape must with Starmerella bacillaris and Saccharomyces cerevisiae. Food Microbiol. 2018, 69, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Jood, I.; Hoff, J.W.; Setati, M.E. Evaluating fermentation characteristics of Kazachstania spp. and their potential influence on wine quality. World J. Microbiol. Biotechnol. 2017, 33, 129. [Google Scholar] [CrossRef] [PubMed]
- Rompkovksi, C.; Agustini, B.C.; Deffert, F.; Amboni Stadtlober, M.G.; Brand, D.; Almeida da Silva, G.; Bordin Bonfim, T.M. Microbial dynamics in industrial-scale wine fermentation employing Hanseniaspora uvarum β-glucosidase-producer strain. J. Food Sci. Technol. 2022, 59, 4. [Google Scholar] [CrossRef] [PubMed]
- Mancic, S.; Stamenković Stojanović, S.; Danilović, B.; Djordjević, N.; Malićanin, M.; Lazić, M.; Karabegović, I. Oenological characterization of native Hanseniaspora uvarum strains. Fermentation 2022, 8, 92. [Google Scholar] [CrossRef]
- Badura, J.; Kiene, F.; Brezina, S.; Fritsch, S.; Semmler, H.; Rauhut, D.; Pretorius, I.S.; von Wallbrunn, C.; van Wyk, N. Aroma profiles of Vitis vinifera L. cv. Gewürztraminer must fermented with co-cultures of Saccharomyces cerevisiae and seven Hanseniaspora spp. Fermentation 2023, 9, 109. [Google Scholar] [CrossRef]
- Martín Russo, V. Hanseniaspora vineae: Caracterización y su Uso en la Vinificación. Master’s Thesis, Universidad de la República, Montevideo, Uruguay, 2016. [Google Scholar]
- Di Maro, E.; Ercolini, D.; Coppola, S. Yeast dynamics during spontaneous wine fermentation of the Catalanesca grape. Int. J. Food Microbiol. 2007, 117, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Erten, H. Relations between elevated temperatures and fermentation behaviour of Kloeckera apiculata and Saccharomyces cerevisiae associated with winemaking in mixed cultures. World J. Microbiol. Biotechnol. 2002, 18, 373–378. [Google Scholar] [CrossRef]
- Henick-Kling, T.; Edinger, W.; Daniel, P.; Monk, P. Selective effects of sulfur dioxide and yeast starter culture addition on indigenous yeast population and sensory characteristics of wine. J. Appl. Microbiol. 1998, 84, 865–876. [Google Scholar] [CrossRef]
- Englezos, V.; Cravero, F.; Torchio, F.; Giascosa, S.; Ortiz-Julien, A.; Gerbi, V.; Rolle, L.; Cocolin, L. Volatile profiles and chromatic characteristicsa of red wines produced with Starmerella bacillaris and Saccharomyces cerevisiae. Food Res. Int. 2018, 109, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.; Zhang, B.; Joseph, L.; Waterhouse, A.L. Effects of initial oxygenation on chemical and aromatic composition of wine in mixed starters of Hanseniaspora vinae and Saccharomyces cerevisiae. Food Microbiol. 2020, 90, 103460. [Google Scholar] [CrossRef] [PubMed]
- Fleet, G.H.; Heard, G.M. Yeasts: Growth during fermentation. In Wine, Microbiology and Biotechnology; Fleet, G.H., Ed.; Harwood Academic Publishers: Lausanne, Switzerland, 1993; pp. 27–54. [Google Scholar]
- Le Jeune, C.; Claude, E.; Demuyter, C.; Lollier, M. Evolution of the population of Saccharomyces cerevisiae from grape to wine in a spontaneous fermentation. Food Microbiol. 2006, 23, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Ocón, E.; Gutiérrez, A.R.; Garijo, P.; López, R.; Santamaría, P. Presence of non-Saccharomyces yeasts in cellar equipments and grape juice during harvest time. Food Microbiol. 2010, 27, 1023–1027. [Google Scholar] [CrossRef] [PubMed]
- Pretorius, I.S. Tailoring wine yeast for the new millenium: Novel approaches to the ancient art of winemaking. Yeast 2000, 16, 675–729. [Google Scholar] [CrossRef] [PubMed]
- Tempère, S.; Marchal, A.; Barbe, J.C.; Bely, M.; Masneuf-Pomarede, I.; Marullo, P.; Albertin, W. The complexity of wine: Clarifying the role of microorganisms. Appl. Microbiol. Biotecnol. 2018, 102, 3995–4007. [Google Scholar] [CrossRef] [PubMed]
- Schütz, M.; Gafner, J. Dynamics of the yeast strain population during spontaneous alcoholic fermentation determined by CHEF gel electrophoresis. Lett. Appl. Microbiol. 1994, 19, 253–257. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).