Abstract
Brettanomyces bruxellensis has been described as the main spoilage microorganism in wines due to its ability to produce volatile phenols, which negatively impact the final product’s organoleptic properties. This yeast can grow and survive in environments that are too nutritionally poor and stressful for other microorganisms, and one of the stressful conditions it can endure is the high alcohol content in wine. In this study, cell wall morphology and the expression of some genes related to its composition were characterized under increasing ethanol concentrations to establish a possible ethanol resistance mechanism. B. bruxellensis LAMAP2480 showed greater resistance to β-1,3-glucanase activity when grown in media supplemented with 5% or 10% ethanol compared with the control assay (without ethanol). Transmission electron microscopy showed no significant differences in cell wall thickness during the different adaptation stages. However, the amount of wall polysaccharides and chitin briefly increased at 1% ethanol but returned to baseline at 5% and 10%. The amount of wall-associated protein increased progressively with each increment in ethanol concentration. In addition, overexpression of the ROM2 and KNR4/SMI1 genes was observed at 10% ethanol. These results suggest that the integrity of the cell wall might play an important role in the adaptation of B. bruxellensis to an ethanol-containing medium.
1. Introduction
The Brettanomyces genus of yeast was first isolated in the British brewing industry, being called Brettanomyces after “British fungus” [1]. Several species of this genus have been recognized, such as Brettanomyces custersianus, Brettanomyces naardenensis, Brettanomyces nanus, Brettanomyces anomalus, and Brettanomyces bruxellensis, the latter being the predominant one in wines [2,3]. Brettanomyces bruxellensis can degrade hydroxycinnamic acids, compounds naturally present in fruits with antimicrobial properties, into volatile substances, removing them from the grape must and thus not affecting its growth [2]. The degradation of these acids produces phenolic aromas, described as animal and horse sweat, among others, known as “Brett flavor” [4]. These sensory qualities cause deterioration of the product, which is reflected in a significant economic impact [5]. One of the most descriptive characteristics of B. bruxellensis is its ability to adapt to stressful conditions where other yeasts cannot proliferate [6]. For example, this species exhibits remarkable resistance to environments with high alcohol concentrations and limited availability of residual sugars and nitrogen sources. Its adaptation to elevated levels of sulfur dioxide (SO2), a chemical compound widely used in the food industry, is facilitated by mechanisms such as active sulfur reduction and efflux, enhanced acetaldehyde production, and the capacity to enter a viable but non-culturable (VBNC) state [7,8]. Industrially, B. bruxellensis is detected in the wine maturation process when the product rests in oak barrels, where the ethanol concentration is above 10% (v/v) [9]. For most microorganisms, a high alcohol concentration alters their growth due to an inhibition of cell division and decreases their cell volume and growth rate. Ethanol also influences cell metabolism and macromolecular biosynthesis by inducing the production of heat shock-like proteins, lowering the rate of RNA and protein accumulation, enhancing the frequency of petite mutations, altering metabolism, denaturing intracellular proteins and glycolytic enzymes, and reducing their activity [10]. The cell wall is the first physical barrier of microorganisms that is affected by any external environmental change [11]. This structure protects against mechanical stress since the combination of the strength and elasticity of the cell wall provides an effective barrier against compression. Likewise, it protects against osmotic shock when there is rapid exposure.
On the other hand, the cell wall is required to establish and maintain the cell shape, which is essential for forming a new cell and its division [12]. The yeast cell wall constitutes 15–30% of its cellular dry weight and 25–50% of its volume, comprising β-1,3 glucan (240 kDa), β-1,6 glucan (24 kDa), mannan bound with protein (100–200 kDa), and chitin (25 kDa) [12]. The composition of the cell wall depends on the strain, culture conditions, physiological state, and growth stage of the yeast [12].
In Saccharomyces cerevisiae, two mechanisms have been described that play a central role in cell wall biogenesis, maintenance, and stress resistance: the cell wall integrity (CWI) pathway and the calcineurin (CN) pathway [13]. CWI is the most studied, and it is activated by a set of plasma membrane-spanning sensors through activation of the cell surface sensor proteins Wsc1-3 and Mtl1 in the face of a stress factor [14]. Surface sensors act as linear nano springs that transmit or detect damage or stress in the cell wall to continuous receptors in the signaling pathway [15]. Cell surface sensors activate Rom2p, a guanine nucleotide exchange factor for the GTP protein (GEP) encoded by the ROM2 gene [16]. The GTP protein is responsible for activating the G-protein and performing the transduction of intracellular signals, such as growth regulation and stress response, in addition to contributing to the activation of the Pkc1 protein [14,16]. This protein plays a fundamental role in the CWI pathway since, among other functions, it allows the activation of the mitogen-activated protein (MAP) kinase cascade of cellular integrity PKC (MAPK) and the subsequent factors that carry out transcription (Rlm1). In this way, it activates the function of the SED1 gene, which encodes the structural glycoprotein GPI [16].
Alternatively, Smi1, the protein encoded by the KNR4/SMI1 gene, appears to relay stress signals by engaging the MAPK cascade, which in turn activates the Rlm1p transcription factor; this protein is also associated with regulating the glucan content of the cell wall [15,17].
In the case of B. bruxellensis, some mechanisms that are involved in its resistance to ethanol have been described. Brettanomyces species, including B. bruxellensis, are Crabtree-positive, capable of fermenting glucose to ethanol even in the presence of oxygen when glucose is abundant [18]. This fermentative capacity may contribute to their persistence in alcoholic environments. Montagner et al. [19] reported that ethanol concentrations could affect cells’ surface properties, affecting adhesion proteins, thus facilitating their bioadhesion capacity. Recently, Di Canito et al. [20] demonstrated that their flocculent character and greater adhesiveness could allow for better survival of B. bruxellensis under stress conditions. It is interesting to know how species of the Brettanomyces genus can adapt to high ethanol concentrations during alcoholic fermentation.
Here, we exposed B. bruxellensis LAMAP2480 to a defined range of ethanol concentrations to (i) measure cell wall modifications with zymolyase treatment and microscopy, and (ii) track expression changes in key cell wall integrity pathway genes. The results showed that the yeast adapts to wine-like ethanol levels by reorganizing its cell wall structure.
2. Materials and Methods
2.1. Media and Growth Conditions
The Brettanomyces bruxellensis strain LAMAP 2480, held in the Biotechnology and Applied Microbiology Laboratory at the Universidad de Santiago de Chile, was maintained on YPD agar (20 g/L glucose, 5 g/L yeast extract, 5 g/L peptone, 20 g/L agar) and incubated at 28 °C for 96 h to obtain fresh colonies. A single colony was transferred to 5 mL of YPD broth and cultivated for 72 h at 28 °C with orbital agitation (250 rpm). This starter culture served as the inoculum for 100 mL of fresh YPD broth adjusted to an initial density of 1 × 106 cells/mL (optical density at 600 nm (OD600) 0.1) and incubated for a further 48 h under identical conditions, thereby establishing the ethanol-free reference culture (0% v/v ethanol).
Progressive adaptation to ethanol was achieved through three successive transfers, each conducted at 28 °C and 250 rpm. First, cells from the reference culture were inoculated at 1 × 106 cells m/L into a 100 mL medium composed of 90 mL YPD and 10 mL synthetic wine (SW), yielding a final ethanol concentration of 1% (v/v). The SW formulation (0.6 g/L glucose, 1.2 g/L fructose, 0.3 g/L trehalose, 2 g/L yeast extract, 1 g/L (NH4)2SO4, 0.4 g/L MgSO4·7H2O, and 2 g/L KH2PO4 in 10% v/v ethanol) was obtained from Coronado et al. [21]. After 8 h, 1 × 106 cells/mL were harvested and transferred into 100 mL medium composed of 50 mL YPD mixed with 50 mL SW, raising the ethanol level to 5% (v/v), and incubated for another 8 h. Finally, 1 × 106 cells/mL from the previous medium were harvested and inoculated into 100 mL of SW + 10% (v/v) ethanol condition and incubated for 24 h.
Cell concentrations were verified at each transfer by Neubauer chamber (Precicolor, HBG, Giessen-Luetzellinden, Germany) counts. This step wise regimen produced a population incrementally acclimated to 10% ethanol while minimizing physiological shock and allowing reproducible comparisons across all adaptation stages.
2.2. Cell Wall Susceptibility Analysis
Structural alterations in the cell wall of B. bruxellensis LAMAP 2480 that arose during step wise ethanol adaptation were evaluated by determining in each culture the cell wall susceptibility to the lytic enzyme zymolyase 20T (AMS Biotechnology, Milton, UK), following Shimoi et al. [22]. For every adaptation stage, 50 mL of culture was harvested under centrifugation at 6000 rpm for 30 min. The resulting pellet was rinsed once with sterile distilled water and resuspended in 1.3 mL of 0.1 M sodium phosphate buffer (pH 7.5). Zymolyase was then added to give a final concentration of 20 µg/mL. Cell lysis was monitored spectrophotometrically as the time-dependent decrease in optical density at 600 nm (OD600); the reduction in OD600 relative to the initial reading was taken as a quantitative measure of cell wall susceptibility.
2.3. Transmission Electron Microscopy (TEM)
To observe changes in the cell wall during the different stages of adaptation to ethanol, 5 mL of culture was centrifuged at 6000× g for 5 min. Then, the pellet obtained was washed with sterile distilled water twice. Subsequently, the pellet was fixed with 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer pH 7.0. As a control, yeast were grown in YPD medium. Pontificia Universidad Católica de Chile provided the TEM service.
2.4. Polysaccharide Composition of the Cell Wall
The polysaccharides were quantified by Dionex liquid chromatography, using glucose, mannose, and glucosamine as standard patterns. The service was provided by Dr. Luis Castillo from the Universidad de La Serena jointly with the Universidad de Guanajuato, Mexico. Cells were collected by centrifugation at 8500× g for 10 min, washed twice with 50 mM sodium phosphate buffer (pH 6.0), and broken with glass beads using a FastPrep machine (Qbiogene, Irvine, CA, USA). The homogenate was centrifuged at 21,500× g for 10 min. The pellet was washed with 1 M NaCl, resuspended in buffer (500 mM Tris-HCl buffer [pH 7.5], 2% (wt/vol) SDS, 0.3 M β-mercaptoethanol, 1 mM EDTA), boiled for 10 min, and freeze-dried [23]. For glucose and mannose quantification, cell walls were hydrolyzed in 2 M trifluoroacetic acid, boiled for 3 h, washed, and centrifuged at 21,500× g for 10 min. Chitin content was determined by hydrolyzing the cell walls in 6 N HCl at 100 °C for 17 h. Quantification of the sugar monomers from the acid-hydrolyzed walls was achieved by high-performance anion exchange chromatography with pulsed amperometric detection in a carbohydrate analyzer from the Dionex-LC system (Surrey, UK) [23]. The total protein concentration was determined using the Bradford method [23,24].
2.5. Evaluation of Some Genes Overexpressed in B. bruxellensis Under Different Ethanol Concentrations
Previously, our group identified the overexpression of orthologous genes of S. cerevisiae in B. bruxellensis when this latter yeast was grown in 10% ethanol. These genes included ROM2, SED1, and KNR4/SMI1. Then, in this research, we continued to deepen our study. The nucleotide sequences of the KNR4/SMI1, ROM2, and SED1 genes of S. cerevisiae S288c, as well as the amino acid sequences of the proteins encoded by the genes of interest, were obtained from the KEGG database (https://www.genome.jp/kegg/, accessed on 17 May 2024) of the Bioinformatics Center, Institute for Chemical Research, Kyoto University. Genomic data of the B. bruxellensis strain LAMAP2480 were obtained from the National Center for Biotechnology Information (NCBI) database (http://www.ncbi.nlm.nih.gov, accessed on 17 May 2024). Local tBLASTn alignments were performed between the amino acid and nucleotide sequences of S. cerevisiae and B. bruxellensis LAMAP2480 to determine a sequence of interest and domains related to proteins involved in the cell wall integrity pathway.
From the alignments performed, primers were designed for the KNR4/SMI1, ROM2, and SED1 genes using the NCBI database (Table 1).

Table 1.
Primers designed for qPCR analysis of the genes of interest.
2.6. RNA Extraction
RNA was extracted from the samples described in point 2.1, according to [25] Godoy et al. (2016). At the end of the lag phase, 50 mL aliquots were taken and then centrifuged at 2850× g for 10 min. The pellet was resuspended in 200 μL of RNA buffer (50 mM Tris-HCl pH 7.4, 100 mM NaCl, 10 mM EDTA), 400 μL of acidic phenol (Winkler, Chile) and glass beads previously washed with HCl. Vortex agitation was then repeated 3 times for 1 min each, and incubation on ice for 1 min. Then, 200 μL of RNA buffer and 40 μL of 10% SDS were added. The samples were incubated for 6 min at 65 °C and centrifuged at 16,000× g for 15 min at 4 °C. The precipitate was then discarded, transferring the supernatant to a new tube, to which 400 μL of acid phenol and 40 μL of 3 M sodium acetate were added. It was then centrifuged at 16,000× g for 15 min at 4 °C. The supernatant was transferred to a new Eppendorf tube, and then 1 mL of cold 96% ethanol was added and left for 2h at −80 °C. The precipitated RNA was centrifuged at 4 °C for 10 min and then continued with the PureLink RNA Mini Kit from Invitrogen Thermo Fisher Scientific (Waltham, MA, USA). The integrity of the RNA was assessed by 1% agarose gel electrophoresis.
2.7. Relative Expression Quantification
For RT-PCR, the RQ1 RNase-Free DNase (Promega, Madison, WI, USA) and M-MLV RT (Promega, USA) protocols were used. The qPCR assays were performed on AriaMx Real-Time PCR equipment (Agilent Technologies, Petaling Jaya, Malaysia) using Agilent Aria 1.8 equipment software. The reactions were performed in a final volume of 20 µL according to the 5x HOT FIREpol EvaGreen qPCR Mix Plus (ROX) protocol (Solis Biodyne, Tartu, Estonia) with the following program: 95 °C for 12 min, 40 amplification cycles of 95 °C for 15 s, 50.5 °C to 55.2 °C (according to Tm primers, Table 1) for 20 s and 72 °C for 20 s. In addition, the dissociation curve at the end of the qPCR cycle was performed with the following program: 95 °C for 15 s, 50.5 °C to 55.2 °C for 1 min, and 95 °C for 15 s.
Each reaction was performed in triplicate for each gene under study, using ACT1 as a reference gene (housekeeping) [26]. Relative quantification of the genes of interest was performed using the mathematical method described by Livak and Schmittgen [27]. This approach evaluates relative changes in gene expression between an experimental condition (synthetic wine with ethanol) and a control condition (YPD medium, ethanol-free reference culture) normalized to an internal reference or housekeeping gene.
To determine the efficiency (E) of the reaction and the correlation coefficient (R2) for the amplifications performed by each gene under study, the linear regression model estimated by Svec et al. [28] was used.
2.8. Statistical Analysis
Relative gene expression levels were quantified using the t-test. One-way ANOVA (p < 0.05) followed by Duncan’s multiple range test was used to analyze the effects of zymolyase on the cell wall of B. bruxellensis, as well as for the transmission electron microscopy (TEM) and the quantification of cell wall polysaccharide content. Statistical analyses were performed using Statgraphics Plus v.19 software (Manugistic Group, Inc., Rockville, MD, USA). Graphs were constructed using GraphPad Prism v.10 software (Boston, MA, USA). All experiments were conducted in triplicate.
3. Results
3.1. Effect of the Zymolyase Enzyme on the Cell Wall of B. bruxellensis Grown at Increasing Ethanol Concentrations
The commercial enzymatic preparation known as zymolyase contains several enzymatic activities that can attack different cell wall polymers. The main one is β-1,3 glucan laminaripentaohydrolase, which hydrolyses linear glucose polymers with β-1,3 linkages and residual protease activity [29,30]. To determine whether the concentration of β-glucans in the yeast cell wall is affected by growth in ethanol medium, B. bruxellensis cultures grown at different concentrations of this alcohol were exposed to a 20 μg/mL solution of zymolyase 20T (Figure 1).
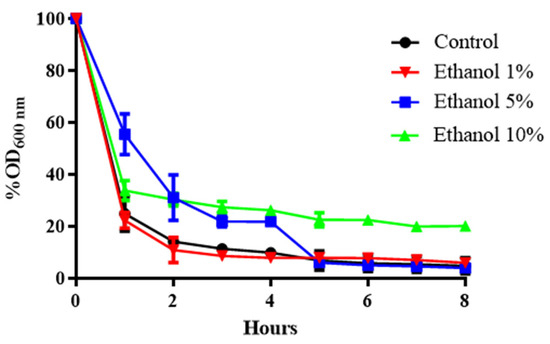
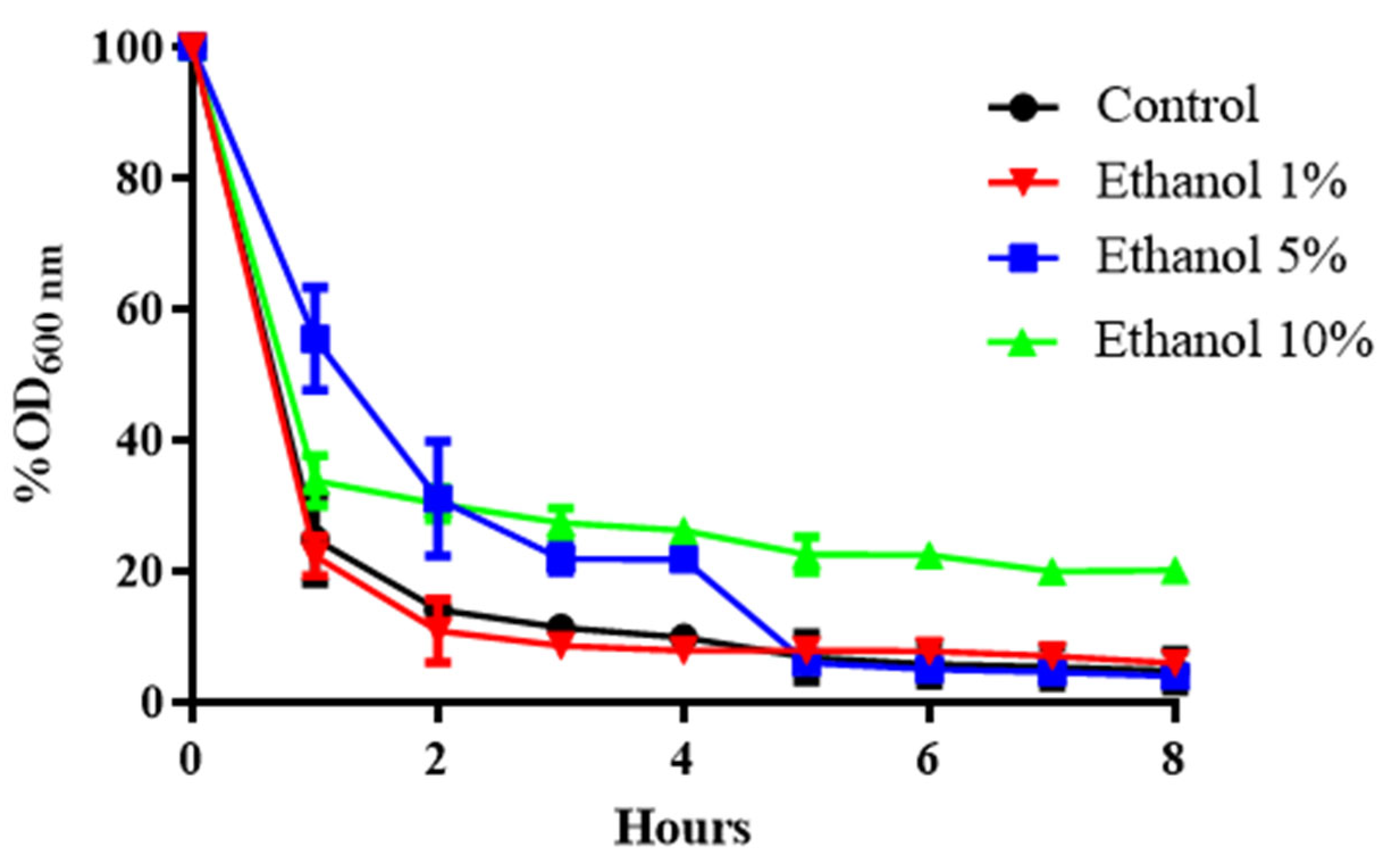
Figure 1.
Susceptibility of the cell wall of B. bruxellensis to the enzyme zymolyase in a sample of yeast grown at different ethanol concentrations. Comparison among cells grown with 1%, 5%, and 10% ethanol. Control: YPD medium. The experiments were performed in triplicate. Statistical analysis was performed using one-way ANOVA (p < 0.05), followed by Duncan’s multiple range test.
Figure 1 shows that as the ethanol concentration in the culture medium of B. bruxellensis increases, the sensitivity to zymolyase decreases. After 2 h at 1% (v/v) ethanol, survival dropped by approximately 90%. When ethanol increased to 5% or 10% (v/v), survival fell by approximately 70% (Figure 1). Likewise, when the cell was previously adapted to ethanol, it was observed that there was greater resistance to exposure to zymolyase (Figure 1). This suggests that when yeast is grown in the presence of alcohol, a change in the composition of glucose polymers with β-1,3 linkages could occur, the links that zymolyase degrades.
3.2. Analysis of the Cell Wall by TEM
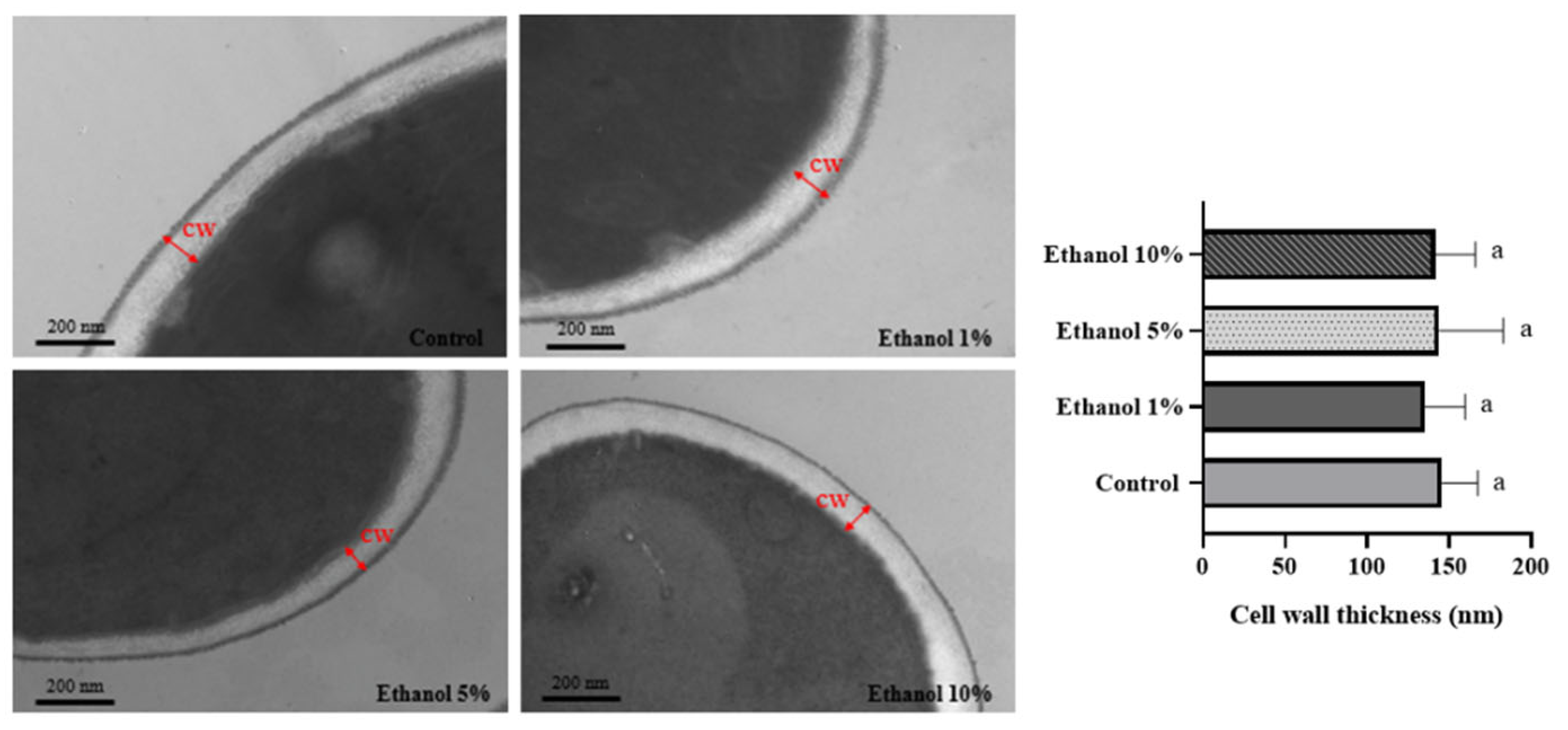
Transmission electron microscopy shows that yeast, such as S. cerevisiae and Kluyveromyces lactis, have a cell wall formed by two layers. The chitin, β-1,3, and β-1,6 glucans composition is responsible for the electron-transparent inner layer and gives it its rigidity. The dense outer layer comprises mannoproteins. Most non-Saccharomyces yeasts can survive under extreme conditions such as high pH, high temperature, etc. [31], where the cell wall plays a crucial role in the mechanical properties. TEM analysis allowed us to determine the cell wall thickness of B. bruxellensis yeasts grown in different ethanol concentrations. Figure 2 shows the results obtained, observing that the cell wall thickness was similar in all the samples analyzed.
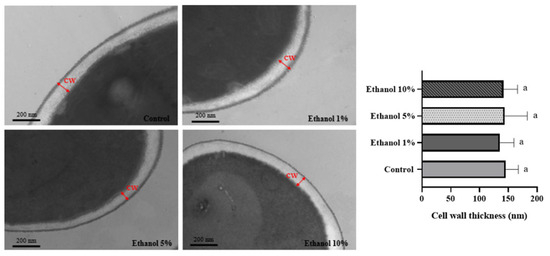
Figure 2.
TEM of B. bruxellensis during the endpoint of the lag phase of the growth curve. Representative images of untreated cells and cells treated with different concentrations of ethanol are shown. Cell wall thickness analysis was measured by taking 11 different points from six cells in each treatment and control. Treatments: synthetic wine (SW) with ethanol 1%, 5%, and 10%. Control: yeast grown in YPD medium. CW: cell wall. The experiments were performed in triplicate. Statistical analysis was performed using one-way ANOVA (p < 0.05), followed by Duncan’s multiple range test. Different letters in the same graphic indicate statistically significant differences (p < 0.05).
3.3. Determination of Cell Wall Polysaccharide Concentration
To quantify the components that constitute the cell wall of B. bruxellensis and determine whether yeast growth at different ethanol concentrations alters this chemical composition, a quantification of mannans, glucans, and chitin, as well as the protein content in the cell wall, was carried out (Figure 3).
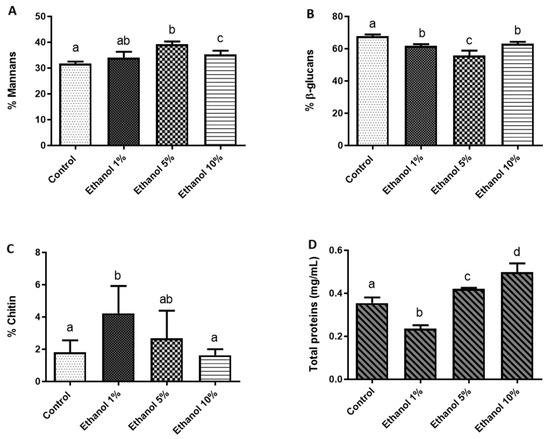
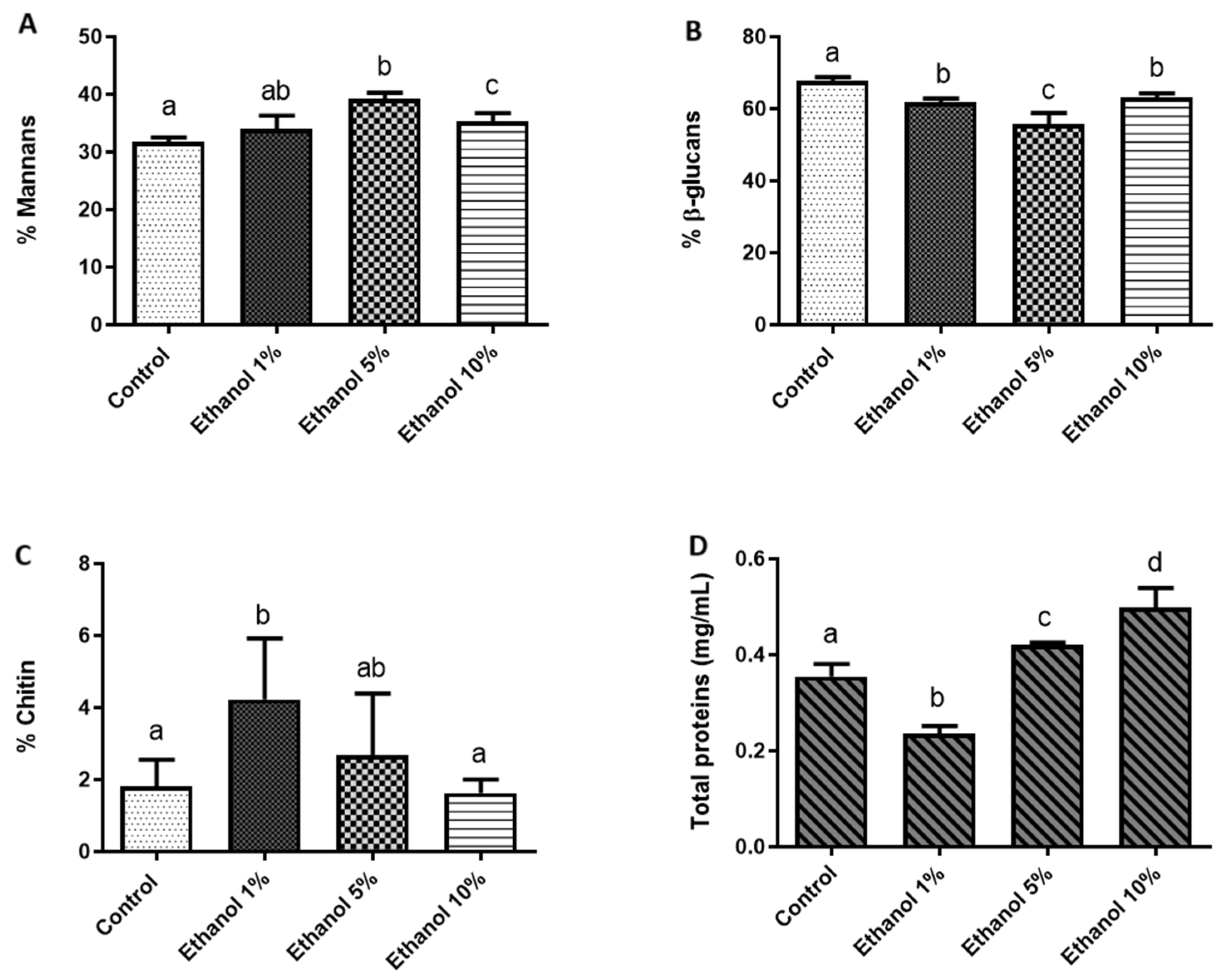
Figure 3.
Percentage of polysaccharides and cell wall proteins of B. bruxellensis. (A) Mannans, (B) glucans, (C) chitin, and (D) total proteins (mg/mL). All quantifications were performed in triplicate. Statistical analysis was performed using one-way ANOVA (p < 0.05), followed by Duncan’s multiple range test. Different letters in the same graphic indicate statistically significant differences (p < 0.05).
Figure 3 shows the ethanol-dependent remodeling of the B. bruxellensis LAMAP2480 cell wall. In YPD medium (nutritive and ethanol-free medium), the wall was dominated by β-glucans (70% of the material quantified), while mannans accounted for 32%, chitin for 2%, and wall-associated proteins for 0.35%. The introduction of 1% (v/v) ethanol elicited an immediate redistribution of these components: β-glucans and total proteins declined, whereas the mannan fraction rose modestly and chitin nearly doubled, indicating an early reinforcement of the wall’s structural scaffold. At 5% ethanol, the trend shifted. Mannans and proteins surpassed the control values, β-glucans underwent a further reduction, and chitin returned to levels indistinguishable from the YPD reference. This pattern suggests compensatory synthesis of mannoproteins to counterbalance the progressive loss of β-glucan polymers. Growth in 10% ethanol accentuated the same response. β-glucans remained significantly lower than in control (YPD), yet both mannans and proteins exceeded control levels, reflecting a continued bias toward a more mannoprotein-rich, β-glucan-poor wall architecture. Chitin again showed no detectable difference from the control, implying that its earlier increase was transitory and confined to the initial ethanol shock.
3.4. Obtaining the orthologous genes KNR4/SMI1, ROM2 and SED1 from S. cerevisiae in B. bruxellensis
Yeast cells sense and respond to physiological stress by evoking an adaptive response. Ethanol is an important inhibitor of yeast growth that works at relatively low concentrations. It inhibits cell division and decreases cell volume and specific growth rates, while a high ethanol concentration reduces cell vitality and increases cell death [32]. Ethanol also influences cell metabolism and macromolecular biosynthesis by inducing the production of heat shock-like proteins, lowering the rate of RNA and protein accumulation, enhancing the frequency of petite mutations, altering metabolism, denaturing intracellular proteins and glycolytic enzymes, and reducing their activity [10]. There is a well-studied signal transduction cascade called cell wall integrity (CWI), which is activated by a set of plasma membrane-spanning sensors in yeast. Some genes activated with increased ethanol concentration in the culture medium have been described.
An example is two genes that code for the Rom1/Rom2 factors (guanine nucleotide exchange factors), which activate the small-G protein Rho1 [33]. It has been demonstrated that ROM2 is required for S. cerevisiae growth in an ethanol-containing medium and is involved in cell wall biosynthesis [34]. In addition, our research group carried out an experiment where B. bruxellensis was grown in SW with 10% ethanol. Under these conditions, we identified an overexpression of orthologous genes of S. cerevisiae in B. bruxellensis. Some of these genes were ROM2, SED1, and KNR4/SMI1. Likewise, a change in the expression of the SED1 gene, which is involved in the metabolism of a glycoprotein, has been described, as well as KNR4/SMI1, which is related to the increase in β-glucan in the cell wall and its assembly [11].
The first genomes of B. bruxellensis were described in the 2000s [35]. Although some genes are already annotated in this genome, they are not yet fully described. Therefore, to evaluate the effect of ethanol on the expression of the genes of interest, it was necessary to suggest a possible location and sequence of KNR4/SMI1, ROM2, and SED1, orthologues of S. cerevisiae S288c in B. bruxellensis LAMAP2480. For this purpose, the nucleotide and amino acid sequences of these genes in S. cerevisiae S288c were searched using the KEGG database (https://www.genome.jp/kegg, accessed on 17 May 2024). To obtain suggestions for the possible location of the genes of interest in the B. bruxellensis LAMAP 2480 genome, a tBLASTn alignment was performed at NCBI with the amino acid sequences of the proteins encoded by the KNR4/SMI1, ROM2, and SED1 genes. In this way, the areas with the highest percentage of identity in the analyzed sequence were identified (Table 2).

Table 2.
NCBI tBLASTn of B. bruxellensis LAMAP2480 sequence against S. cerevisiae Knr4p/Smi1p, Rom2p and Sed1p amino acid sequences.
In the case of the KNR4/SMI1 gene, the database identified four possible similar regions in the genome sequence of B. bruxellensis LAMAP2480, with a level of gene coverage in the genome of 67%, with an identity percentage of 30.62%. This alignment showed the highest level of identity, which was optimal for the research objective. As for the ROM2 gene, two segments were obtained with a high level of homology between the amino acid sequences of the gene and the Brettanomyces genome. The identity percentage between the gene and the genome segment was 45.08%, with a total coverage of 69%. For the SED1 gene, the encoded amino acid sequences of this gene were analyzed, obtaining a similarity between two genome segments. By identifying the possible sequences of the KNR4/SMI1, ROM2, and SED1 genes in B. bruxellensis, primers were developed for subsequent experiments.
3.5. Gene Expression Analysis
The effect of ethanol concentration on the relative expression of these genes was evaluated using qPCR (Figure 4).
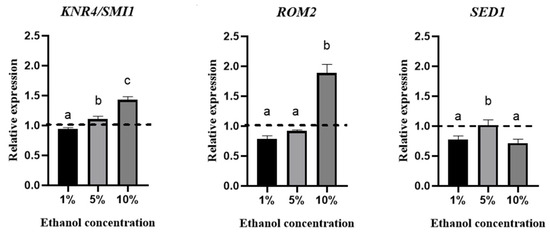
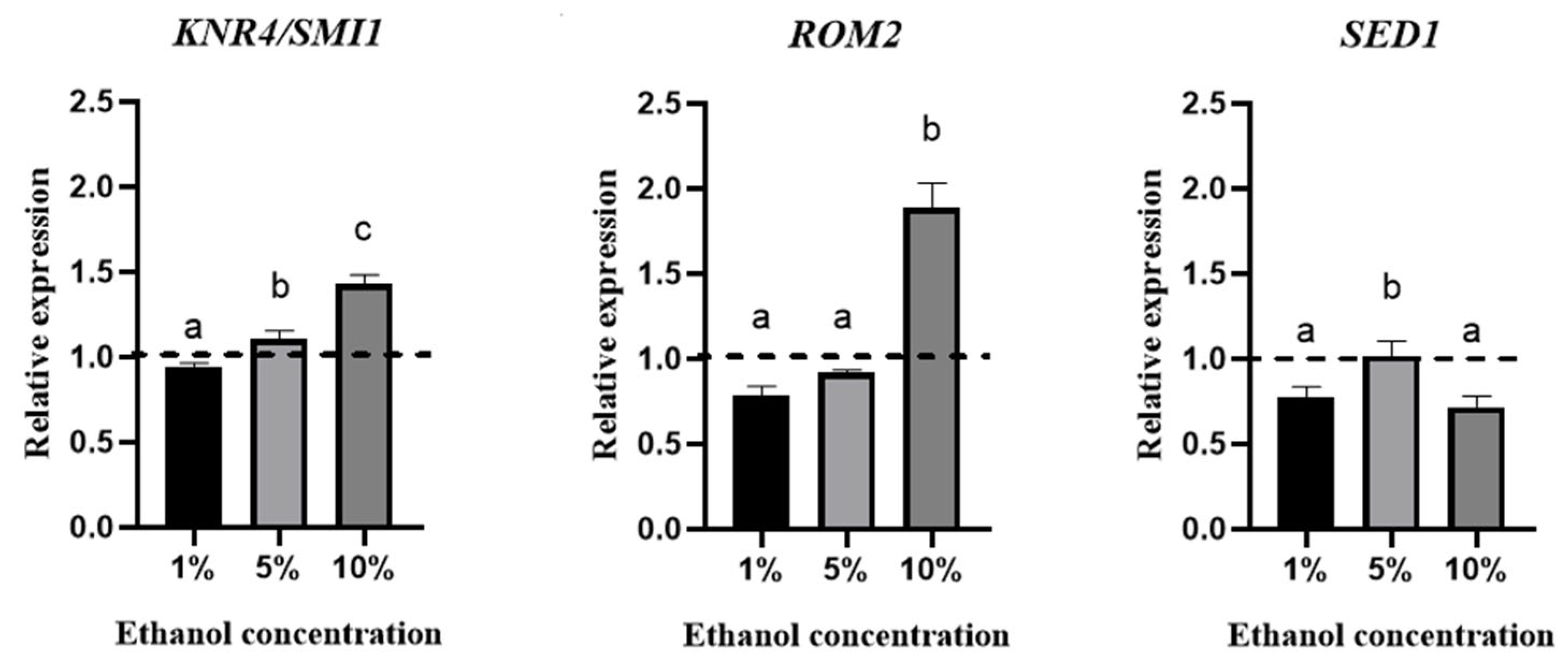
Figure 4.
Relative expression of KNR4/SMI1, ROM2, and SED1 genes at the endpoint of the lag phase at different ethanol concentrations (1%, 5%, and 10%). The line above 1 is considered overexpression. The experiments were performed in triplicate, and statistical analysis was performed using t tests. Different letters in the same graphic indicate statistically significant differences (p < 0.05).
This figure shows overexpression of the KNR4/SMI1 gene in culture media containing 5% and 10% (v/v) ethanol. In the case of the ROM2 gene, overexpression was only observed when the yeasts were grown in the presence of 10% (v/v) ethanol. No overexpression was observed for the SED1 gene under the conditions studied.
4. Discussion
The wine fermentation process begins with a varied population of yeasts selected as the alcohol concentration increases. This is how a few yeast genera remain active in substrates with concentrations above 10% (v/v) of ethanol. Adapting these genera to high ethanol concentrations is crucial for their survival. Several studies have reported that the cell wall is essential since changes in cell composition and loss of rigidity have been observed when subjected to high alcohol concentrations [11,36]. The cell wall is the first physical barrier against the different culture conditions for yeasts and is essential for cell survival [37].
For B. bruxellensis, there are no studies that have analyzed the composition of its cell wall or the possible adaptation mechanisms of this yeast to high alcohol concentrations, considering that this microorganism usually proliferates during the maturation of wines, where there is at least 10% (v/v) alcohol [5].
In the present work, the structural changes in the cell wall and CWI-related gene expression in cells grown in the presence of ethanol were evaluated. First, a sensitivity test was performed with the enzyme zymolyase, which has β-1,3 glucanase activity (Figure 1). The results indicated a lower sensitivity of the cell wall to the activity of this enzyme as the ethanol concentration increased. Similar results have been described in S. cerevisiae when it was grown in a medium supplemented with 6% (v/v) ethanol, observing a lower sensitivity to β-1,3 glucanase activity [38]. This suggests that the cell wall of both S. cerevisiae and B. bruxellensis changes its chemical composition as the ethanol concentration in the culture medium increases, resulting in an adaptation mechanism to this stress factor.
On the other hand, it has been described that in S. cerevisiae, no significant changes in cell wall thickness are observed in cells grown in a medium supplemented with 9% (v/v) ethanol [36]. A similar observation was made in the present study (Figure 2). This would indicate that there are no changes in the spatial configuration of the cell wall components when varying the culture medium that the yeast faces. The yeast cell wall is organized into two main layers, composed of four macromolecules: cell wall proteins, β-1,6 glucans, β-1,3 glucans, and chitin [39]
To evaluate whether ethanol stress reshapes the cell wall architecture, we quantified the main polysaccharide fractions. Rising ethanol concentrations led to a drop in chitin and a concomitant rise in total protein content (Figure 3). Mannan levels also trended upward during adaptation, reaching a peak at 5% (v/v) ethanol. In the case of glucans, β-glucans stayed below control values even at 10% ethanol (Figure 3). Opposite results have been reported in S. cerevisiae, where there are no significant differences in the percentage of mannans, glucans, or chitin when grown in a medium supplemented with 9% (v/v) ethanol [40]. However, Orlean [41] indicated that an increase of ethanol in the culture medium leads to remodeling of the cell wall architecture to allow it to become more robust. This includes an increase in cell wall components and a change in the cross-linking between them. Some mannoproteins can have a structural role or mediate social activity by serving as mating agglutinins, or they might promote the formation of biofilms. Mannoprotein would have a role in yeast adaptation to this stress [42]. On the other hand, Uscanga and Francois [39] demonstrated that cell growth with ethanol was almost completely refractory to zymolyase, indicating it might produce more β-1,6 glucan than β-1,3 glucan. β-1,6 glucan will be an important component of the cell wall in stress conditions. Bekirian et al. [43] showed that β-1,6 glucan has a pivotal role in determining the architectural arrangement of polysaccharides in the cell wall. This will influence growth, drug sensitivity, cell morphology, and filamentation. β-1,6 glucan biosynthesis is stimulated via a compensatory pathway when there is a defect in cell wall mannan biosynthesis. Then, the variation in the concentration of these two cell wall compounds in the B. bruxellensis cell wall is related to the mechanism that yeast use in stress conditions.
Our results suggest that B. bruxellensis would respond to ethanol differently from that observed in S. cerevisiae. Valdivia and Schekman [44] reported that S. cerevisiae, in response to heat, decreases the synthesis of β-glucans and increases the export of chitin synthases from chitosomes to the plasma membrane. Notably, the changes induced by ethanol are identical to those caused by heat stress, suggesting a “functional overlap” between heat- and ethanol-induced cell damage [45].
In previous work, we observed that when B. bruxellensis was grown in a medium containing ethanol, cell wall-related genes such as ROM2, KNR4/SMI1, and SED1 were overexpressed. In the current study, we confirmed the overexpression of the ROM2 gene (Figure 4) when the yeast was grown in a medium supplemented with 10% (v/v) ethanol. In this regard, genes required for growth in S. cerevisiae under ethanol stress have been identified, demonstrating that ROM2 is essential for its growth [35]. It has been described that the Rom2p protein is fundamental for activating the CWI pathway and for the synthesis of β-glucans. S. cerevisiae mutants in the ROM2 gene have reduced its β-1,3 glucan synthase activity [46]. Likewise, the overexpression of ROM2 in S. cerevisiae triggers the accumulation of β-1,3 glucan in secretory vesicles to be exported to the cell wall. Additionally, there is an increase in ROM2 gene expression in yeast cells grown in a medium supplemented with 1 M sorbitol, defined as a polyalcohol [47]. Our results showed that when yeast grows with 10% (v/v) ethanol, there is a slight increase in β-glucans. However, this somewhat contradicts the sensitivity of B. bruxellensis cells to the enzyme zymolyase, which decreases when this microorganism grows in a medium supplemented with 10% (v/v) ethanol. Comparing different responses to different stressors reveals not only the existence of specific transcriptional adaptation profiles for each situation but also the presence of a standard signature that is induced in all these stress situations [29].
On the other hand, in S. cerevisiae, the Knr4/Smi1 protein represents a conserved family of fungus-specific proteins. It was initially identified in Hansenula mrakii during a study about genes affecting cell wall β-1,3 glucan synthesis. This gene would be related to increased chitin concentration and decreased β-glucans in the yeast cell wall [13]. The KNR4/SMI1 gene has been described to encode a phosphoprotein in several cellular processes, such as cell wall maintenance, cell cycle, osmoregulation, and spore formation [48]. This gene is required to appropriately target the RIm1p and Swi4p transcription factors by the mitogen-activated protein kinase (MAPK) Slt2, which is involved in cell wall remodeling [13]. The overexpression of KNR4/SMI1 in a wild-type S. cerevisiae strain caused increased resistance to drugs that affect the cell wall. Figure 4 shows the expression of the KNR4/SMI1 gene when B. bruxellensis was grown in different concentrations of ethanol, observing that at both 5% and 10% ethanol there was an overexpression of this gene. A similar response was reported by S. cerevisiae when it was grown in a medium with 5% (v/v) of ethanol for 30 min, observing an overexpression of this gene [49].
In the case of the SED1 gene, it has been described as necessary in cell wall integrity since it encodes the Sed1p protein, which is involved in the recovery of proteins from the endoplasmic reticulum of the secretory and structural pathway of the cell wall. This is the main stress-induced glycoprotein (GPI) [50]. In S. cerevisiae, the expression of SED1 occurs mainly in the stationary phase of cell growth [22]. When the SED1 gene was interrupted, it was observed that it does not affect the cell in the exponential growth phase, but in the stationary phase it is crucial for lytic resistance. By mutating this gene in S. cerevisiae, an increase in sensitivity to zymolyase was observed compared to the wild-type strain [22].
On the other hand, the overexpression of SED1 in S. cerevisiae cells exposed to 5% (v/v) ethanol for one hour was observed as a phenomenon of adaptation to ethanol during the first phase of the growth curve (lag phase) [51]. SED1 is upregulated under thermal, oxidative, nutritional, and hyperosmotic stress. These findings suggest that SED1 has an important function of protection against any factor that induces extreme conditions in the cell [12,22]. In the case of B. bruxellensis, overexpression of this gene was not observed when the yeasts were exposed to 1, 5, or 10% (v/v) ethanol. Moreover, there was repression in this last condition (10% ethanol). The expression of the SED1 gene was not detected after the adaptation time of the growth curve of S. cerevisiae [51]. Our study considered the expression of these genes at the end of the lag phase, so it is important to consider the growth stage in a future study.
5. Conclusions
Our findings demonstrate that B. bruxellensis adapts to wine-like ethanol levels by strategically remodeling its cell wall rather than thickening it. A temporary increase in chitin was observed at 1% ethanol during a stepwise ethanol concentration increase from 0% to 10% (v/v), as shown by quantitative chromatography, followed by a return to its initial levels and concurrent rises by mannans of 20% and by wall-associated proteins of 60%. This compositional shift translated into functionally stronger walls: susceptibility to the β-1,3-glucanase zymolyase dropped by more than half, yet transmission electron microscopy confirmed that the wall thickness remained unchanged.
At the molecular level, we identified the B. bruxellensis orthologues of ROM2 and KNR4/SMI1 and observed ≥2-fold over-expression of both genes at 10% ethanol, while SED1 expression stayed constant. This is the first evidence that the conserved cell wall integrity pathway operates in this species under ethanol stress. Together, the biochemical, structural, and transcriptomic data converge on a single adaptive mechanism: ethanol triggers a controlled depletion of chitin and enrichment of mannoproteins, orchestrated by the ROM2/KNR4 axis, to reinforce the wall’s mechanical resilience and likely restrict ethanol ingress—ultimately enabling B. bruxellensis to thrive in high-ethanol environments.
Author Contributions
Conceptualization, M.A.G., L.G. and L.C.; Formal analysis, L.H.-C., Y.C., V.P. and H.M.M.-M.; Funding acquisition, M.A.G.; Methodology, L.G., L.C., L.H.-C., N.R.-T., C.G.-P. and H.M.M.-M.; Supervision, L.G., C.G.-P. and M.A.G.; Writing—preparation of the original draft, review and editing, L.G., N.R.-T., C.G.-P. and M.A.G. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Universidad de Santiago de Chile, USACH with the DICYT 082371GM Grant, Grant 2023_1 of the Technological Faculty and Fondecyt Regular 1221209 Grant.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Claussen, N.H. On a Method for the Application of Hansen’s Pure Yeast System in the Manufacturing of Well-Conditioned English Stock Beers. J. Inst. Brew. 1904, 10, 308–331. [Google Scholar] [CrossRef]
- Harris, V.; Ford, C.M.; Jiranek, V.; Grbin, P.R. Dekkera and Brettanomyces Growth and Utilisation of Hydroxycinnamic Acids in Synthetic Media. Appl. Microbiol. Biotechnol. 2008, 78, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Da-Silva, E.; Surribas, A.; Gambari, C.; Granès, D.; Pic, L.; Barthelmebs, L. Development of a Molecular Diagnostic Test for the Specific Detection of Brettanomyces bruxellensis in Red Wine. Int. J. Food Microbiol. 2023, 407, 110394. [Google Scholar] [CrossRef] [PubMed]
- Schumaker, M.R.; Diako, C.; Castura, J.C.; Edwards, C.G.; Ross, C.F. Influence of Wine Composition on Consumer Perception and Acceptance of Brettanomyces Metabolites Using Temporal Check-All-That-Apply Methodology. Food Res. Int. 2019, 116, 963–972. [Google Scholar] [CrossRef]
- Miranda, J.; Miot-Sertier, C.; Olazabal, L.; Albertin, W.; Richard, T.; Da Costa, G.; Rouger, C.; Dols-Lafargue, M. Bordeaux Red Wines Display Variable Intrinsic Ability to Support Brettanomyces bruxellensis Growth. Food Control 2024, 155, 110067. [Google Scholar] [CrossRef]
- Chandra, M.; Branco, P.; Prista, C.; Malfeito-Ferreira, M. Role of P-Coumaric Acid and Micronutrients in Sulfur Dioxide Tolerance in Brettanomyces bruxellensis. Beverages 2023, 9, 69. [Google Scholar] [CrossRef]
- Louw, M.; du Toit, M.; Alexandre, H.; Divol, B. Comparative Morphological Characteristics of Three Brettanomyces bruxellensis Wine Strains in the Presence/absence of Sulfur Dioxide. Int. J. Food Microbiol. 2016, 238, 79–88. [Google Scholar] [CrossRef]
- Serpaggi, V.; Remize, F.; Recorbet, G.; Gaudot-Dumas, E.; Sequeira-Le Grand, A.; Alexandre, H. Characterization of the “Viable but Nonculturable” (VBNC) State in the Wine Spoilage Yeast Brettanomyces. Food Microbiol. 2012, 30, 438–447. [Google Scholar] [CrossRef]
- Oelofse, A.; Pretorius, I.S.; Du Toit, M. Significance of Brettanomyces and Dekkera during Winemaking: A Synoptic Review. S. Afr. J. Enol. Vitic. 2008, 29, 128–144. [Google Scholar] [CrossRef]
- Paschos, T.; Xiros, C.; Christakopoulos, P. Simultaneous Saccharification and Fermentation by Co-Cultures of Fusarium oxysporum and Saccharomyces cerevisiae Enhances Ethanol Production from Liquefied Wheat Straw at High Solid Content. Ind. Crops Prod. 2015, 76, 793–802. [Google Scholar] [CrossRef]
- Sahana, G.R.; Balasubramanian, B.; Joseph, K.S.; Pappuswamy, M.; Liu, W.-C.; Meyyazhagan, A.; Kamyab, H.; Chelliapan, S.; Joseph, B.V. A Review on Ethanol Tolerance Mechanisms in Yeast: Current Knowledge in Biotechnological Applications and Future Directions. Process Biochem. 2024, 138, 1–13. [Google Scholar] [CrossRef]
- Klis, F.M.; Boorsma, A.; De Groot, P.W.J. Cell Wall Construction in Saccharomyces cerevisiae. Yeast 2006, 23, 185–202. [Google Scholar] [CrossRef]
- Batista, M.; Donker, E.I.M.; Bon, C.; Guillien, M.; Caisso, A.; Mourey, L.; François, J.-M.; Maveyraud, L.; Zerbib, D. The Conserved Yeast Protein Knr4 Involved in Cell Wall Integrity Is a Multi-Domain Intrinsically Disordered Protein. J. Mol. Biol. 2023, 435, 168048. [Google Scholar] [CrossRef] [PubMed]
- Sanz, A.B.; García, R.; Rodríguez-Peña, J.M.; Arroyo, J. The CWI Pathway: Regulation of the Transcriptional Adaptive Response to Cell Wall Stress in Yeast. J. Fungi 2017, 4, 1. [Google Scholar] [CrossRef]
- Ibe, C.; Munro, C.A. Fungal Cell Wall Proteins and Signaling Pathways Form a Cytoprotective Network to Combat Stresses. J. Fungi 2021, 7, 739. [Google Scholar] [CrossRef]
- Li, X.; Ohmori, T.; Irie, K.; Kimura, Y.; Suda, Y.; Mizuno, T.; Irie, K. Different Regulations of and Expression by Ccr4, Pop2, and Dhh1 in the Cell Wall Integrity Pathway. mSphere 2016, 1, e00250-16. [Google Scholar] [CrossRef]
- Bakir, G.; Dahms, T.E.S.; Martin-Yken, H.; Bechtel, H.A.; Gough, K.M. CellWall Remodeling in the Absence of Knr4 and Kre6 Revealed by Nano-FourierTransform Infrared Spectroscopy. Appl. Spectrosc. 2024, 78, 355–364. [Google Scholar] [CrossRef]
- Schifferdecker, A.J.; Dashko, S.; Ishchuk, O.P.; Piškur, J. The Wine and Beer Yeast Dekkera bruxellensis. Yeast 2014, 31, 323–332. [Google Scholar] [CrossRef]
- Le Montagner, P.; Bakhtiar, Y.; Miot-Sertier, C.; Guilbaud, M.; Albertin, W.; Moine, V.; Dols-Lafargue, M.; Masneuf-Pomarède, I. Effect of Abiotic and Biotic Factors on Brettanomyces bruxellensis Bioadhesion Properties. Food Microbiol. 2024, 120, 104480. [Google Scholar] [CrossRef]
- Di Canito, A.; Foschino, R.; Vigentini, I. Flocculation Mechanisms in: Influence of Ethanol and Sulfur Dioxide on Gene Expression. Curr. Res. Microb. Sci. 2025, 8, 100372. [Google Scholar] [CrossRef]
- Coronado, P.; Aguilera, S.; Carmona, L.; Godoy, L.; Martínez, C.; Ganga, M.A. Comparison of the Behaviour of Brettanomyces bruxellensis Strain LAMAP L2480 Growing in Authentic and Synthetic Wines. Antonie Van. Leeuwenhoek 2015, 107, 1217–1223. [Google Scholar] [CrossRef] [PubMed]
- Shimoi, H.; Kitagaki, H.; Ohmori, H.; Iimura, Y.; Ito, K. Sed1p Is a Major Cell Wall Protein of Saccharomyces cerevisiae in the Stationary Phase and Is Involved in Lytic Enzyme Resistance. J. Bacteriol. 1998, 180, 3381–3387. [Google Scholar] [CrossRef] [PubMed]
- Mora-Montes, H.M.; Bates, S.; Netea, M.G.; Díaz-Jiménez, D.F.; López-Romero, E.; Zinker, S.; Ponce-Noyola, P.; Kullberg, B.J.; Brown, A.J.P.; Odds, F.C.; et al. Endoplasmic Reticulum Alpha-Glycosidases of Candida albicans Are Required for N Glycosylation, Cell Wall Integrity, and Normal Host-Fungus Interaction. Eukaryot. Cell 2007, 6, 2184–2193. [Google Scholar] [CrossRef]
- Fleet, G.H. Composition and Structure of Yeast Cell Walls. Curr. Top. Med. Mycol. 1985, 1, 24–56. [Google Scholar] [CrossRef]
- Godoy, L.; Vera-Wolf, P.; Martinez, C.; Ugalde, J.A.; Ganga, M.A. Comparative Transcriptome Assembly and Genome-Guided Profiling for Brettanomyces bruxellensis LAMAP2480 during P-Coumaric Acid Stress. Sci. Rep. 2016, 6, 34304. [Google Scholar] [CrossRef]
- Nardi, T.; Remize, F.; Alexandre, H. Adaptation of Yeasts Saccharomyces cerevisiae and Brettanomyces bruxellensis to Winemaking Conditions: A Comparative Study of Stress Genes Expression. Appl. Microbiol. Biotechnol. 2010, 88, 925–937. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Svec, D.; Tichopad, A.; Novosadova, V.; Pfaffl, M.W.; Kubista, M. How Good Is a PCR Efficiency Estimate: Recommendations for Precise and Robust qPCR Efficiency Assessments. Biomol. Detect. Quantif. 2015, 3, 9–16. [Google Scholar] [CrossRef]
- Arroyo, J.; Bermejo, C.; García, R.; Rodríguez-Peña, J.M. Genomics in the Detection of Damage in Microbial Systems: Cell Wall Stress in Yeast. Clin. Microbiol. Infect. 2009, 15 (Suppl. S1), 44–46. [Google Scholar] [CrossRef]
- Rodríguez-Peña, J.M.; Díez-Muñiz, S.; Bermejo, C.; Nombela, C.; Arroyo, J. Activation of the Yeast Cell Wall Integrity MAPK Pathway by Zymolyase Depends on Protease and Glucanase Activities and Requires the Mucin-like Protein Hkr1 but Not Msb2. FEBS Lett. 2013, 587, 3675–3680. [Google Scholar] [CrossRef]
- Schiavone, M.; François, J.M.; Zerbib, D.; Capp, J.-P. Emerging Relevance of Cell Wall Components from Non-Conventional Yeasts as Functional Ingredients for the Food and Feed Industry. Curr. Res. Food Sci. 2023, 7, 100603. [Google Scholar] [CrossRef] [PubMed]
- Birch, R.M.; Walker, G.M. Influence of Magnesium Ions on Heat Shock and Ethanol Stress Responses of Saccharomyces cerevisiae. Enzym. Microb. Technol. 2000, 26, 678–687. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.C.T.; Schneper, L.; Russo, M.; Fernandes, A.A.R.; Broach, J.R.; Fernandes, P.M.B. Comparative Transcriptome Analysis of Industrial and Laboratory Saccharomyces cerevisiae Strains after Sequential Stresses. Fermentation 2024, 10, 395. [Google Scholar] [CrossRef]
- Takahashi, T.; Shimoi, H.; Ito, K. Identification of Genes Required for Growth under Ethanol Stress Using Transposon Mutagenesis in Saccharomyces cerevisiae. Mol. Genet. Genom. 2001, 265, 1112–1119. [Google Scholar] [CrossRef]
- Woolfit, M.; Rozpedowska, E.; Piskur, J.; Wolfe, K.H. Genome Survey Sequencing of the Wine Spoilage Yeast Dekkera (Brettanomyces) Bruxellensis. Eukaryot. Cell 2007, 6, 721–733. [Google Scholar] [CrossRef]
- Schiavone, M.; Formosa-Dague, C.; Elsztein, C.; Teste, M.-A.; Martin-Yken, H.; De Morais, M.A., Jr.; Dague, E.; François, J.M. Evidence for a Role for the Plasma Membrane in the Nanomechanical Properties of the Cell Wall as Revealed by an Atomic Force Microscopy Study of the Response of Saccharomyces cerevisiae to Ethanol Stress. Appl. Environ. Microbiol. 2016, 82, 4789–4801. [Google Scholar] [CrossRef]
- Cansado, J.; Soto, T.; Franco, A.; Vicente-Soler, J.; Madrid, M. The Fission Yeast Cell Integrity Pathway: A Functional Hub for Cell Survival upon Stress and Beyond. J. Fungi 2021, 8, 32. [Google Scholar] [CrossRef]
- Teixeira, M.C.; Raposo, L.R.; Mira, N.P.; Lourenço, A.B.; Sá-Correia, I. Genome-Wide Identification of Saccharomyces cerevisiae Genes Required for Maximal Tolerance to Ethanol. Appl. Environ. Microbiol. 2009, 75, 5761–5772. [Google Scholar] [CrossRef]
- Aguilar-Uscanga, B.; François, J.M. A Study of the Yeast Cell Wall Composition and Structure in Response to Growth Conditions and Mode of Cultivation. Lett. Appl. Microbiol. 2003, 37, 268–274. [Google Scholar] [CrossRef]
- Schiavone, M.; Vax, A.; Formosa, C.; Martin-Yken, H.; Dague, E.; François, J.M. A Combined Chemical and Enzymatic Method to Determine Quantitatively the Polysaccharide Components in the Cell Wall of Yeasts. FEMS Yeast Res. 2014, 14, 933–947. [Google Scholar] [CrossRef]
- Orlean, P. Architecture and Biosynthesis of the Saccharomyces cerevisiae Cell Wall. Genetics 2012, 192, 775–818. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, R.A.; Bourbon-Melo, N.; Sá-Correia, I. The Cell Wall and the Response and Tolerance to Stresses of Biotechnological Relevance in Yeasts. Front. Microbiol. 2022, 13, 953479. [Google Scholar] [CrossRef] [PubMed]
- Bekirian, C.; Valsecchi, I.; Bachellier-Bassi, S.; Scandola, C.; Guijarro, J.I.; Chauvel, M.; Mourer, T.; Gow, N.A.R.; Aimanianda, V.K.; d’Enfert, C.; et al. β-1,6-Glucan Plays a Central Role in the Structure and Remodeling of the Bilaminate Fungal Cell Wall. Elife 2024, 13, 100569. [Google Scholar] [CrossRef]
- Valdivia, R.H.; Schekman, R. The Yeasts Rho1p and Pkc1p Regulate the Transport of Chitin Synthase III (Chs3p) from Internal Stores to the Plasma Membrane. Proc. Natl. Acad. Sci. USA 2003, 100, 10287–10292. [Google Scholar] [CrossRef]
- Piper, P.W. The Heat Shock and Ethanol Stress Responses of Yeast Exhibit Extensive Similarity and Functional Overlap. FEMS Microbiol. Lett. 1995, 134, 121–127. [Google Scholar] [CrossRef]
- Sekiya-Kawasaki, M.; Abe, M.; Saka, A.; Watanabe, D.; Kono, K.; Minemura-Asakawa, M.; Ishihara, S.; Watanabe, T.; Ohya, Y. Dissection of Upstream Regulatory Components of the Rho1p Effector, 1,3-Beta-Glucan Synthase, in Saccharomyces cerevisiae. Genetics 2002, 162, 663–676. [Google Scholar] [CrossRef]
- Gasch, A.P.; Spellman, P.T.; Kao, C.M.; Carmel-Harel, O.; Eisen, M.B.; Storz, G.; Botstein, D.; Brown, P.O. Genomic Expression Programs in the Response of Yeast Cells to Environmental Changes. Mol. Biol. Cell 2000, 11, 4241–4257. [Google Scholar] [CrossRef]
- Durand, F.; Dagkessamanskaia, A.; Martin-Yken, H.; Graille, M.; Van Tilbeurgh, H.; Uversky, V.N.; François, J.M. Structure-Function Analysis of Knr4/Smi1, a Newly Member of Intrinsically Disordered Proteins Family, Indispensable in the Absence of a Functional PKC1-SLT2 Pathway in Saccharomyces cerevisiae. Yeast 2008, 25, 563–576. [Google Scholar] [CrossRef]
- Lewis, J.A.; Broman, A.T.; Will, J.; Gasch, A.P. Genetic Architecture of Ethanol-Responsive Transcriptome Variation in Saccharomyces cerevisiae Strains. Genetics 2014, 198, 369–382. [Google Scholar] [CrossRef]
- Arnthong, J.; Ponjarat, J.; Bussadee, P.; Deenarn, P.; Prommana, P.; Phienluphon, A.; Charoensri, S.; Champreda, V.; Zhao, X.-Q.; Suwannarangsee, S. Enhanced Surface Display Efficiency of β-Glucosidase in Saccharomyces cerevisiae by Disruption of Cell Wall Protein-Encoding Genes YGP1 and CWP2. Biochem. Eng. J. 2022, 179, 108305. [Google Scholar] [CrossRef]
- Chandler, M.; Stanley, G.A.; Rogers, P.; Chambers, P. A Genomic Approach to Defining the Ethanol Stress Response in the Yeast Saccharomyces cerevisiae. 2004, 54, 427–454. 54.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).