Enhancing Agricultural Sustainability by Improving the Efficiency of Lignocellulosic Biomass Utilization in the Ruminant Diet via Solid-State Fermentation with White-Rot Fungi: A Review
Abstract
1. Introduction
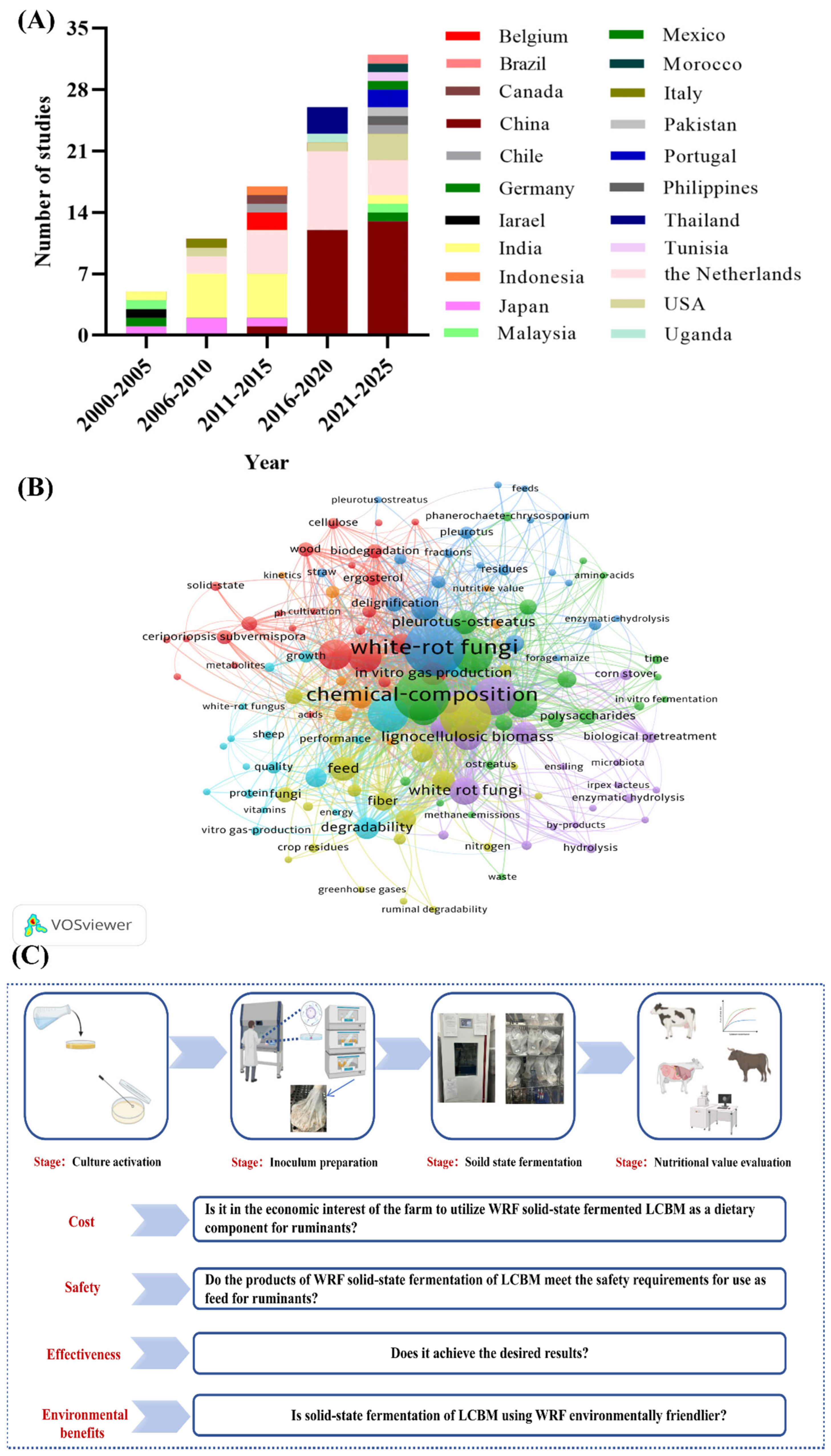
2. Global Research Trends
3. Pros and Cons of Utilizing LCBM in Ruminants
4. Key Technical Factors for LCBM in Solid-State Fermentation at WRF
4.1. Fungal
4.2. Substrate
4.3. Environmental Parameters
| Strain/Straw | Size (cm) | Temperature (°C) | Humidity (%RH) | Strain Additions | Time (day) | Moisture (%) | Sterilization Conditions | Ref. |
|---|---|---|---|---|---|---|---|---|
| Wheat straw | ||||||||
| P. ostreatus | 5–10 | 25 | 78 | 4% | 21 | infuse | 100 °C (1 h) | [43] |
| P. ostreatus and T. versicolor | 1.5–2 | 30 | - | 0.5% | 30 | 30% | 121 °C (15 min) | [44] |
| C. subvermispora and L. edodes | 0.5 | 24 | - | 10% (Barley grains) | 39 and 52 | 40% | 121 °C (1 h) | [45] |
| P. chrysosporium, P. ostreatus and I. lacteus | 0.1 | 28 | - | 3 agar | 7, 14, 21, and 49 | 70% | 121 °C (20 min) | [46] |
| P. ostreatus | 2.5 | 24 | 75–85 | 3% | 0, 10, 20, and 30 | 70% | Lime, steam, and formaldehyde sterilization | [47] |
| Rice straw | ||||||||
| P. ostreatus | 2–3 | 25 | 75–80 | 3% (millet grain) | 30 | 50% | 121 °C (25 min) | [48] |
| P. chrysosporium and P. ostreatus | 2–3 | 25 | 75–80 | 5% (millet grain) | 30 | infuse | 121 °C (45 min) | [49] |
| P. ostreatus | 2–3 | 22 | 80–90 | 3–5% | 35 | infuse | Alternative silage sterilization | [50] |
| C. subvermispora, L. edodes, P. eryngii and P. ostreatus | 2–5 | 24 | 75 | 6 g | 21 and 42 | infuse | 121 °C (1 h) | [51] |
| C.subvermispora, L. edodes and P. eryngii | 3–5 | 24, 30, 35, and 40 | - | 5% | 28, 42, 49, and 56 | 75% | 121 °C (1 h) | [52] |
| Corn straw | ||||||||
| P. ostreatus | 2 | 24 | 70 | 1% | 21, 28, and 35 | - | 121 °C (1 h) | [53] |
| L. edodes and P. eryngii | 3 | 24 | 70 | 2.5% | 21, 42, and 63 | - | 121 °C (1 h) | [54] |
| P. ostreatus, L. edodes, H. erinaceus, P. eryngii, and F. filiformis | 2–3 | 24 | 75 | 8%, 10% | 14, 21, 28, 35, and | - | 121 °C (2 h) | [55] |
| P. diamor, P. eryngii, P. sajor-caju and P. citrinopileatus | 2–3 | 25 | 70–80 | 10% | 21 | - | 121 °C (1 h) | [56] |
| C. subvermispora, L. edodes, P. eryngii, and P. ostreatus | 2–5 | 24 | 75 | 6 g | 21 and 42 | infuse | 121 °C (1 h) | [51] |
| Oher straw | ||||||||
| P. chrysosporium, C. subvermispora, L. edode, and P. acerina/Canola straw | 1–2 | 25 | - | 3% | 10, 20, and 30 | 50% | 121 °C (15 min) | [57] |
| A. bisporus, P. djamor, C. indica, and P. ostreatus/Bagasse | 2 | 24 | 75–85 | 3% | 21 and 56 | 75% | 90 °C (2 h) | [58] |
| L. edodes, P. eryngii, and P. citrinopileatus/Grage stalk | 0.3 | 28 | 85 | 2.5% | 28, 35, and 42 | infuse | 121 °C (30 min) | [59] |
| P. citrinopileatus/Cowpea straw | 2 | 28 | 75 | 4% | 22 | 75 | 121 °C (30 min) | [60] |
| P. ostreatus and P. chrysosporium/White tea straw | 2–3 | 25 | 60 | 6% | 7, 14, 21, and 28 | 70 | 121 °C (20 min) | [61] |
5. Effects of WRF Solid-State Fermentation of LCBM
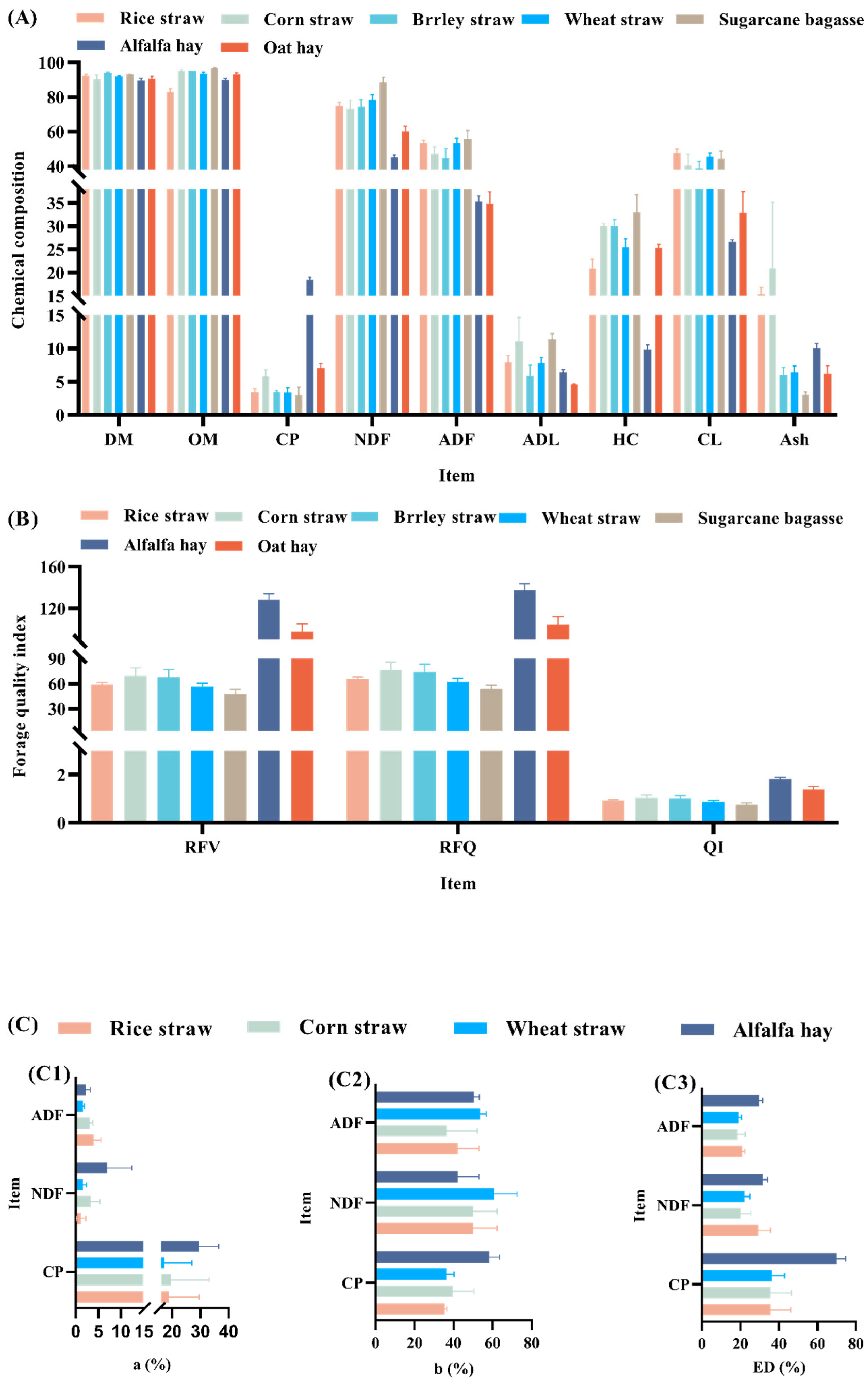
5.1. Chemical Nutrient Composition of LCBM
5.2. Experimental Evaluation of Rumen In Vitro Fermentation of LCBM After Solid-State Fermentation
5.3. Experimental Evaluation of Rumen In Vivo Fermentation of LCBM After Solid State Fermentation
6. CH4 Emissions
7. Future Perspectives on the Limitations of WRF Solid-State Fermentation for LCBM Treatment
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- World Food and Agriculture—Statistical Yearbook 2024; FAO: Rome, Italy, 2024; ISBN 978-92-5-139255-3.
- Han, Y. Exploring Biomimetic Potential of Ruminant Digestion Strategies for Lignocellulosic Biomass Utilization: A Comprehensive Review. Renew. Sustain. Energy Rev. 2023, 188, 113887. [Google Scholar] [CrossRef]
- Saravanan, A.; Yaashikaa, P.R.; Kumar, P.S.; Thamarai, P.; Deivayanai, V.C.; Rangasamy, G. A Comprehensive Review on Techno-Economic Analysis of Biomass Valorization and Conversional Technologies of Lignocellulosic Residues. Ind. Crops Prod. 2023, 200, 116822. [Google Scholar] [CrossRef]
- Chatterjee, S.; Venkata Mohan, S. Fungal Biorefinery for Sustainable Resource Recovery from Waste. Bioresour. Technol. 2022, 345, 126443. [Google Scholar] [CrossRef] [PubMed]
- Firkins, J.L. Invited Review: Advances in Rumen efficiency resented as Part of the ARPAS Symposium: New Advances in Dairy Efficiency at the American Dairy Science Association Virtual Annual Meeting, June 2020. Appl. Anim. Sci. 2021, 37, 388–403. [Google Scholar] [CrossRef]
- Gao, Q.; Liu, H.; Wang, Z.; Lan, X.; An, J.; Shen, W.; Wan, F. Recent Advances in Feed and Nutrition of Beef Cattle in China—A Review. Anim. Biosci. 2023, 36, 529–539. [Google Scholar] [CrossRef]
- Van Kuijk, S.J.A.; Sonnenberg, A.S.M.; Baars, J.J.P.; Hendriks, W.H.; Cone, J.W. Fungal Treated Lignocellulosic Biomass as Ruminant Feed Ingredient: A Review. Biotechnol. Adv. 2015, 33, 191–202. [Google Scholar] [CrossRef]
- Adesogan, A.T.; Arriola, K.G.; Jiang, Y.; Oyebade, A.; Paula, E.M.; Pech-Cervantes, A.A.; Romero, J.J.; Ferraretto, L.F.; Vyas, D. Symposium Review: Technologies for Improving Fiber Utilization. J. Dairy Sci. 2019, 102, 5726–5755. [Google Scholar] [CrossRef]
- Sun, X.; Dou, Z.; Shurson, G.C.; Hu, B. Bioprocessing to Upcycle Agro-Industrial and Food Wastes into High-Nutritional Value Animal Feed for Sustainable Food and Agriculture Systems. Resour. Conserv. Recycl. 2024, 201, 107325. [Google Scholar] [CrossRef]
- Nayan, N.; Sonnenberg, A.S.M.; Hendriks, W.H.; Cone, J.W. Prospects and Feasibility of Fungal Pretreatment of Agricultural Biomass for Ruminant Feeding. Anim. Feed Sci. Technol. 2020, 268, 114577. [Google Scholar] [CrossRef]
- Donthu, N.; Kumar, S.; Mukherjee, D.; Pandey, N.; Lim, W.M. How to Conduct a Bibliometric Analysis: An Overview and Guidelines. J. Bus. Res. 2021, 133, 285–296. [Google Scholar] [CrossRef]
- Xiao, P.; Wu, D.; Wang, J. Bibliometric Analysis of Global Research on White Rot Fungi Biotechnology for Environmental Application. Environ. Sci. Pollut. Res. 2022, 29, 1491–1507. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wei, L.; Wang, W. Review: Challenges and Prospects for Milk Production in China After the 2008 Milk Scandal. Appl. Anim. Sci. 2021, 37, 166–175. [Google Scholar] [CrossRef]
- Wang, X.; Shen, W.; Wu, P.; Wang, C.; Li, J.; Wang, D.; Yue, W. How Food Consumption Trends Change the Direction of Sheep Breeding in China. Animals 2024, 14, 3047. [Google Scholar] [CrossRef] [PubMed]
- Dahal, B.R.; DeLong, K.L.; Gao, S.; Grebitus, C.; Muhammad, A. Factors Affecting Chinese Consumers’ Beef Purchase Frequency. Agribusiness 2025, 41, 521–543. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, X.; Liu, Y.; Dong, P.; Liang, R.; Hopkins, D.L.; Holman, B.W.B.; Luo, X.; Zhu, L.; Yang, Z.; et al. Chinese Consumer Perception and Purchasing Behavior of Beef—Mainly in North and East China. Meat Sci. 2025, 220, 109696. [Google Scholar] [CrossRef]
- Chen, A.; Moradi, S.; Huang, J.; Xu, S.; Sismey, M.; Hort, J. Older Chinese Adults’ Milk Consumption Habits: A Study Across 5 Cities. J. Dairy Sci. 2024, 107, 3515–3530. [Google Scholar] [CrossRef]
- Chen, J.; Yang, C.-C.; Lin, Y. Effects of the COVID-19 Pandemic on Dairy Consumption Trends: An Empirical Investigation of Accounting Data in China. Foods 2024, 13, 741. [Google Scholar] [CrossRef]
- Clauss, M.; Hummel, J. Physiological Adaptations of Ruminants and Their Potential Relevance for Production Systems. R. Bras. Zootec. 2017, 46, 606–613. [Google Scholar] [CrossRef]
- Weimer, P.J. Redundancy, Resilience, and Host Specificity of the Ruminal Microbiota: Implications for Engineering Improved Ruminal Fermentations. Front. Microbiol. 2015, 6, 296. [Google Scholar] [CrossRef]
- Weimer, P.J. Degradation of Cellulose and Hemicellulose by Ruminal Microorganisms. Microorganisms 2022, 10, 2345. [Google Scholar] [CrossRef]
- Wang, B.; Sun, H.; Wang, D.; Liu, H.; Liu, J. Constraints on the Utilization of Cereal Straw in Lactating Dairy Cows: A Review from the Perspective of Systems Biology. Anim. Nutr. 2022, 9, 240–248. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Kong, F.; Wang, W.; Li, S. Comparison of Nutritional Components, Ruminal Degradation Characteristics and Feed Value from Different Cultivars of Alfalfa Hay. Animals 2023, 13, 734. [Google Scholar] [CrossRef]
- Foster, J.L.; Smith, W.B.; Rouquette, F.M.; Tedeschi, L.O. Forages and Pastures Symposium: An Update on in Vitro and in Situ Experimental Techniques for Approximation of Ruminal Fiber Degradation. J. Anim. Sci. 2023, 101, skad097. [Google Scholar] [CrossRef]
- Wang, E.; Wang, J.; Lv, J.; Sun, X.; Kong, F.; Wang, S.; Wang, Y.; Yang, H.; Cao, Z.; Li, S.; et al. Comparison of Ruminal Degradability, Indigestible Neutral Detergent Fiber, and Total-Tract Digestibility of Three Main Crop Straws with Alfalfa Hay and Corn Silage. Animals 2021, 11, 3218. [Google Scholar] [CrossRef]
- Li, Q.; Xue, B.; Zhao, Y.; Wu, T.; Liu, H.; Yi, X.; Sun, C.; Wang, Z.; Zou, H.; Yan, T. In Situ Degradation Kinetics of 6 Roughages and the Intestinal Digestibility of the Rumen Undegradable Protein. J. Anim. Sci. 2018, 96, 4835–4844. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gao, J.; Cheng, C.; Lv, J.; Lambo, M.T.; Zhang, G.; Li, Y.; Zhang, Y. Nutritional Values of Industrial Hemp Byproducts for Dairy Cattle. Animals 2022, 12, 3488. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Zhang, L.-X.; Tu, Y.; Zhang, N.-F.; Si, B.-W.; Ma, T.; Diao, Q.-Y. Improving the in Situ Ruminal Degradability of Maize Stalk Using Fungal Inoculants in Dorper × Thin-Tailed Han Crossbred Ewes. Small Rumin. Res. 2016, 144, 119–125. [Google Scholar] [CrossRef]
- Du, S.; Xu, M.; Yao, J. Relationship between Fibre Degradation Kinetics and Chemical Composition of Forages and By-Products in Ruminants. J. Appl. Anim. Res. 2016, 44, 189–193. [Google Scholar] [CrossRef]
- Guo, L.; Yao, D.; Li, D.; Lin, Y.; Bureenok, S.; Ni, K.; Yang, F. Effects of Lactic Acid Bacteria Isolated From Rumen Fluid and Feces of Dairy Cows on Fermentation Quality, Microbial Community, and In Vitro Digestibility of Alfalfa Silage. Front. Microbiol. 2020, 10, 2998. [Google Scholar] [CrossRef]
- Jeranyama, P.; Garcia, A.D. Understanding Relative Feed Value (RFV) and Relative Forage Quality (RFQ). SDSU Extension Extra Archives. 2004. Available online: https://openprairie.sdstate.edu/extension_extra/352 (accessed on 16 June 2025).
- Bo, P.T.; Dong, Y.; Zhang, R.; Soe Htet, M.N.; Hai, J. Optimization of Alfalfa-Based Mixed Cropping with Winter Wheat and Ryegrass in Terms of Forage Yield and Quality Traits. Plants 2022, 11, 1752. [Google Scholar] [CrossRef]
- Zuo, S.; Niu, D.; Jiang, D.; Tian, P.; Li, R.; Wu, W. Effect of White-Rot Fungal Treatments on the In Vitro Rumen Degradability of Two Kinds of Corn Stover. BioResources 2019, 14, 895–899. [Google Scholar] [CrossRef]
- Niu, D.; Zuo, S.; Ren, J.; Li, C.; Zheng, M.; Jiang, D.; Xu, C. Effect of Wheat Straw Types on Biological Delignification and In Vitro Rumen Degradability of Wheat Straws during Treatment with Irpex lacteus. Anim. Feed Sci. Technol. 2020, 267, 114558. [Google Scholar] [CrossRef]
- Cano Y Postigo, L.O.; Jacobo-Velázquez, D.A.; Guajardo-Flores, D.; Garcia Amezquita, L.E.; García-Cayuela, T. Solid-State Fermentation for Enhancing the Nutraceutical Content of Agrifood by-Products: Recent Advances and Its Industrial Feasibility. Food Biosci. 2021, 41, 100926. [Google Scholar] [CrossRef]
- De Menezes, L.H.S.; Oliveira, P.C.; Do Espírito Santo, E.L.; Gonçalves, M.S.; Bilal, M.; Ruiz, H.A.; Da Silva, E.G.P.; Salay, L.C.; De Oliveira, J.R.; Franco, M. Solid-State Fermentation as a Green Technology for Biomass Valorization: Optimization Techniques for Bioprocess—An Overview. Bioenerg. Res. 2023, 17, 42–58. [Google Scholar] [CrossRef]
- Niu, D.; Zuo, S.; Ren, J.; Huhetaoli; Zheng, M.; Jiang, D.; Xu, C. Novel Strategy to Improve the Colonizing Ability of Irpex lacteus in Non-Sterile Wheat Straw for Enhanced Rumen and Enzymatic Digestibility. Appl. Microbiol. Biotechnol. 2020, 104, 1347–1355. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Li, R.; Wang, Y.; Cui, X.; Niu, D.; Yang, F.; Xu, C. Ensiling with Rumen Fluid Promoted Irpex lacteus Colonization on the Non-Sterile Naked Oat Straw for Enhanced Lignocellulose Degradation and Enzymatic Hydrolysis. Biochem. Eng. J. 2022, 183, 108462. [Google Scholar] [CrossRef]
- Sufyan, A.; Khan, N.A.; Akbar, A.; Tang, S.; Tan, Z. Scaling-up Fungal Pretreatment of Lignocellulose Biomass: Impact on Nutritional Value, Ruminal Degradability, Methane Production, and Performance of Lactating Dairy Cows. Livest. Sci. 2024, 285, 105499. [Google Scholar] [CrossRef]
- Farrell, J. Temperature Effects on Microorganisms. Annu. Rev. Microbiol. 1967, 21, 101–120. [Google Scholar] [CrossRef]
- Wang, D.; Luo, C.; Li, C.; Zhang, S.; Lu, N.; Yang, Z.; Yu, X.; Cao, Z.; Yang, H.; Li, S.; et al. Study on the Relationship between Fermentation-Accumulated Temperature and Nutrient Loss of Whole-Plant Corn Silage. Agronomy 2022, 12, 2752. [Google Scholar] [CrossRef]
- Benavides, V.; Ciudad, G.; Pinto-Ibieta, F.; Robledo, T.; Rubilar, O.; Serrano, A. Enhancing Laccase and Manganese Peroxidase Activity in White-Rot Fungi: The Role of Copper, Manganese, and Lignocellulosic Substrates. Agronomy 2024, 14, 2562. [Google Scholar] [CrossRef]
- Fazaeli, H.; Mahmodzadeh, H.; Azizi, A.; Jelan, Z.A.; Liang, J.B.; Rouzbehan, Y.; Osman, A. Nutritive Value of Wheat Straw Treated with Pleurotus Fungi. Asian Australas. J. Anim. Sci 2004, 17, 1681–1688. [Google Scholar] [CrossRef]
- Shrivastava, B.; Thakur, S.; Khasa, Y.P.; Gupte, A.; Puniya, A.K.; Kuhad, R.C. White-Rot Fungal Conversion of Wheat Straw to Energy Rich Cattle Feed. Biodegradation 2011, 22, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Sonnenberg, A.S.M.; Hendriks, W.H.; Cone, J.W. Preservation of Ceriporiopsis Subvermispora and Lentinula Edodes Treated Wheat Straw under Anaerobic Conditions: Preservation of Fungal-Treated Wheat Straw. J. Sci. Food Agric. 2018, 98, 1232–1239. [Google Scholar] [CrossRef] [PubMed]
- Niu, D.; Zuo, S.; Jiang, D.; Tian, P.; Zheng, M.; Xu, C. Treatment Using White Rot Fungi Changed the Chemical Composition of Wheat Straw and Enhanced Digestion by Rumen Microbiota In Vitro. Anim. Feed Sci. Technol. 2018, 237, 46–54. [Google Scholar] [CrossRef]
- Sufyan, A.; Khan, N.A.; AbuGhazaleh, A.; Ahmad, N.; Tang, S.; Tan, Z. Novel Techniques for the Mass Production of Nutritionally Improved, Fungus-Treated Lignocellulosic Biomass for Ruminant Nutrition. J. Sci. Food Agric. 2023, 104, 2215–2224. [Google Scholar] [CrossRef]
- Datsomor, O.; Yan, Q.; Wang, K.; Mohamed, S.; Opoku-Mensah, L.; Zhao, G.; Miao, L. Effect of Ammoniated and/or Basidiomycete White-Rot Fungi Treatment on Rice Straw Proximate Composition, Cell Wall Component, and In Vitro Rumen Fermentation Characteristics. Fermentation 2022, 8, 228. [Google Scholar] [CrossRef]
- Datsomor, O.; Zhao, G.-Q.; Lin, M. Effect of Ligninolytic Axenic and Coculture White-Rot Fungi on Rice Straw Chemical Composition and In Vitro Fermentation Characteristics. Sci. Rep. 2022, 12, 1129. [Google Scholar] [CrossRef]
- Kholif, A.E.; Gouda, G.A.; Patra, A.K. The Sustainable Mitigation of In Vitro Ruminal Biogas Emissions by Ensiling Date Palm Leaves and Rice Straw with Lactic Acid Bacteria and Pleurotus ostreatus for Cleaner Livestock Production. J. Appl. Microbiol. 2022, 132, 2925–2939. [Google Scholar] [CrossRef]
- Tuyen, D.V.; Phuong, H.N.; Cone, J.W.; Baars, J.J.P.; Sonnenberg, A.S.M.; Hendriks, W.H. Effect of Fungal Treatments of Fibrous Agricultural By-Products on Chemical Composition and In Vitro Rumen Fermentation and Methane Production. Bioresour. Technol. 2013, 129, 256–263. [Google Scholar] [CrossRef]
- Hai, T.T.; Peer, A.V.; Cone, J.W.; Schonewille, J.T.; Baars, J.J.P.; Phung, L.D.; Hendriks, W.H. Incubation Temperature Affects Growth and Efficacy of White-Rot Fungi to Improve the Nutritive Value of Rice Straw. Anim. Prod. Sci. 2024, 64, AN23403. [Google Scholar] [CrossRef]
- Khan, N.A.; Hussain, S.; Ahmad, N.; Alam, S.; Bezabhi, M.; Hendriks, W.H.; Yu, P.; Cone, J.W. Improving the Feeding Value of Straws with Pleurotus ostreatus. Anim. Prod. Sci. 2015, 55, 241. [Google Scholar] [CrossRef]
- He, Y.; Dijkstra, J.; Sonnenberg, A.S.M.; Mouthier, T.M.B.; Kabel, M.A.; Hendriks, W.H.; Cone, J.W. The Nutritional Value of the Lower Maize Stem Cannot Be Improved by Ensiling nor by a Fungal Treatment. Anim. Feed Sci. Technol. 2019, 247, 92–102. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, F.; Fang, Y.; Zhou, D.; Wang, S.; Wu, D.; Wang, L.; Zhong, R. High-Potency White-Rot Fungal Strains and Duration of Fermentation to Optimize Corn Straw as Ruminant Feed. Bioresour. Technol. 2020, 312, 123512. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Luo, Y.; Luo, L.; Zhang, H.; Liao, Y.; Gou, C. Enhancement of the Nutritional Value of Fermented Corn Stover as Ruminant Feed Using the Fungi Pleurotus spp. Sci. Rep. 2021, 11, 11961. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Gong, J.; Zhou, S.; OuYang, K.; Song, X.; Fu, C.; Xu, L.; Qu, M. Effect of Fungal Treatments of Rape Straw on Chemical Composition and In Vitro Rumen Fermentation Characteristics. Bioresources 2015, 10, 622–637. [Google Scholar] [CrossRef]
- Khan, N.A.; Khan, M.; Sufyan, A.; Saeed, A.; Sun, L.; Wang, S.; Nazar, M.; Tan, Z.; Liu, Y.; Tang, S. Biotechnological Processing of Sugarcane Bagasse through Solid-State Fermentation with White Rot Fungi into Nutritionally Rich and Digestible Ruminant Feed. Fermentation 2024, 10, 181. [Google Scholar] [CrossRef]
- Costa-Silva, V.; Anunciação, M.; Andrade, E.; Fernandes, L.; Costa, A.; Fraga, I.; Barros, A.; Marques, G.; Ferreira, L.; Rodrigues, M. Biovalorization of Grape Stalks as Animal Feed by Solid State Fermentation Using White-Rot Fungi. Appl. Sci. 2022, 12, 6800. [Google Scholar] [CrossRef]
- Andrade, E.; Mendes-Ferreira, A.; Botelho, S.; Marques, G.; Cone, J.W.; Rodrigues, M.; Ferreira, L. Preservation of Fungal-Treated Cowpea Straw in Association with Discarded Apple by Ensilage Process. Waste Biomass Valor. 2021, 12, 5533–5543. [Google Scholar] [CrossRef]
- Yan, Q.; Lin, M.; Huang, Y.; Datsomor, O.; Wang, K.; Zhao, G. Effects of Solid-State Fermentation Pretreatment with Single or Dual Culture White Rot Fungi on White Tea Residue Nutrients and In Vitro Rumen Fermentation Parameters. Fermentation 2022, 8, 557. [Google Scholar] [CrossRef]
- Olagunju, L.K.; Isikhuemhen, O.S.; Dele, P.A.; Anike, F.N.; Alabi, J.O.; Ike, K.A.; Shaw, Y.; Brice, R.M.; Orimaye, O.E.; Wuaku, M.; et al. The Impact of Three White-Rot Fungi on Nutrient Availability, Greenhouse Gas Emissions, and Volatile Fatty Acid Production in Myceliated Sorghum. Foods 2024, 13, 2199. [Google Scholar] [CrossRef]
- Chi, Y.; Hatakka, A.; Maijala, P. Can Co-Culturing of Two White-Rot Fungi Increase Lignin Degradation and the Production of Lignin-Degrading Enzymes? Int. Biodeterior. Biodegrad. 2007, 59, 32–39. [Google Scholar] [CrossRef]
- Tuyen, V.D.; Cone, J.W.; Baars, J.J.P.; Sonnenberg, A.S.M.; Hendriks, W.H. Fungal Strain and Incubation Period Affect Chemical Composition and Nutrient Availability of Wheat Straw for Rumen Fermentation. Bioresour. Technol. 2012, 111, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Sufyan, A.; Ahmad, N.; Shahzad, F.; Embaby, M.G.; AbuGhazaleh, A.; Khan, N.A. Improving the Nutritional Value and Digestibility of Wheat Straw, Rice Straw, and Corn Cob Through Solid State Fermentation Using Different Pleurotus Species. J. Sci. Food Agric. 2021, 102, 2445–2453. [Google Scholar] [CrossRef]
- Nasehi, M.; Torbatinejad, N.M.; Zerehdaran, S.; Safaie, A.R. Effect of Solid-State Fermentation by Oyster Mushroom (Pleurotus florida) on Nutritive Value of Some Agro by-Products. J. Appl. Anim. Res. 2017, 45, 221–226. [Google Scholar] [CrossRef]
- Timm, T.G.; Amâncio, B.R.; Loregian, K.E.; Magnani, E.; Helm, C.V.; De Lima, E.A.; Marcondes, M.I.; Branco, R.H.; De Paula, E.M.; Benedeti, P.D.B.; et al. Peach Palm Shells (Bactris gasipaes Kunth) Bioconversion by Lentinula Edodes: Potential as New Bioproducts for Beef Cattle Feeding. Bioresour. Technol. 2024, 394, 130292. [Google Scholar] [CrossRef]
- Hutson, S.M.; Sweatt, A.J.; LaNoue, K.F. Branched-Chain Amino Acid Metabolism: Implications for Establishing Safe Intakes. J. Nutr. 2005, 135, 1557S–1564S. [Google Scholar] [CrossRef]
- Conceição, A.A.; Mendes, T.D.; Mendonça, S.; Quirino, B.F.; Almeida, E.G.D.; Siqueira, F.G.D. Nutraceutical Enrichment of Animal Feed by Filamentous Fungi Fermentation. Fermentation 2022, 8, 402. [Google Scholar] [CrossRef]
- Cherdthong, A.; Seankamsorn, A.; Suriyapha, C.; Chanjula, P.; Wanapat, M. Effect of Beta-glucan Supplementation on Feed Intake, Digestibility of Nutrients and Ruminal Fermentation in Thai Native Beef Cattle. Anim. Physiol. Nutr. 2018, 102, 1509–1514. [Google Scholar] [CrossRef]
- Peng, Q.; Cheng, L.; Kang, K.; Tian, G.; Al-Mamun, M.; Xue, B.; Wang, L.; Zou, H.; Gicheha, M.G.; Wang, Z. Effects of Yeast and Yeast Cell Wall Polysaccharides Supplementation on Beef Cattle Growth Performance, Rumen Microbial Populations and Lipopolysaccharides Production. J. Integr. Agric. 2020, 19, 810–819. [Google Scholar] [CrossRef]
- Liang, J.; Zhang, R.; Chang, J.; Chen, L.; Nabi, M.; Zhang, H.; Zhang, G.; Zhang, P. Rumen Microbes, Enzymes, Metabolisms, and Application in Lignocellulosic Waste Conversion—A Comprehensive Review. Biotechnol. Adv. 2024, 71, 108308. [Google Scholar] [CrossRef]
- O’Hara, E.; Neves, A.L.A.; Song, Y.; Guan, L.L. The Role of the Gut Microbiome in Cattle Production and Health: Driver or Passenger? Annu. Rev. Anim. Biosci. 2020, 8, 199–220. [Google Scholar] [CrossRef] [PubMed]
- Copelin, J.E.; Firkins, J.L.; Socha, M.T.; Lee, C. Effects of Diet Fermentability and Supplementation of 2-Hydroxy-4-(Methylthio)-Butanoic Acid and Isoacids on Milk Fat Depression: 1. Production, Milk Fatty Acid Profile, and Nutrient Digestibility. J. Dairy Sci. 2021, 104, 1591–1603. [Google Scholar] [CrossRef] [PubMed]
- Allison, M.J.; Bryant, M.P.; Doetsch, R.N. Volatile Fatty Acid Growth Factor for Cellulolytic Cocci of Bovine Rumen. Science 1958, 128, 474–475. [Google Scholar] [CrossRef] [PubMed]
- Redoy, M.R.A.; Ahmed, S.; Bonilla Urbina, J.; Kleinschmit, D.H.; Socha, M.T.; Salunke, P.; Uddin, M.E. Supplementation of Isoacids to Lactating Dairy Cows Fed Low- or High-Forage Diets: Effects on Performance, Digestibility, and Milk Fatty Acid Profile. J. Dairy Sci. 2025, 108, 1408–1418. [Google Scholar] [CrossRef]
- Wang, Y.; Gou, C.; Chen, L.; Liao, Y.; Zhang, H.; Luo, L.; Ji, J.; Qi, Y. Solid-State Fermentation with White Rot Fungi (Pleurotus Species) Improves the Chemical Composition of Highland Barley Straw as a Ruminant Feed and Enhances In Vitro Rumen Digestibility. J. Fungi 2023, 9, 1156. [Google Scholar] [CrossRef]
- Zuo, S.; Niu, D.; Zheng, M.; Jiang, D.; Tian, P.; Li, R.; Xu, C. Effect of Irpex lacteus, Pleurotus ostreatus and Pleurotus cystidiosus Pretreatment of Corn Stover on Its Improvement of the In Vitro Rumen Fermentation. J. Sci. Food Agric. 2018, 98, 4287–4295. [Google Scholar] [CrossRef]
- Abid, K.; Boudagga, S.; Abid, O.; Najar, T.; Jaouani, A. Bioconversion of Grape Pomace Waste into Suitable Alternative Feed for Ruminants with Pleurotus cornucopiae and Ganoderma resinaceum via Solid-State Fermentation Bioprocess. Biomass Conv. Bioref. 2023, 15, 1–10. [Google Scholar] [CrossRef]
- Chanjula, P.; Petcharat, V.; Cherdthong, A. Effects of Fungal (Lentinussajor-Caju) Treated Oil Palm Frond on Performance and Carcass Characteristics in Finishing Goats. Asian-Australas J. Anim. Sci. 2017, 30, 811–818. [Google Scholar] [CrossRef]
- Xiang, H.; Zhao, X.; Fang, Y.; Wang, F.; Liang, R.; Sun, X.; Wang, S.; Zhong, R. Feeding Fungal-Pretreated Corn Straw Improves Health and Meat Quality of Lambs Infected with Gastrointestinal Nematodes. Animals 2020, 10, 1659. [Google Scholar] [CrossRef]
- Zailan, M.Z.; Salleh, S.M.; Abdullah, S.; Yaakub, H. Effect of Feeding Pleurotus Pulmonarius-treated Empty Fruit Bunch on Nutrient Digestibility and Milk Fatty Acid Profiles in Goats. Trop. Anim. Health Prod. 2023, 55, 402. [Google Scholar] [CrossRef]
- Hamchara, P.; Chanjula, P.; Cherdthong, A.; Wanapat, M. Digestibility, Ruminal Fermentation, and Nitrogen Balance with Various Feeding Levels of Oil Palm Fronds Treated with Lentinus Sajor-Caju in Goats. Asian-Australas. J. Anim. Sci. 2018, 31, 1619–1626. [Google Scholar] [CrossRef] [PubMed]
- Laconi, E.B.; Jayanegara, A. Improving Nutritional Quality of Cocoa Pod (Theobroma cacao) Through Chemical and Biological Treatments for Ruminant Feeding: In Vitro and In Vivo Evaluation. Asian-Australas. J. Anim. Sci. 2015, 28, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Mizrahi, I.; Wallace, R.J.; Moraïs, S. The Rumen Microbiome: Balancing Food Security and Environmental Impacts. Nat. Rev. Microbiol. 2021, 19, 553–566. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Cao, Y.; Li, Q.; Li, Q.; Yang, X.; Wang, R.; Zhang, X.; Tan, Z.; Lin, B.; Wang, M. Mitigating Enteric Methane Emissions: An Overview of Methanogenesis, Inhibitors and Future Prospects. Anim. Nutr. 2025, 21, 84–96. [Google Scholar] [CrossRef]
- Jahromi, M.F.; Liang, J.B.; Mohamad, R.; Goh, Y.M.; Shokryazdan, P.; Ho, Y.W. Lovastatin-Enriched Rice Straw Enhances Biomass Quality and Suppresses Ruminal Methanogenesis. BioMed Res. Int. 2013, 2013, 397934. [Google Scholar] [CrossRef]
- Ábrego-Gacía, A.; Poggi-Varaldo, H.M.; Robles-González, V.; Ponce-Noyola, T.; Calva-Calva, G.; Ríos-Leal, E.; Estrada-Bárcenas, D.; Mendoza-Vargas, A. Lovastatin as a Supplement to Mitigate Rumen Methanogenesis: An Overview. J. Anim. Sci. Biotechnol. 2021, 12, 123. [Google Scholar] [CrossRef]
- Svobodová, K. Bioreactors Based on Immobilized Fungi: Bioremediation Under Non-Sterile Conditions. Appl. Microbiol. Biotechnol. 2018, 102, 39–46. [Google Scholar] [CrossRef]
- Chen, J.; Cai, Y.; Wang, Z.; Xu, Z.; Li, J.; Ma, X.; Zhuang, W.; Liu, D.; Wang, S.; Song, A.; et al. Construction of a Synthetic Microbial Community Based on Multiomics Linkage Technology and Analysis of the Mechanism of Lignocellulose Degradation. Bioresour. Technol. 2023, 389, 129799. [Google Scholar] [CrossRef]
- Lin, L. Bottom-up Synthetic Ecology Study of Microbial Consortia to Enhance Lignocellulose Bioconversion. Biotechnol. Biofuels Bioprod. 2022, 15, 14. [Google Scholar] [CrossRef]
| Strain/Straw | DM | CP | NDF | ADF | ADL | HC | CL | OM | RFV | RFQ | QI | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wheat straw | ||||||||||||
| P. ostreatus | −19 | +25.8 | −15.6 | −24.3 | −38.8- | −38.2 | −21.4 | −20.6 | +8.51 | +4.75 | +3.31 | [64] |
| I. lacteus | −22.34 | +25.5 | −14.00 | −4.00 | −43.08 | −46.03 | −20.13 | −22.2 | +15.40 | +24.51 | +22.19 | [46] |
| P. eryngii | −12.15 | +16.35 | −5.2 | −2.32 | −20.36 | −14.57 | +1.32 | −12.46 | +7.61 | +16.18 | +13.89 | [65] |
| Pleurotus florida | −8.69 | +49.73 | −11.54 | −15.13 | −10.40 | −2.13 | −16.15 | −3.09 | +30.02 | +33.52 | +22.16 | [66] |
| P. chrysosporium | −45.17 | +34.43 | −28.26 | −28.04 | −24.00 | −61.00 | −61.90 | −47.96 | +39.27 | +48.77 | +45.54 | [46] |
| Rice straw | ||||||||||||
| P. ostreatus | −5.92 | +60.88 | −16.53 | −10.5 | −31.89 | −28.1 | −6.75 | −9.63 | +22.09 | +27.77 | +25.44 | [48] |
| C. eriporiopsis | - | +22.93 | −22.83 | +3 | −84.59 | −58.83 | +12.32 | −3.51 | +21.48 | +35.88 | +33.20 | [52] |
| L. edodes | - | +20.85 | −20.71 | +1.73 | −73.61 | −48.60 | +6.53 | −2.74 | +21.49 | +34.60 | +31.84 | [52] |
| P. eryngii | −4.50 | +19.30 | −21.00 | −7.70 | −41.10 | −43.00 | −2.90 | −4.90 | +36.67 | +31.05 | +19.34 | [51] |
| P. chrysosporium | −13.14 | +22.83 | −29.74 | −31.56 | −24.26 | −19.83 | −23.93 | −22.68 | +35.74 | +43.73 | +40.45 | [49] |
| Corn stover | ||||||||||||
| P. ostreatus | −10.50 | +66.19 | −18.35 | −18.13 | −44.67 | −18.52 | −9.07 | −1.92 | +22.67 | +27.16 | +24.62 | [53] |
| P. eryngii | +0.24 | +31.21 | −15.91 | −16.72 | −33.12 | −15.03 | - | −3.90 | +21.74 | +25.83 | +23.65 | [55] |
| F. filigormis | +0.31 | +33.99 | −21.04 | −13.54 | −20.14 | −29.17 | - | −2.87 | +25.53 | +32.42 | +29.91 | [55] |
| L. edodes | +0.61 | +36.29 | −17.79 | −12.14 | −29.88 | −23.90 | - | −2.80 | +22.01 | +26.47 | +24.26 | [55] |
| P. diamor | - | +33.19 | −7.00 | −5.80 | −15.03 | −9.74 | −6.43 | −7.52 | +11.24 | +11.80 | +10.55 | [56] |
| Other LCBM | ||||||||||||
| L. edodes/Rape straw | −16.8 | +22.82 | −17.0 | −14.3 | −9.40 | −24.9 | −15.7 | −17.2 | +30.34 | +38.51 | +34.97 | [57] |
| P. ostreatus/Bagasse | −15.10 | +84.3 | −31.70 | −26.50 | −41.50 | −41.80 | −23.00 | +23.30 | +29.64 | +44.19 | +38.92 | [58] |
| P. citrinopileatus/ Grape Stalks | −33.96 | +51.81 | −2.93 | −8.37 | −19.29 | −36.75 | −2.51 | −4.66 | +5.53 | +8.94 | +7.80 | [59] |
| T. versicolor/Sorghum | +3.99 | +28.50 | −23.73 | +23.19 | +20.32 | −64.49 | +23.65 | +3.10 | +13.61 | +26.05 | +24.07 | [62] |
| P. chrysosporium/White tea straw | −5.03 | +0.68 | −7.89 | −13.98 | −25.64 | +2.47 | −6.47 | −0.06 | +11.15 | +11.64 | +11.19 | [61] |
| Strain | LCBM | Animal | pH | NH3-N | T-Gas | TVFA | A-Acid | P-Acid | Ib-Acid | B-Acid | Iv-Acid | V-Acid | DMD | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P. sajor-caju | Barely straw | Yak | - | +23.70 | - | +7.05 | +3.00 | −3.39 | - | +6.81 | - | - | +11.88 | [77] |
| I.lacteus | Corn straw | Cow | - | - | - | +7.28 | −2.18 | +10.95 | −12.63 | −5.79 | −7.63 | −16.67 | - | [78] |
| P. ostreatus | Corn straw | Cow | - | - | - | +0.93 | −1.25 | +2.71 | +17.15 | −4.06 | +6.14 | −4.17 | - | [78] |
| L. edodes | Corn straw | Goat | −2.47 | +19.04 | +19.73 | +12.64 | −0.88 | +0.56 | - | +4.40 | - | +14.43 | +16.53 | [55] |
| P.chrysosporium | Rape straw | Cattle | - | - | - | +18.68 | +23.31 | - | - | - | - | 11.90 | - | [57] |
| P. ostreatus | Rice straw | cow | −0.31 | - | +29.39 | +36.40 | −6.62 | +22.24 | +35.09 | −27.64 | +78.43 | +68.63 | +17.59 | [48] |
| P. cornucopiae | Grape pomace | Lamb | - | - | - | +22.61 | - | - | - | - | - | - | +11.08 | [79] |
| P. ostreatus | Sugarcane bagasse | Cow | - | - | +28.00 | - | - | - | - | - | - | - | +20.80 | [58] |
| I.lacteus | Wheat straw | Cattle | −1.01 | +14.32 | +28.70 | +5.39 | −2.11 | +10.48 | −3.17 | −8.86 | −5.99 | −6.86 | - | [46] |
| Strain | LCBM | Animal | DMI | OMI | CPI | NDFI | ADFI | DMAD | OMAD | CPAD | NDFAD | ADFAD | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P. ostreatus | Wheat straw | Cow | +5.94 | +4,72 | +6.57 | −2.41 | −2.27 | +8.14 | +8.21 | +14.01 | +15.57 | +17.58 | [39] |
| P. pulonarius | Emp fruit bunch | Goat | −2.34 | - | +2.91 | +3.18 | +10.05 | +10.86 | - | +8.66 | +13.82 | +21.12 | [82] |
| P. sajor-caju | Oil palm frond | Goat | −3.74 | +0.96 | - | −12.5 | +3.23 | +10.13 | +10.03 | +11.98 | +10.68 | +29.90 | [83] |
| P. sajor-caju | Oil palm frond | Goat | −2.13 | −1.19 | +0.92 | −5.73 | −4.60 | +5.07 | +5.35 | - | - | - | [80] |
| P. chrysosporium | Cocoa pod | Cow | +11.19 | +43.20 | +6.09 | +5.99 | +5.94 | +9.82 | +18.26 | +24.28 | +30.55 | +13.57 | [84] |
| Crinipellis spp. | Wheat straw | Cattle | +14.37 | +11.54 | - | - | - | +9.63 | +9.94 | +7.71 | +14.10 | +28.65 | [82] |
| Strain | LCBM | Animal | Milk Yield | FCM Yield | Fat | Lactose | Protein | Total Solid | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| P. ostreatus | Wheat straw | Cow | +6.63 | +5.81 | +5.10 | +7.02 | +3.67 | +5.92 | [39] |
| P. pulonarius | Emp fruit bunch | Goat | −1.43 | - | +4.87 | −0.65 | +5.71 | +1.75 | [82] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, Q.; Datsomor, O.; Zhao, W.; Chen, W.; Wei, C.; Wei, D.; Gao, X.; Qin, C.; Gu, Q.; Zou, C.; et al. Enhancing Agricultural Sustainability by Improving the Efficiency of Lignocellulosic Biomass Utilization in the Ruminant Diet via Solid-State Fermentation with White-Rot Fungi: A Review. Microorganisms 2025, 13, 1708. https://doi.org/10.3390/microorganisms13071708
Yan Q, Datsomor O, Zhao W, Chen W, Wei C, Wei D, Gao X, Qin C, Gu Q, Zou C, et al. Enhancing Agricultural Sustainability by Improving the Efficiency of Lignocellulosic Biomass Utilization in the Ruminant Diet via Solid-State Fermentation with White-Rot Fungi: A Review. Microorganisms. 2025; 13(7):1708. https://doi.org/10.3390/microorganisms13071708
Chicago/Turabian StyleYan, Qi, Osmond Datsomor, Wenhao Zhao, Wenjie Chen, Caixiang Wei, Deshuang Wei, Xin Gao, Chenghuan Qin, Qichao Gu, Caixia Zou, and et al. 2025. "Enhancing Agricultural Sustainability by Improving the Efficiency of Lignocellulosic Biomass Utilization in the Ruminant Diet via Solid-State Fermentation with White-Rot Fungi: A Review" Microorganisms 13, no. 7: 1708. https://doi.org/10.3390/microorganisms13071708
APA StyleYan, Q., Datsomor, O., Zhao, W., Chen, W., Wei, C., Wei, D., Gao, X., Qin, C., Gu, Q., Zou, C., & Lin, B. (2025). Enhancing Agricultural Sustainability by Improving the Efficiency of Lignocellulosic Biomass Utilization in the Ruminant Diet via Solid-State Fermentation with White-Rot Fungi: A Review. Microorganisms, 13(7), 1708. https://doi.org/10.3390/microorganisms13071708








