Animal Models of Pathogenic New World Arenaviruses
Abstract
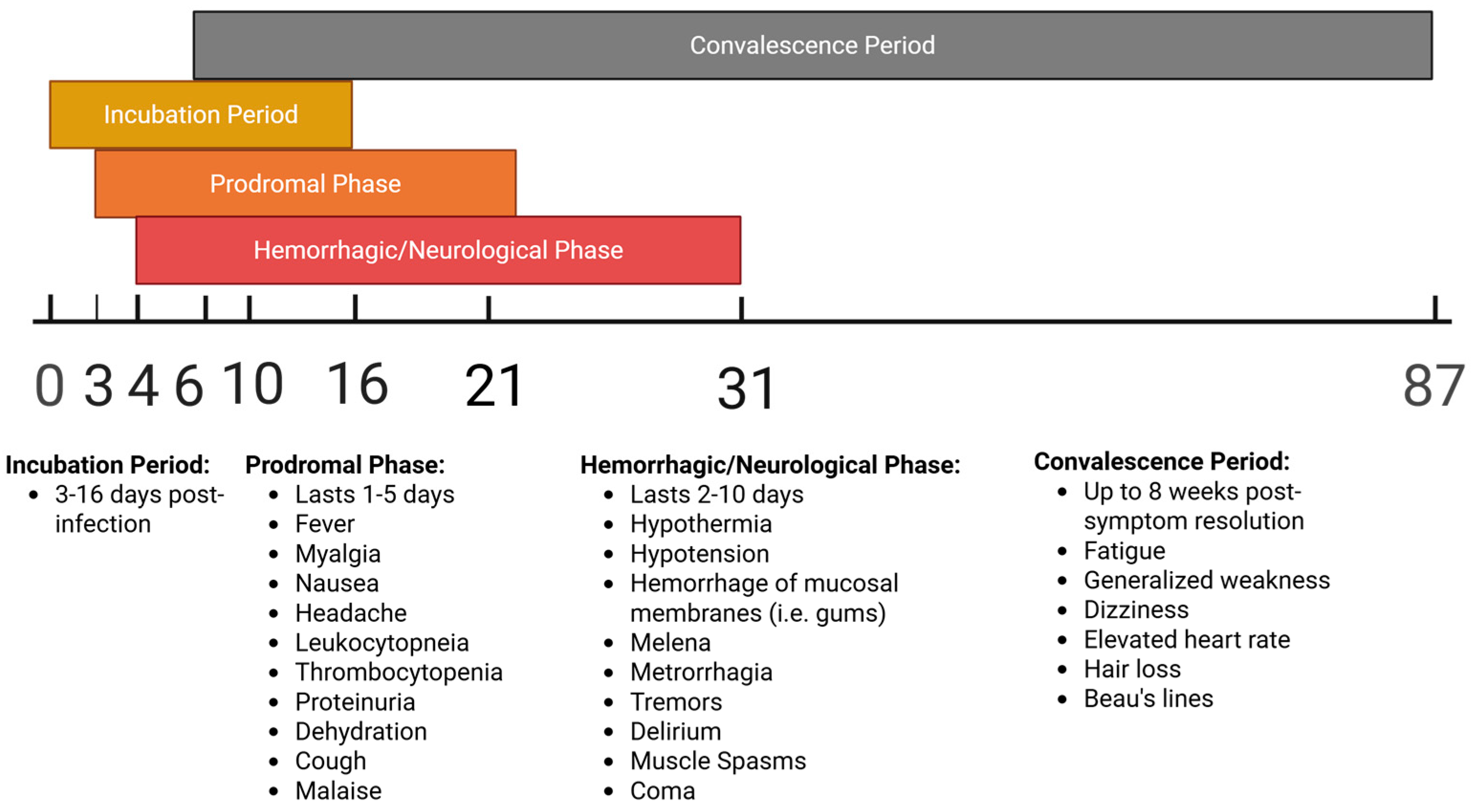
1. Introduction
2. Junín Virus (JUNV)
2.1. Background
2.2. Animal Models of Experimental Infection with Junín Virus
2.2.1. Mice (Mus musculus)
2.2.2. Table 1: Experimental JUNV Infection of Mice (Mus musculus)
| Age, Animal Strain (Noted If Applicable) | Virus Strain, Passage History, Inoculum, Route of Infection | Clinical Disease | Time-to-Death (Days Post-Infection) | Mortality Rate (%) | Source |
|---|---|---|---|---|---|
| Suckling white mice, aged 1 day | XJ strain (prototype), passaged twice or more in suckling guinea pigs, unknown viral titer i.c. | Encephalitis, incoordination with gait, tremors, convulsions, terminal hind-limb paralysis | Undisclosed | >0% (unspecified) | [17] |
| Adult white mice, age unspecified | Not reported | N/A | 0% (unspecified) | ||
| XJ strain (prototype), passaged twice or more in suckling guinea pigs, unknown viral titer i.p. | Undisclosed | >0% (unspecified) | |||
| Suckling Rockland, CFW, C3H, CF1, or Balb/c mice (outbred, immunocompetent), aged 1–10 days | Unspecified strain, passage history, and viral titer (known to be 103 LD50 for 2-day-old mice only), i.c., s.c., and/or i.p. | Encephalitis, tremors, lateralized gait, convulsions, hind-limb paralysis | Within 5 days of symptom onset (12–17, estimated) | 95–100% (unspecified), all routes and mouse strains/ages mentioned | [35] |
| Newborn, thymectomized Rockland, CFW, or Balb/c mice (outbred, immunosuppressed), unknown age | No reported clinical signs | Unspecified | ~0% (unspecified) | ||
| Juvenile Rockland, C3H, Balb/c, CFW and CF1 mice (outbred, immunocompetent), aged 15–30 days | No reported clinical signs | Unspecified | <95% (unspecified) | ||
| Suckling CFW mice (outbred, immunocompetent), aged 1–30 days | XJ strain (prototype), passaged twice in guinea pigs and 13 times in suckling mouse brain, 5000 LD50 i.c. | Tremors, lateralized gait, convulsions, hind-limb paralysis | <14 | 100% (unspecified), 1, 3, 5, and 10-day-old mice | [36] |
| 85% (unspecified), 15 and 20-day-old mice | |||||
| 33% (unspecified), 25-day-old mice | |||||
| 7% (unspecified), 30-day-old mice | |||||
| XJ strain (prototype), passaged twice in guinea pigs and 13 times in suckling mouse brain, 5000 LD50 i.p. | Tremors, lateralized gait, convulsions, hind-limb paralysis | <14 | 100% (unspecified), 1-day-old mice | ||
| 16% (unspecified), 3-day-old mice | |||||
| 5% (unspecified), 6-day-old mice | |||||
| No reported clinical signs | N/A | 0% (unspecified), 9, 12, 15-day-old mice | |||
| Newborn Rockland mice (outbred, immunocompetent), aged 1–2 days | RC strain, passaged at least once in suckling mouse brain, 1000 (suckling mouse) LD50 i.c. | Neurological manifestations of disease (not specified further) | 12–17 | 100% (unspecified) | [39] |
| Newborn thymectomized Rockland mice (outbred, immunodeficient), aged 1–2 days | No reported clinical signs | Not reported | Near 0% (unspecified) | ||
| Adult NIH pathogen free, nude mice with thymus (immunocompetent), aged 60 days | XJ strain (prototype), passaged 27 times in guinea pigs and 32 times in suckling mice, 1000 TCID50, i.c. | No clinical signs reported | Not reported | 7.2% (4/55) | [40] |
| Adult NIH pathogen free, nude, thymectomized mice (immunosuppressed), aged 60 days | Not reported | 3.6% (2/55) | |||
| Adult C3H/HeJ (inbred, immunocompetent) mice, aged 45–180 days | XJ strain, passaged in suckling mouse brain (number of passages unclear), 1600 PFU i.c. | Tremor, ataxia, hyperkinesia | 8.6 | 100% (unspecified) | [38] |
| XJ strain, passaged in suckling mouse brain (number of passages unclear), 160 PFU i.c. | Excitability, hunched posture, hair standing, weight loss, fatigue, hypothermia, unresponsiveness, opistotonic neurological signs (3-month-old mice) | 11.6 | 90% (unspecified) | ||
| Clinical signs not clearly indicated (45, 60, 120, 150, 180-day-old mice) | Not reported | >80% (unspecified), 45–120-day-old mice | |||
| ~40% (unspecified), 150-day-old mice | |||||
| ~10% (unspecified), 180-day-old mice | |||||
| XJ strain, passaged in suckling mouse brain (number of passages unclear), 160 PFU i.p. | No clinical signs reported | N/A | 0% (unspecified), 3-month-old mice | ||
| XJ strain, passaged in suckling mouse brain (number of passages unclear), 160 PFU i.m. | |||||
| XJ strain, passaged in suckling mouse brain (number of passages unclear), 160 PFU s.c. | |||||
| XJ strain, passaged in suckling mouse brain (number of passages unclear), 16 PFU i.c. | Tremor, ataxia, hyperkinesia | 10.3 | 100% (unspecified), 3-month-old mice | ||
| XJ strain, passaged in suckling mouse brain (number of passages unclear), 1.6 PFU i.c. | 11 | 20% (unspecified), 3-month-old-mice | |||
| XJ strain, passaged in suckling mouse brain (number of passages unclear), 0.16 PFU i.c. | No clinical signs reported | N/A | 0% (unspecified) | ||
| Suckling C3H/HeJ (inbred, immunocompetent) mice, aged 1–15 days | XJ strain, passaged in suckling mouse brain (number of passages unclear), 160 PFU i.c. | Tremor, ataxia, hyperkinesia | Not reported | 100% (unspecified), 1, 2, 3, 7-day-old mice | |
| 80% (unspecified), 15-day-old mice | |||||
| Adult C57BL, Balb/c, C3H/HeJ × BALB/cJ, and BALB/cJ × C3H/HeJ (inbred, immunocompetent) mice, aged 3 months | Not discussed (C57BL and Balb/c mice) | Not reported (C57BL and Balb/c mice) | 10% (unspecified), C57BL and Balb/c mice | ||
| No clinical signs reported (C3H/HeJ × BALB/cJ, and BALB/cJ × C3H/HeJ mice) | N/A (C3H/HeJ × BALB/cJ, and BALB/cJ × C3H/HeJ mice) | 0% (unspecified), C3H/HeJ × BALB/cJ, and BALB/cJ × C3H/HeJ mice | |||
| Suckling albino (outbred) mice, aged 2–14 days | CbaFHA5069, passaged 5 times in suckling mice, unspecified viral titer i.c. | Not reported | Not reported | >0% (unspecified), all ages of mice | [37] |
| Adult IFN-α/β/γ R—/— mice (inbred, immune system suppressed) mice, aged 4–8 weeks | Romero strain, unclear passage history, 1 × 104 PFU i.p. | Weight loss, scruffy coat, terminal decrease in body temperature | 13.45 | 100% (13/13) | [41] |
| Adult Strain 129 twice backcrossed with C57BL/6 mice (in-bred, immune system competent) mice, aged 4–8 weeks | No clinical manifestations observed | N/A | 0% (0/23) | ||
| Adult hTfR1 HET mice, aged 3 weeks (inbred, immunocompetent) | Romero strain, passaged once in Vero cells, 105 CCID50 (as measured in Vero cells), i.p. | Stagnation of weight gain, neurological signs of infection (e.g., unresponsiveness | 14 | >0% (1/unspecified) | [42] |
| Adult hTfR1 HOM mice, 3 weeks of age (inbred, immunocompetent | Romero strain, passaged once in Vero cells, 103–105 CCID50 (as measured in Vero cells), i.p. | Weight loss, lethargy, ruffling of fur, tremors, paralysis, abdominal distension, bleeding, encephalitis | 13–16 | 100% (unspecified) | |
| 18 | |||||
| 18 | 71% (5/7) | ||||
| Adult hTfR1 HOM mice, 4 weeks of age (inbred, immunocompetent) | Romero strain, passaged once in Vero cells, 105 CCID50 (as measured in Vero cells), i.p. | 13 | 16.6% (1/6) | ||
| Adult hTfR1 HOM mice, 5 weeks of age (inbred, immunocompetent) | No clinical signs reported | N/A | 0% (0/3 or 0/unspecified) | ||
| Adult hTfR1 HOM mice, 6 weeks of age (inbred, immunocompetent | |||||
| Adult hybrid C57BL/6 × AG129 mice aged 3 weeks (immunosuppressed) | |||||
| Adult IFN- α/β R—/— mice, aged 3 weeks (inbred, immune system suppressed) | Romero strain, passaged once in Vero cells, 104 CCID50 (as measured in Vero cells), i.p. | ||||
| Adult IFN- α/β/γ R—/— mice aged 3 weeks (inbred, immune system suppressed) | |||||
| Adult hTfR1 HOM IFN- α/β R—/— mice (inbred, immune system suppressed) | Romero strain, passaged once in Vero cells, 103 CCID50 (as measured in Vero cells), i.p. | ||||
| Adult hTfR1 HOM IFN- α/β/γ R—/— mice aged 3 weeks (inbred, immune system suppressed) | |||||
| Newborn CFW mice (outbred, immunocompetent), aged 0–1 day | RC strain passaged in suckling mouse brain multiple times, unknown viral titer | Tremor, convulsions, paralysis | 12–19 | 100% (50/50) | [43] |
| Newborn, thymectomized CFW mice (outbred, immunocompetent), aged 0–1 day | No reported clinical signs | N/A | 0% (0/25) |
2.2.3. Rats (Rattus)
2.2.4. Table 2: Experimental JUNV Infection of Rats (Rattus)
| Age, Animal Strain (Noted If Applicable) | Virus Strain, Passage History, Inoculum, Route of Infection | Clinical Disease | Time to Death (Days Post-Infection) | Mortality Rate (%) | Source |
|---|---|---|---|---|---|
| Suckling Wistar (outbred) rats, aged 2–3 days | XJ (prototype) strain, passaged in suckling mouse brain, 1000 LD50, i.c. | Weight loss, diarrhea, conjunctivitis, lateralization of gait, thinning of hair (and increased dullness of coat) | N/A | 0% (unspecified) | [44] |
| Suckling Wistar (outbred) rats, aged 5 days | Not reported | 31% (unspecified) | |||
| Suckling Wistar (outbred) rats, aged 7 days | 91% (unspecified) | ||||
| Suckling Wistar (outbred) rats, aged 10 days | Weight loss, diarrhea, conjunctivitis, lateralization of gait, thinning of hair (and increased dullness of coat), hyperexcitation, balance issues, cyanosis, tremors, and convulsions | 12–13 | 93% (unspecified) | ||
| Suckling Wistar (outbred) rats, aged 12 days | Weight loss, diarrhea, conjunctivitis, lateralization of gait, thinning of hair (and increased dullness of coat) | Not reported | 91% (unspecified) | ||
| Suckling Wistar (outbred) rats, aged 14 days | ~30% (unspecified) | ||||
| Suckling Wistar (outbred) rats, aged 16 days | 27% (unspecified) | ||||
| Juvenile Wistar (outbred) rats, aged 18 days | 29% (unspecified) | ||||
| Juvenile Wistar (outbred) rats, aged 19 days | No clinical manifestations of infection reported | N/A | 0% (unspecified) | ||
| Juvenile Wistar (outbred) rats, aged 26 days | |||||
| Juvenile Wistar (outbred) rats, aged 28 days | |||||
| Juvenile Wistar (outbred) rats, aged 33 days | |||||
| Suckling rats, aged 2 days | XJ strain, passaged twice in guinea pigs and 15 times in hamsters, 103 LD50, i.p. | Unspecified neurological signs | Not reported | 85% (unspecified) | [45] |
| Suckling Wistar rats (outbred), aged 1 day | XJ strain (prototype), passaged in suckling mice brain, 103 LD50, i.p. | Encephalitis; tremors; hyper-excitability; lateralized gait; hind-limb paralysis | 19.9 | 69.2% (27/39) | [46] |
| Suckling Wistar rats (outbred), aged 2 days | 20.49 (pooled average between three experiments) | 85.3% (93/109) (pooled average between three experiments) | |||
| Suckling Wistar rats (outbred), aged 2 days | XJ strain (prototype), passaged in suckling mice brain, 103 LD50, i.c. | Not reported | Not reported | 6.7% (1/15) | |
| Suckling Buffalo/Sim rats (inbred), aged 2 days | 7.1% (1/14) | ||||
| Suckling Buffalo/Sim rats (inbred), aged 2 days | XJ strain (prototype), passaged in suckling mice brain, 103 LD50, i.p. | 88% (44/50) | |||
| Suckling Wistar rats (outbred), aged 2 days | XJ strain (prototype), passaged in suckling mice brain, 102 LD50, i.p. | Encephalitis; tremors; hyper-excitability; lateralized gait; hind-limb paralysis | 24.11 | 50% (9/18) | |
| Suckling Wistar rats (outbred), aged 2 days | XJ strain (prototype), passaged in suckling mice brain, 104 LD50, i.p. | 18.7 | 70% (14/20) | ||
| Suckling Wistar rats (outbred), aged 2 days | XJ strain (prototype), passaged in suckling mice brain, 105 LD50, i.p. | 19.25 | 63.2% (12/19) | ||
| Suckling Wistar rats (outbred), aged 3 days | XJ strain (prototype), passaged in suckling mice brain, 103 LD50, i.p. | 22.22 | 34.6% (9/26) | ||
| Suckling Wistar rats (outbred), aged 4 days | 18 | 13.9% (5/36) | |||
| Suckling Wistar rats (outbred), aged 5 days | 23 | 12.9% (4/31) | |||
| Suckling Wistar rats (outbred), aged 6 days | No clinical manifestations of infection reported | N/A | 0% (0/31) | ||
| Suckling Wistar rats (outbred), aged 7 days | 0% (0/23) | ||||
| Suckling Wistar rats (outbred), aged 10 days | 0% (0/56) (pooled data from two experiments) | ||||
| Suckling Buffalo/Sim rats (inbred), aged 10 days | 0% (0/14) | ||||
| Suckling Wistar rats (outbred), aged 10 days | XJ strain (prototype), passaged in suckling mice brain, 103 LD50, i.c. | Not reported | Not reported | 95.2% (20/21) | |
| Suckling Buffalo/Sim rats (inbred), aged 10 days | 81.3% (13/16) | ||||
| Suckling Wistar rats (outbred), aged 16 days | XJ strain (prototype), passaged in suckling mice brain, 103 LD50, i.p. | No clinical manifestations of infection reported | N/A | 0% (0/36) (pooled data from two experiments) | |
| Suckling Buffalo/Sim rats (inbred), aged 16 days | 0% (0/8) | ||||
| Suckling Wistar rats (outbred), aged 16 days | XJ strain (prototype), passaged in suckling mice brain, 103 LD50, i.c. | 0% (0/12) | |||
| Suckling Buffalo/Sim rats (inbred), aged 16 days | 0% (0/10) | ||||
| Juvenile Wistar rats (outbred), aged 26 days | XJ strain (prototype), passaged in suckling mice brain, 103 LD50, i.p. | 0% (0/29) (pooled data from two experiments) | |||
| Juvenile Wistar rats (outbred), aged 26 days | XJ strain (prototype), passaged in suckling mice brain, 103 LD50, i.c. | 0% (0/10) | |||
| Juvenile Buffalo/Sim rats (inbred), aged 26 days | 0% (0/12) | ||||
| Juvenile Buffalo/Sim rats (inbred), aged 26 days | XJ strain (prototype), passaged in suckling mice brain, 103 LD50, i.p. | 0% (0/13) | |||
| Suckling Wistar (outbred) rats, aged 2 days | XJ strain (prototype), passaged at least once in suckling mouse brain, 100,000 Vero TCID50, i.c. | Up to 30 days post-infection: listlessness, tremors, hind-limb paresis and/or paralysis | Not reported | 5% (unspecified) | [48] |
| 31–280 days post-infection: no clinical manifestations of disease reported | 10% (unspecified) | ||||
| 281–780 days post-infection: tremors, lateralization of gait, hind-limb paralysis, blindness | N/A | 0% (unspecified) | |||
| Suckling Buffalo/Sim (inbred) rats, aged 8–12 days | XJ strain (prototype), passaged in suckling mice brain, 103 PFU, i.c. | Neurological manifestations; encephalitis | Not reported | 90–100% (unspecified) | [47] |
2.2.5. Hamsters (Cricetinae)
2.2.6. Table 3: Experimental JUNV Infection of Hamsters (Cricetinae)
| Age, Animal Strain (Noted If Applicable) | Virus Strain, Passage History, Inoculum, Route of Infection | Clinical Disease | Time to Death (Days Post-Infection) | Mortality Rate (%) | Source |
|---|---|---|---|---|---|
| Suckling hamsters, aged 2–5 days | Cba Lye/63, passaged 8 times in suckling mice, dilutions of the LD50: LD50/100, LD50/10, LD50, 10 LD50, 100 LD50, 1000 LD50, 10,000 LD50 i.c. | Suckling hamsters: Unspecified | 12.05 (suckling hamsters) | >0% (unspecified) | [49] |
| Young hamsters: Lack of coordination in gait; excitability; hind-limb paralysis; prostration, underdevelopment | Unspecified (young hamsters) | ||||
| Juvenile hamsters, 7–19 days of age | Cba FHA 5045H, passaged twice in suckling hamster, dilutions of the LD50: LD50/100, LD50/10, LD50, 10 LD50, 100 LD50, 1000 LD50, 10,000 LD50 i.c. | Suckling hamsters: Unspecified | 12.04 (suckling hamsters) | ||
| Young hamsters: Lack of coordination in gait; excitability; hind-limb paralysis; prostration | Unspecified (young hamsters) | 50% (2/4) (young hamsters at each of 1000 or 10,000 LD50) | |||
| Cba An 9446, passaged 3 times in suckling mice, dilutions of the LD50: LD50/100, LD50/10, LD50, 10 LD50, 100 LD50, 1000 LD50, 10,000 LD50 i.c. | Suckling hamsters: Unspecified | 13.82 (suckling hamsters) | |||
| Young hamsters: Lack of coordination in gait; excitability; hind-limb paralysis; prostration, underdevelopment | 22 (young hamsters at 1000 or 10,000 LD50) | ||||
| Suckling hamsters, 2 days of age | XJ strain (prototype), unclear passage history, passaged many times in suckling guinea pigs and at least 17 times in suckling mice brains, 1000 PFU i.p. | Unspecified | 12 | >0% (unspecified) | [50] |
2.2.7. Guinea Pigs (Cavia porcellus)
2.2.8. Table 4: Experimental JUNV Infection of Guinea Pigs (Cavia porcellus)
| Age, Animal Strain (Noted If Applicable) | Virus Strain, Passage History, Inoculum, Route of Infection | Clinical Disease | Time-to-Death (Days Post-Infection) | Mortality Rate (%) | Source |
|---|---|---|---|---|---|
| Unspecified age and strain | XJ strain (prototype), undescribed passage history, unknown viral titer i.p. or s.c. | Petechiae | 12–15 | 100% (unspecified) | [17] |
| Adult, outbred guinea pigs, unspecified age | CbaFHA5069, passaged 5 times in suckling mouse brain, 0.80 log10 PFU i.c. | Unspecified | 10–12 | >0% (unspecified) | [37] |
| Suckling/juvenile outbred guinea pigs, aged 11 days | CbaIV4454, passaged 5 times in suckling mice, 0.70 log10 PFU i.c. | Hind-limb paralysis | 10–26 | ||
| Unspecified age and strain | XJ strain (prototype), undescribed passage history, 100 LD50 s.c., i.p., i.m., i.n., i.c., or oral | Fever, weight loss, terminal hypothermia, petechiae | 11–17 | 100% (unspecified) | [35] |
| Adult, pregnant guinea pigs (outbred), age unspecified | XJ strain (prototype), undescribed passage history, 103 LD50 i.m. | Not reported | 9–15 | 100% (5/5) | [54] |
| Adult Hartley guinea pigs (outbred), age unspecified) | XJ strain (prototype), undescribed passage history, 5 × 103 LD50 i.m. | Initial fever, terminal hypothermia, petechiae | 12.5 | 100% (10/10) | [59] |
| Adult Hartley guinea pigs (outbred), aged 1 year | Romero strain, passaged once in Vero cells, 7.5 × 103 PFU i.p. | Shock, encephalitis, mucosal hemorrhage, coma, convulsions, paralysis | 14–17 | 100% (4/4) | [60] |
| Juvenile Hartley guinea pigs (outbred), aged 5–10 weeks | Romero strain, passaged once in Vero cells, 1.5 × 103, 2.5 × 103, or 6.0 × 103 PFU i.p. | 9–19 (1.5 × 103 PFU) | 100% (3/3) | ||
| 13–15 (2.5 × 103 PFU) | |||||
| 12–17 (6.0 × 103 PFU) | 100% (4/4) | ||||
| XJ strain (prototype), passaged 37 times in suckling mouse brain, passaged once in Vero cells, 1 × 103–5 × 105 PFU i.p. | Fever | N/A | 0% (0/17) | ||
| Juvenile strain 13 (inbred) guinea pigs, aged 8–20 weeks | XJ strain (prototype), passaged 37 times in suckling mouse brain, passaged once in Vero cells, 1 × 103–1.5 × 103 PFU i.p. | 0% (0/9) | |||
| Outbred guinea pigs, unspecified age | Espindola strain, passaged a low number of times in Vero cells, 103 PFU, undisclosed route of infection | Primarily hemorrhagic manifestations of infection | 17.3 | 100% (10/10) | [57] |
| Ledesma strain, passaged a low number of times in Vero cells, 103 PFU, undisclosed route of infection | 19.0 | ||||
| Romero strain, passaged a low number of times in Vero cells, 103 PFU, undisclosed route of infection | 14.5 | ||||
| P3551 strain, passaged a low number of times in Vero cells, 103 PFU, undisclosed route of infection | Mixed between hemorrhagic and neurological (non-suppurative encephalitis) clinical signs | 21.1 | 80% (8/10) | ||
| Coronel strain, passaged a low number of times in Vero cells, 103 PFU, undisclosed route of infection | Primarily neurological (non-suppurative encephalitis) clinical signs | 30.0 | 10% (1/10) | ||
| Suarez strain, passaged a low number of times in Vero cells, 103 PFU, undisclosed route of infection | 24.5 | 40% (4/10) | |||
| Adult Hartley (outbred) guinea pigs, age unspecified | Coronel (referred to as P3827 in pa-per), passaged twice in MRC-5 cells and once in Vero cells, 5000 PFU i.p. | Hind-limb paralysis | 28 | 20% (4/20) | [61] |
| P3551, passaged twice in MRC-5 cells and once in Vero cells, 5000 PFU i.p. | 21.1 | 73.5% (11/15) | |||
| P3684, passaged twice in MRC-5 cells and once in Vero cells, 5000 PFU i.p. | 27.8 | 40% (4/10) | |||
| Espindola (referred to as P3790 in paper), passaged twice in MRC-5 cells and once in Vero cells, 5000 PFU i.p. | Fatigue, weight loss, anorexia | 17.3 | 100% (20/20) | ||
| Romero (referred to as P3235 in pa-per), passaged twice in MRC-5 cells and once in Vero cells, 5000 PFU i.p. | 14.5 | ||||
| Ledesma (referred to as P3406 in paper), passaged twice in MRC-5 cells and once in Vero cells, 5000 PFU i.p. | Not specified | 19 | 88.9% (16/18) | ||
| Outbred guinea pigs, unspecified age | XJ strain (prototype), passaged twice in guinea pigs, 13 times in suckling mice, 25 additional times in guinea pigs and 19 additional times in suckling mice,300,000 or 30,000 TCID50 oral or 300,000, 30,000, or 3000 TCID50 i.n. | Not reported | Unspecified | 100% (6/6)—300,000 or 30,000 TCID50 i.n. | [51] |
| 83% (5/6)—3000 TCID50 i.n. | |||||
| 40% (2/5)—300,000 TCID50 oral | |||||
| 60% (3/5)—30,000 TCID50 oral | |||||
| Unspecified age and strain | XJ strain (prototype), passaged at least once in guinea pigs, 107 LD50 i.m. | Not reported | <20 | >0% (unspecified) | [52] |
| Unspecified age and strain | XJ strain (prototype), passaged at least once in guinea pigs, 100 LD50 i.m. | Not reported | ~14 | 100% (un-specified) * * Unknown proportion implied to have died naturally, most sacrificed during coagulation studies | [53] |
| Outbred guinea pigs, unspecified age | XJ strain (prototype), passaged at least once in mouse brain, 103 PFU i.m. | Not reported | Not reported | Not reported | [55] |
| Outbred guinea pigs, unspecified age | XJ strain (prototype), passaged at least once in mouse brain, 103 LD50 i.c. | Weight loss, terminal cachexia, hypothermia | 11.3 | 100% (12/12) | [56] |
2.2.9. Common Marmosets (Callithrix jacchus)
2.2.10. Table 5: Experimental JUNV Infection of Common Marmosets (Callithrix jacchus)
| Age, Animal Strain (Noted If Applicable) | Virus Strain, Passage History, Inoculum, Route of Infection | Clinical Disease | Time-to-Death (Days Post-Infection) | Mortality Rate (%) | Source |
|---|---|---|---|---|---|
| Adults, age undisclosed | XJ strain (prototype), passaged in suckling mouse and guinea pig, 1000 LD50 (guinea pig) i.m. | General depression, anorexia, dehydration, adipsia, weight loss, petechiae, abdominal erythematous rash, ecchymosis, tremors, hyperexcitability, convulsions, terminal hypothermia, gingival hemorrhages and hematomas | 22 * * Average of terminally moribund and natural death marmosets | 66.7% (4/6) * * One died spontaneously, three were sacrificed when terminally moribund | [68] |
| XJ strain (prototype), passaged 27 times in guinea pigs, 30 times in suckling mouse brain, 1000 LD50 (guinea pig) i.m. | Anorexia, weight loss, hyperexcitability, tremors, terminal hypothermia | 23 | 10% (1/10) | [69] | |
| XJ strain (prototype), passage history unspecified, 1000 LD50 i.m. | Weight loss, general depression, anorexia, meningoencephalitis, tremor, post-stimulation clonic spasms of head and trunk, terminal hypothermia, unspecified hemorrhagic symptoms, gingival hematomas, difficulty walking | 100% (2/2) | [70,71] |
2.2.11. Capuchin Monkeys (Cebus sp.)
2.2.12. Table 6: Experimental JUNV Infection of Capuchin Monkeys (Cebus sp.)
| Age, Animal Strain (Noted If Applicable) | Virus Strain, Passage History, Inoculum, Route of Infection | Clinical Disease | Time-to-Death (Days Post-Infection) | Mortality Rate (%) | Source |
|---|---|---|---|---|---|
| Adult Cebus sp., age unspecified | XJ strain (prototype), passaged 12 times in guinea pigs, passaged 13 times in suckling mice, and passaged an additional 27 times in guinea pigs, 104 LD50 (as determined in guinea pigs) i.m. | Congested mouth, gingivitis, polyadenopathy, elevated body temperature, weight loss, photophobia, tremors | N/A | 0% (0/4) | [75] |
| P3551 strain, passaged twice in fetal rhesus macaque lung cells, passaged once in MRC-5 cells, 2.8 × 105 PFU i.m. | Anorexia, possible mild lethargy and temperature increase | [58] | |||
| Romero strain, passaged twice in MRC-5 cells, 1.42 × 104 PFU i.m. | Anorexia, possible mild lethargy |
2.2.13. Cynomolgus Macaques (Macaca fascicularis)
2.2.14. Table 7: Experimental JUNV Infection of Cynomolgus Macaques (Macaca fascicularis)
| Age, Animal Strain (Noted If Applicable) | Virus Strain, Passage History, Inoculum, and Route of Infection | Clinical Disease | Time-to-Death (Days Post-Infection) | Mortality Rate (%) | Source |
|---|---|---|---|---|---|
| Adult (3–6 years of age) | Romero strain, undisclosed passage history, 5000 PFU i.v. | Weight loss; transient fever; facial edema; diarrhea; petechial rash; weakness; ataxia; intention tremors; and seizures | 18.7 | 100% (3/3) | [76] |
| Espindola strain, undisclosed passage history, 5000 PFU i.v. | 16.7 | ||||
| Unspecified age | XJ strain (prototype), passaged twice in guinea pigs, unknown viral inoculum and route of infection | None reported | N/A | Not reported | [17] |
2.2.15. Rhesus Macaques (Macaca mulatta)
2.2.16. Table 8: Experimental JUNV Infection of Rhesus Macaques (Macaca mulatta)
| Age, Animal Strain (Noted If Applicable) | Virus Strain, Passage History, Inoculum, Route of Infection | Clinical Disease | Time-to-Death (Days Post-Infection) | Mortality Rate (%) | Source |
|---|---|---|---|---|---|
| Adults, age undisclosed | Espindola strain, passaged 3 times in MRC-5 cells, 4.1–4.5 log10 PFU, i.m. | Petechiae, ecchymoses, bleeding from mucosal membranes, terminal dehydration and weight loss | 33 | 100% (8/8) | [23,77] |
| Ledesma strain, passaged three times in MRC-5 cells, 4.1–4.5 log10 PFU, i.m. | Tremors, ataxia, paresis | 71% (5/7) | |||
| Espindola strain, passaged low number of times in Vero cells, unspecified titer and route of infection | Hemorrhagic manifestations, no further specification provided | Not reported | 100% (3/3) | [57] | |
| Ledesma strain, passaged low number of times in Vero cells, unspecified titer and route of infection | Neurological manifestations, no further specification provided | ||||
| Romero strain, passaged low number of times in Vero cells, unspecified titer and route of infection | Mild clinical signs observed, no further specification provided | N/A | 0% (0/3) | ||
| P3551 strain, passaged low number of times in Vero cells, unspecified titer and route of infection | Mixed neurological and hemorrhagic manifestations of infection, no further specification provided | Not reported | 66.7% (2/3) | ||
| Romero strain, passaged 3 times in MRC-5 cells, unspecified titer and route of infection | Anorexia, fatigue, diarrhea or constipation, flushing of the face | N/A | 0% (0/4) | [80] | |
| Espindola strain, passaged 3 times in MRC-5 cells, unspecified titer and route of infection | Progressive anorexia, malaise, diarrhea or constipation, facial erythema, malar or circumocular rash, conjunctivitis, oral ulcers, petechiae, gingival bleeding, serosanguinous nasal discharge, terminal hypothermia, dehydration and wasting, possible purulent conjunctivitis and oral ulcers | Not reported | 100% (3/3) | ||
| Ledesma strain, passaged 3 times in MRC-5 cells, unspecified titer and route of infection | 66.7% (2/3) | ||||
| P3551 strain, passaged twice in rhesus fetal lung cells, unspecified titer and route of infection | |||||
| Espindola strain, passaged twice in MRC-5 cells and once in Vero cells, 104 PFU aerosolized | Anorexia, fatigue, weight loss, erythematous rash, gingival bleeding, gingival hemorrhage, bleeding from mucous membranes, wasting | 29.5 | 100% (2/2) | [78] | |
| Espindola strain, passaged twice in MRC-5 cells and once in Vero cells, 102 PFU aerosolized | 31.3 | 100% (3/3) |
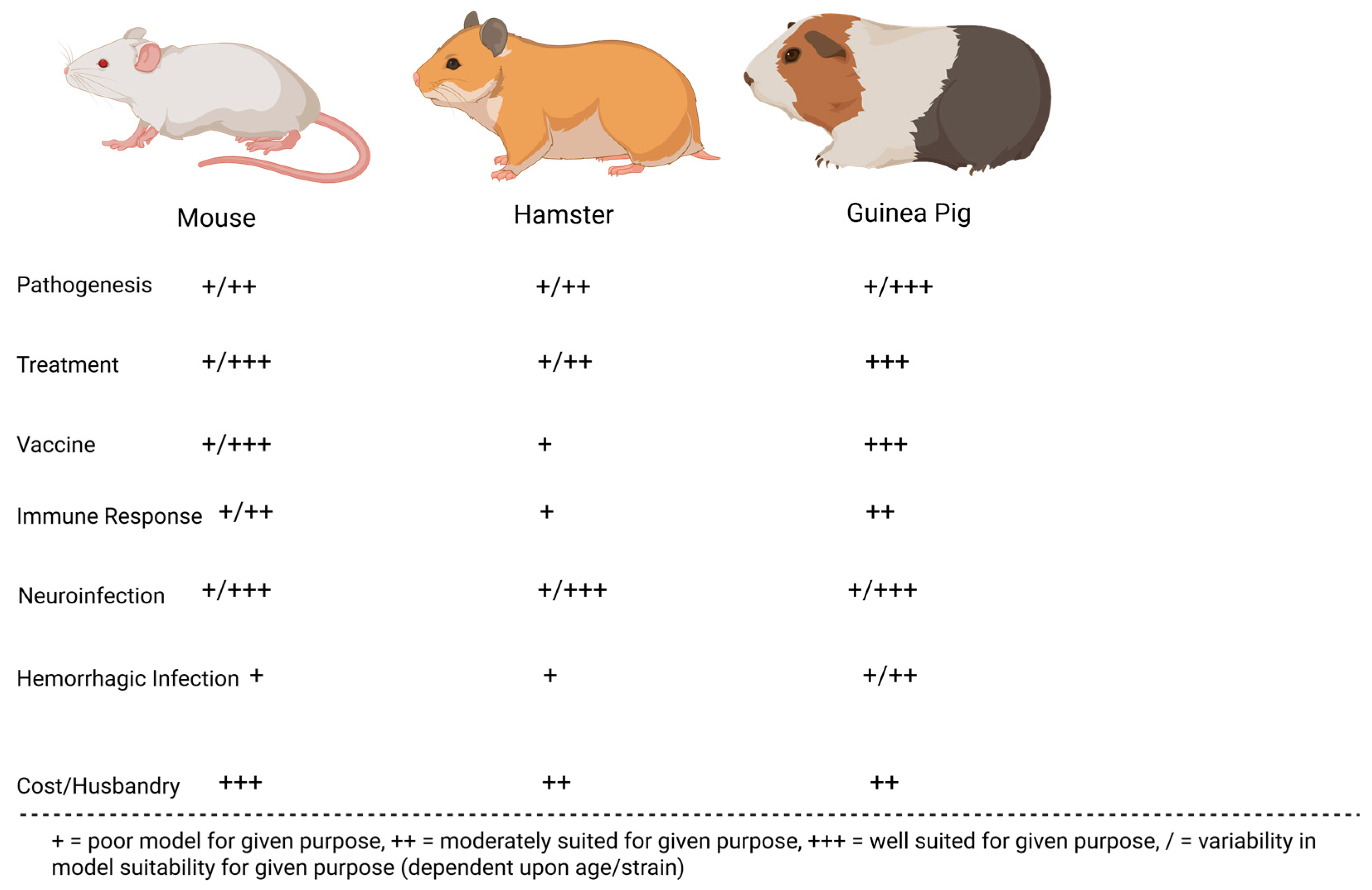
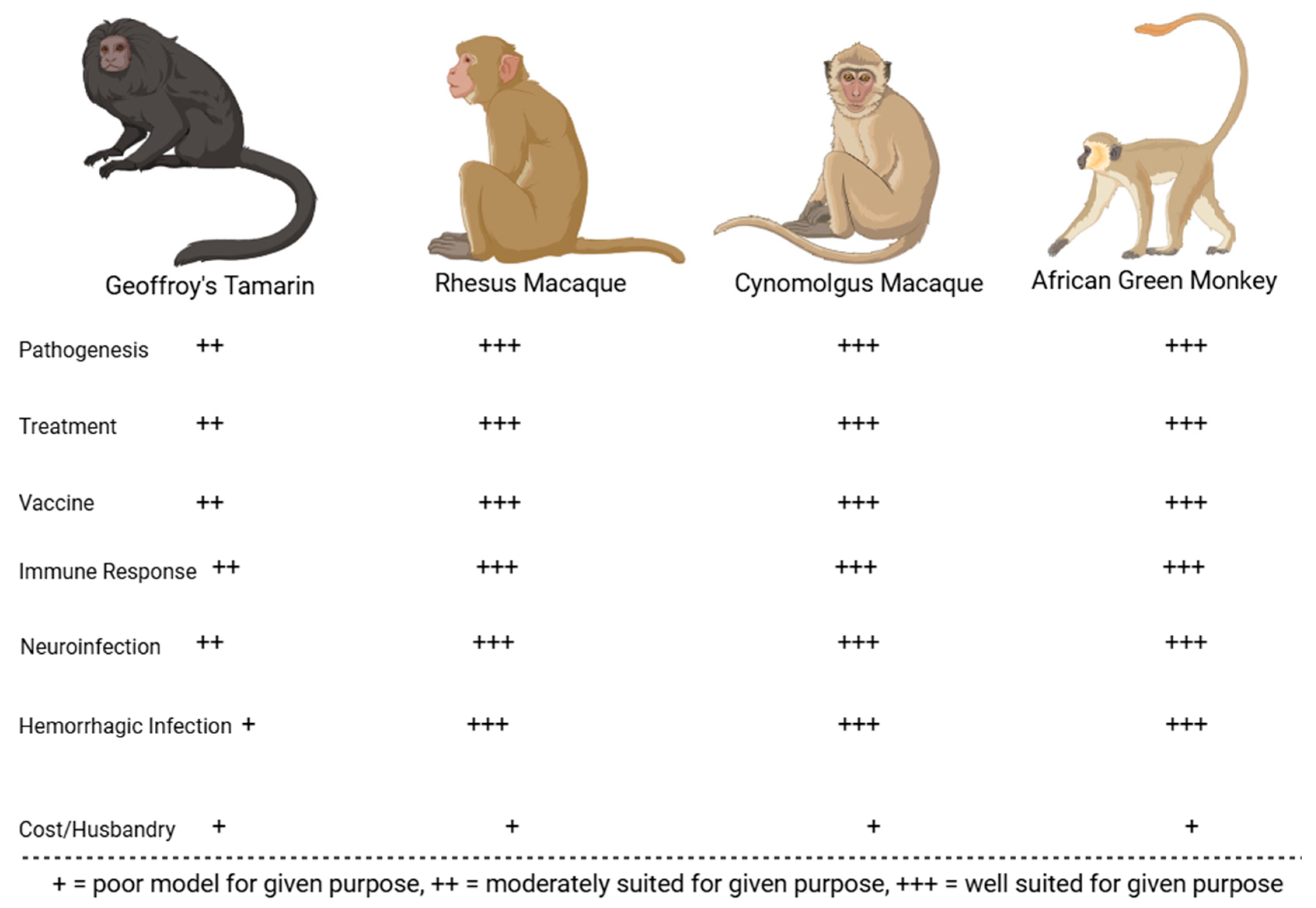
2.3. Summary of JUNV Animal Models
3. Machupo Virus (MACV)
3.1. Background
3.2. Animal Models of Experimental Infection with Machupo Virus
3.2.1. Mice (Mus musculus)
3.2.2. Table 9: Experimental MACV Infection of Mice (Mus musculus)
| Age, Animal Strain (Noted If Applicable) | Virus Strain, Passage History, Inoculum, Route of Infection | Clinical Disease | Time to Death (Days Post-Infection) | Mortality Rate (%) | Source |
|---|---|---|---|---|---|
| Suckling NIH general purpose Swiss (outbred, immunocompetent) mice, aged 2 days or less | Carvallo (prototype), passaged eight or fewer times in suckling hamster or suckling mice, unknown viral titer of inoculum, i.c., i.p., or combined i.c./i.p. | Growth retardation; rough fur; dullness of fur coat; lack of coordination; tonic-clonic convulsions; ataxia; apnea; rigidity | Unspecified | >0% (unspecified) | [88,92] |
| Suckling BALB/c (inbred, immunocompetent) mice, aged 0–7 days | Carvallo (prototype), passaged eight or fewer times in suckling hamster or suckling mice, 103 Hamster LD50 (for all other strains), i.c. | ||||
| Suckling C3H/HCN (inbred, immunocompetent) mice, aged 0–7 days | |||||
| Suckling AKR (inbred, immunocompetent) mice, aged 0–7 days | |||||
| Suckling DBA/2 (inbred, immunocompetent) mice, aged 0–7 days | |||||
| Suckling C57/6 (inbred, immunocompetent) mice, aged 0–7 days | |||||
| Adult NIH general purpose Swiss (outbred, immunocompetent) mice, aged 5 weeks or greater | Carvallo (prototype), passaged eight or fewer times in suckling hamster or suckling mice, unknown viral titer of inoculum, i.c. or i.p. | No clinical signs reported | Unspecified | 0% (unspecified) | |
| BALB/c (inbred, competent immune system) mice of unspecified age | Unspecified strain and passage history, unspecified viral titer, i.c. | Unspecified | 8–9 | 100% (unspecified) | [89] |
| C57B1/6 (inbred, competent immune system) mice of unspecified age | N/A | 0% (unspecified) | |||
| Suckling Swiss Webster Strain (outbred, competent immune system) mice, aged 3 days or less | Cochabamba, second passage from suckling hamster brain, 9 × 103 PFU, i.c. | Growth retardation, tremors, convulsions | 9–16 | >0% (unspecified) | [86] |
| Suckling Swiss Webster Strain (outbred, competent immune system) mice, aged 3 days or less | Carvallo (prototype), second or third passage from suckling hamster brain, 2.1 × 103 PFU, i.c. | ||||
| Adult STAT-1 knockout (inbred, immunosuppressed) mice, aged 6–12 weeks | Carvallo (prototype), passaged once in Vero cells, 1000 PFU, i.p. | Ruffled appearance, hunched posture, lethargy | 7.3 | 100% (6/6) | [91] |
| Carvallo (prototype), passaged once in Vero cells, 1000 PFU, i.n. | 20 | 25% (1/4) | |||
| Carvallo (prototype), passaged once in Vero cells, 1000 PFU, s.c. | 10.5 | 66.7% (4/6) | |||
| IFN-α/β/γ R—/— mice (C5BL/6 background) (inbred, immuno-suppressed) | Carvallo (prototype), passaged once in Vero cells, 10,000 PFU, i.p. | Weight loss, neurological impairment (partial paralysis, hunched posture, labored breathing, awkward gait), hypothermia 1–2 days prior to death | 22–34 | 84% (11/13) | [90] |
| C57BL/6 (inbred, competent immune system) | No clinical signs reported | N/A | 0% (0/10) | ||
| Newborn thymectomized Rockland mice (outbred, immunodeficient), aged 1–2 days | Carvallo (prototype) passaged at least once in suckling mouse brain, 1000 (suckling mouse) LD50 i.c. | No clinical signs reported | Unspecified | Near 0% (unspecified) | [39] |
| Newborn Rockland mice (outbred, immunodeficient), aged 1–2 days | Unspecified neurological clinical signs | 12–17 | 100% (unspecified) |
3.2.3. Hamsters (Cricetinae)
3.2.4. Table 10: Experimental MACV Infection of Hamsters (Cricetinae)
| Age, Animal Strain (Noted If Applicable) | Virus Strain, Passage History, Inoculum, Route of Infection | Clinical Disease | Time to Death (Days Post-Infection) | Mortality Rate (%) | Source |
|---|---|---|---|---|---|
| Suckling hamster, aged less than 5 days | Carvallo (prototype), obtained directly from blood of infected individuals, unknown viral titer, i.p. or i.c. | Growth retardation; rough fur; dullness of fur coat; lack of coordination; tonic-clonic convulsions; ataxia; apnea; rigidity; underdevelopment | Unspecified | >0% (unspecified) | [92] |
| Juvenile hamster, aged greater than 4 weeks | Carvallo (prototype), obtained directly from blood of infected individuals, unknown viral titer, i.p. or i.c. | No clinical manifestations of infection observed | N/A | 0% (unspecified) | |
| Suckling hamsters, aged less than 5 days | Carvallo (prototype), passaged twice in suckling hamster brain, 101.69 PFU, i.c. | Not reported | 7–11 | 100% (7/7) | [93] |
| Cochabamba, passaged twice in suckling hamster brain, 101.02 PFU, i.c. | Non-suppurative encephalitis | 10–17 | 100% (6/6) | ||
| Suckling hamsters, aged less than 6 days | Carvallo (prototype), passaged eight or fewer times in suckling hamster or suckling mice, unknown viral titer of inoculum, i.c., i.p., or combined i.p./i.c. | Growth retardation; rough fur; dullness of fur coat; lack of coordination; tonic-clonic convulsions; ataxia; apnea; rigidity | Unspecified | >0% (unspecified) | [88,92] |
| Adult hamsters, aged more than 5 weeks | Carvallo (prototype), passaged eight or fewer times in suckling hamster or suckling mice, unknown viral titer of inoculum, i.c., i.p., i.n., or oral | No clinical manifestations of infection reported | N/A | 0% (unspecified) | |
| Adult hamsters, aged between 5 and 6 weeks | Carvallo (prototype), passaged twice in hamsters (presumed suckling hamster brain), 104 Suckling Hamster LD50 | [85] |
3.2.5. Guinea Pigs (Cavia porcellus)
3.2.6. Table 11: Experimental MACV Infection of Guinea Pigs (Cavia porcellus)
| Age, Animal Strain (Noted If Applicable) | Virus Strain, Passage History, Inoculum, Route of Infection | Clinical Disease | Time to Death (Days Post-Infection) | Mortality Rate (%) | Source |
|---|---|---|---|---|---|
| Adult C-13 guinea pigs (inbred), age unspecified | Unspecified strain, unknown passage history and viral titer; undisclosed route of infection | Reportedly nondescript; no reported hemorrhagic or neurological manifestations | Unspecified | >0% (unspecified) | [95] |
| Adult Hartley (outbred) guinea pigs, age unspecified | Carvallo (prototype), second or third passage from suckling hamster brain, 1.4 × 104 PFU, i.p. | Weight loss, inactivity | 18–23 | 84% (21/25) | [86] |
| Cochabamba, second passage from suckling hamster brain | Not reported | N/A | 0% (0/6) | ||
| Suckling or Adult Hartley (outbred) guinea pigs, aged 5 days or less or unspecified age, respectively | 1000 PFU Carvallo (suckling), i.p. | Weight loss, inactivity | 18–23 | 84% (21/25) | |
| 100 PFU Carvallo (suckling), i.p. | 64.7% (11/17) | ||||
| 10 PFU Carvallo (suckling), i.p. | 100% (11/11) | ||||
| 1 PFU Carvallo (suckling), i.p. | 33% (6/18) | ||||
| 1.4 × 104 PFU Carvallo (adults), i.p. | 67% (4/6) | ||||
| 6.6 × 105 PFU Cochabamba (adults), i.p. | 0% (0/6) | ||||
| Unspecified PFU, Carvallo (suckling), i.c. | 87.5% (49/56) | ||||
| Unspecified PFU, Cochabamba (suckling), i.c. | 17.2% (5/29) | ||||
| 7000 PFU Cochabamba (suckling), i.p. | 30.4% (7/23) | ||||
| 700 PFU Cochabamba (suckling), i.p. | 16.6% (2/12) | ||||
| 70 PFU Cochabamba (suckling), i.p. | 20% (3/15) | ||||
| 7 PFU Cochabamba (suckling), i.p. | 4.5% (1/22) | ||||
| Dunkin-Hartley (outbred) guinea pigs of unspecified age | Chicava, passaged twice in Vero E6 cells, 10, 100 or 1000 PFU, aerosolized | Piloerection, fever, loss of appetite, erythema of haired skin (axillary, inguinal, ear tips), dyspnea, intermittent diarrhea (possibly bloody). Neurological signs—16–20 days post-infection, head tilt and ataxia; rapid breathing and respiratory difficulties; weight loss. | <30 | 100% (10/10) | [94] |
| Young adult Hartley (outbred) guinea pigs, 6–8 weeks of age | Chicava, passaged twice in Vero E6 or Neuro-2A cells, 104 PFU, i.p. | Mild fever, weight loss, vomiting and hind-limb paralysis (in some cases) | 19–22 | 100% (4/4) | [96] |
| Guinea pigs, unspecified age and strain | Carvallo, passaged five times in guinea pig spleen, 2 PFU (and higher, unspecified doses), unspecified route of infection | Not reported | Not reported | 100% (unspecified) | [23] |
3.2.7. Geoffroy’s Tamarins (Sanguinus geoffroyi)
3.2.8. Table 12: Experimental MACV Infection of Geoffroy’s Tamarin (Sanguinus geoffroyi)
| Age, Animal Strain (Noted If Applicable) | Virus Strain, Passage History, Inoculum, Route of Infection | Clinical Disease | Time to Death (Days Post-Infection) | Mortality Rate (%) | Source |
|---|---|---|---|---|---|
| Undisclosed age | Carvallo (prototype), passaged a maximum of eight times in suckling hamsters or suckling mice, 105.7 suckling hamster LD50, or 1:10, 1:100, 1:1000, 1:10,000. 1:100,000, or 1:1,000,000 dilutions thereof, s.c. | Sick appearance, anorexia, weakness, inactivity, decrease in bodily temperature 1–3 days prior to death | 11 (undiluted dose) | 100% (2/2) | [88] |
| 13.5 (1:10 dilution) | |||||
| 13 (1:100 dilution) | |||||
| 18 (1:1000 dilution) | |||||
| 20 (1:10,000 dilution) | |||||
| 16 (1:100,000 dilution) | |||||
| Undisclosed (1:1,000,000 dilution) | N/A | 0% (0/2) | |||
| Carvallo (prototype), passaged a maximum of eight times in suckling hamsters or suckling mice, 105.7 suckling hamster LD50, application to scarified skin or corneal instillation | Sick appearance, anorexia, weakness, inactivity, decrease in bodily temperature 1–3 days prior to death | 17 (application to scarified skin) | 100% (3/3) | ||
| Unspecified (corneal instillation) | 33% (1/3) | ||||
| Carvallo (prototype), passaged a maximum of eight times in suckling hamsters or suckling mice, 105.7 suckling hamster LD50, i.n. or oral | No reported clinical signs | N/A | 0% (unspecified) | ||
| Unspecified age | Unspecified strain and passage history | Anorexia, tremors, shock | 8–20 | >0% (unspecified) | [88,95] |
3.2.9. African Green Monkeys (Chlorocebus aethiops) (AGMs)
3.2.10. Cynomolgus Macaques (Macaca fascicularis)
3.2.11. Table 13: Experimental MACV Infection of Cynomolgus Macaques (Macaca fascicularis)
| Age, Animal Strain (Noted If Applicable) | Virus Strain, Passage History, Inoculum, Route of Infection | Clinical Disease | Time to Death (Days Post-Infection) | Mortality Rate (%) | Source |
|---|---|---|---|---|---|
| Adult macaques, age unspecified | Carvallo (prototype), third/fourth passage in suckling hamster brain, 1000 PFU, s.c. | Initial phase: conjunctivitis, depression, anorexia, fever, dehydration, initial constipation followed by diarrhea, nasal discharge (at times bloody), clonic spasms Neurological phase: Severe intention tremors, nystagmus, lack of coordination, paresis, coma | 17.0 | 6/7 (86%) (total, both phases of infection) | [99] |
| 5/7 (71%) (initial phase of infection) | |||||
| 1/2 (50%) (neurological phase of infection) | |||||
| Adult male macaques, aged 2.5 years | Carvallo (prototype), passaged in Vero E6 cells, 3000 FFU, s.c. | Epistaxis, petechiae, non-specific febrile clinical signs, melena, hematochezia, balance disorders, reduced activity, dehydration, weight loss | 11–18 | 6/6 (100%) | [101,102] |
| Adult macaques, age unspecified | Chicava, passaged twice in Vero E6 cells, 1000 PFU, i.m. | Reduced appetite, weakness, depression, mild fever, dehydration, rash (axillary and inguinal regions), hematuria, epistaxis. Tremors, ataxia, facial edema, darkened hard plate, black/purple discoloration of the lips | 18.3 | 100% (4/4) | [100] |
| Chicava, passaged twice in Vero E6 cells, 100 PFU, aerosolized | Similar clinical signs as in i.m. group, inclusion of mild-moderate nonsuppurative encephalitis/gliosis | 17.3 | 75% (3/4) | ||
| Chicava, passaged twice in Vero E6 cells, 1000 PFU, aerosolized | 19.5 | 50% (2/4) | |||
| Chicava, passaged twice in Vero E6 cells, 10,000 PFU, aerosolized | 21.3 | 75% (3/4) |
3.2.12. Rhesus Macaques (Macaca mulatta)
3.2.13. Table 14: Experimental MACV Infection of Rhesus macaques (Macaca mulatta)
| Age, Animal Strain (Noted If Applicable) | Virus Strain, Passage History, Inoculum, Route of Infection | Clinical Disease | Time to Death (Days Post-Infection) | Mortality Rate (%) | Source |
|---|---|---|---|---|---|
| Young adult macaques, unspecified age | Carvallo (prototype), third/fourth passage in suckling hamster, 1000 PFU s.c. | Initial phase: conjunctivitis, depression, anorexia, fever, dehydration, initial constipation followed by diarrhea (majority), nasal discharge (at times bloody) (half), clonic spasms, erythematous facial and abdominal rash, terminal progressive anorexia, dehydration and depression Neurological phase: severe intention tremors, nystagmus, lack of coordination, paresis, coma | 19.3 | 45/47 (96%) (total macaques, initial and neurological phases of illness) | [99] |
| 39/47 (83%) (total macaques, initial phase) | |||||
| 6/8 (75%) (total macaques, neurological phase of illness) | |||||
| Mature macaques, unspecified age | 30.5 | 2/4 (50%) (mature, 5–8 kg rhesus macaques, initial phase of illness) | |||
| 37/43 (86%) (young, 2.5–4 kg rhesus macaques, initial phase of illness) | |||||
| 2/2 (100%) (mature, 5–8 kg rhesus macaques, neurological phase of illness) | |||||
| 6/8 (75%) (young, 2.5–4 kg rhesus macaques, acute phase of illness) | |||||
| Young adult macaques, age unspecified | Carvallo (prototype), third/fourth passage in suckling hamster, 103 PFU s.c. | Skin petechiae, exudative rash, epistaxis | 19.5 | 100% (4/4) | [105] |
| Carvallo (prototype), third/fourth passage in suckling hamster, 10 PFU s.c. | 37 | 25% (1/4) | |||
| Carvallo (prototype), third/fourth passage in suckling hamster, 105 PFU s.c. | Not recorded | 14.5 | 100% (4/4) | ||
| Carvallo (prototype), passaged in suckling hamster brain, 1000 PFU s.c. | Initial: diarrhea, depression, anorexia, necrosis of the skin Neurological: lack of coordination, mucopurulent nasal discharge, alopecia, diarrhea, emaciation, tremors, paresis and convulsions, lateral curvature of the spine, paralysis, muscle atrophy, dermatitis of the skin and abdomen, weakness | 36.8 | 100% (4/4) | [103] | |
| Carvallo (prototype), third passage in suckling hamster brain, 103 PFU s.c. | Fever, depression, anorexia, diarrhea, clonic spasms, adipsia, nasal discharge, facial and abdominal rashes, dehydration, purulent nasal discharge, bleeding from the nares and skin, terminal hypothermia | 19.5 | 100% (4/4) | [104] | |
| Carvallo (prototype), third passage in suckling hamster, 105 PFU s.c. | 14.3 | ||||
| Carvallo (prototype), third passage in suckling hamster brain, 10 PFU s.c. | No reported clinical signs | N/A | 0% (0/4) | ||
| Carvallo (prototype), fourth passage in suckling hamster brain, 1000 PFU s.c. | Lethargy, decreased appetite, dehydration, epistaxis, skin petechiae, bleeding gums, terminal hypotension and hypothermia | 13–21 | 100% (14/14) | [106] |
3.3. Summary of MACV Animal Models
4. Guanarito Virus (GTOV)
4.1. Background
4.2. Animal Models of Experimental Infection with Guanarito Virus
4.2.1. Mice (Mus musculus)
4.2.2. Guinea Pigs (Cavia porcellus)
4.2.3. Table 15: Successful Rodent Animal Models of Experimental GTOV Infection
| Species, Age, Animal Strain (Noted If Applicable) | Virus Strain, Passage History, Inoculum, Route of Infection | Clinical Disease | Time-to-Death (Days Post-Infection) | Mortality Rate (%) | Source |
|---|---|---|---|---|---|
| Suckling CD-1 (outbred) mice (Mus musculus), aged 3–6 days | INH-95551 (prototype), passaged once in Vero cells, unknown viral titer, i.c. | Lethargy, ataxia, runting, hind-limb paralysis | 12+ | >0% (majority, unspecified) | [116] |
| Adult mice (Mus musculus), age/strain unspecified | INH-95551 (prototype), 10% suspension of infected suckling mouse brain, unknown viral titer, i.p. | None reported | N/A | 0% (0/unspecified) | |
| Adult strain 13 (inbred) guinea pig (Cavia porcellus), age unspecified | INH-95551 (prototype), passaged twice in suckling mouse brain, 103.4 PFU, s.c. | Minimal hemorrhagic manifestations, moribund state | 11–14 | 100% (10/10) | |
| INH-95551 (prototype), passaged twice in suckling mice brains and once in Vero cells, 1.0–3.4 log10 PFU, s.c. | 12–14 (killed when moribund) | 100% (2/2) | [117] | ||
| Adult Hartley (outbred) guinea pig (Cavia porcellus), age unspecified | 100% (6/6) | ||||
| Hartley (outbred) guinea pig (Cavia porcellus) | INH-95551 (prototype), passaged in Vero cells, 2000 PFU, i.p. | Weight loss | N/A | 0% (0/16)* *(animals sacrificed for pathology data collection) | [118] |
| Weight loss, elevated body temperature | 15 | 100% (unspecified) | [119] |
4.2.4. Cynomolgus Macaques (Macaca fascicularis)
4.2.5. Rhesus Macaques (Macaca mulatta)
4.2.6. Table 16: Successful NHP Animal Models of Experimental GTOV Infection
| Species, Age, Animal Strain (Noted If Applicable) | Virus Strain, Passage History, Inoculum, Route of Infection | Clinical Disease | Time-to-Death (Days Post-Infection) | Mortality Rate (%) | Source |
|---|---|---|---|---|---|
| Adult rhesus macaque (Macaca mulatta), age unspecified | INH-95551 (prototype), passaged twice in suckling mice brains, log10 3.4 PFU, s.c. | Lethargy, reduced appetite, fever | N/A | 0% (0/3) | [116] |
| Adult cynomolgus macaque (Macaca fascicularis), aged 3 years | INH-95551 (prototype), passaged once in Vero cells, 3000 FFU, s.c. | Weight loss, reduced activity, fever, epistaxis, petechiae, melena, hematochezia | 11–18 | 33.3% (1/3) | [101,102] |
4.3. Summary of GTOV Animal Models
5. Chapare Virus (CHAPV)
5.1. Background
5.2. Animal Models of Experimental Infection with Chapare Virus
5.2.1. Guinea Pigs (Cavia porcellus)
5.2.2. Cynomolgus Macaques (Macaca fascicularis)
5.3. Summary of CHAPV Animal Models
6. Sabiá Virus (SABV)
6.1. Background
6.2. Animal Models of Experimental Infection with Sabiá Virus
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NWAV | New World Arenavirus |
| OWAV | Old-World Arenavirus |
| MACV | Machupo Virus |
| GTOV | Guanarito Virus |
| CHAPV | Chapare Virus |
| SABV | Sabiá Virus |
| JUNV | Junín Virus |
| WWAV | Whitewater Arroyo Virus |
| LASV | Lassa Virus |
| NHP | Non-Human Primate |
| PFU | Plaque-Forming Units |
| FFU | Focus-Forming Units |
| i.p. | Intraperitoneal |
| i.c. | Intracerebral |
| s.c. | Subcutaneous |
| i.n. | Intranasal |
| i.m. | Intramuscular |
| TCID50 | Tissue Culture Infectious Dose, 50% |
| CCID50 | Cell Culture Infectious Dose, 50% |
| LD50 | Lethal Dose, 50% |
| BHF | Bolivian Hemorrhagic Fever |
| VHF | Venezuelan Hemorrhagic Fever |
| CHF | Chapare Hemorrhagic Fever |
| AHF | Argentinian Hemorrhagic Fever |
| MCMs | Medical countermeasures |
References
- Sarute, N.; Ross, S.R. New World Arenavirus Biology. Annu. Rev. Virol. 2017, 4, 141–158. [Google Scholar] [CrossRef]
- Delgado, S.; Erickson, B.R.; Agudo, R.; Blair, P.J.; Vallejo, E.; Albariño, C.G.; Vargas, J.; Comer, J.A.; Rollin, P.E.; Ksiazek, T.G.; et al. Chapare Virus, a Newly Discovered Arenavirus Isolated from a Fatal Hemorrhagic Fever Case in Bolivia. PLoS Pathog. 2008, 4, e1000047. [Google Scholar] [CrossRef] [PubMed]
- Garry, R.F. Lassa Fever—The Road Ahead. Nat. Rev. Microbiol. 2023, 21, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Silva-Ramos, C.R.; Montoya-Ruíz, C.; Faccini-Martínez, Á.A.; Rodas, J.D. An Updated Review and Current Challenges of Guanarito Virus Infection, Venezuelan Hemorrhagic Fever. Arch. Virol. 2022, 167, 1727–1738. [Google Scholar] [CrossRef] [PubMed]
- Patterson, M.; Grant, A.; Paessler, S. Epidemiology and Pathogenesis of Bolivian Hemorrhagic Fever. Curr. Opin. Virol. 2014, 5, 82–90. [Google Scholar] [CrossRef]
- Vilibic-Cavlek, T.; Savic, V.; Ferenc, T.; Mrzljak, A.; Barbic, L.; Bogdanic, M.; Stevanovic, V.; Tabain, I.; Ferencak, I.; Zidovec-Lepej, S. Lymphocytic Choriomeningitis—Emerging Trends of a Neglected Virus: A Narrative Review. Trop. Med. Infect Dis. 2021, 6, 88. [Google Scholar] [CrossRef]
- Simulundu, E.; Mweene, A.S.; Changula, K.; Monze, M.; Chizema, E.; Mwaba, P.; Takada, A.; Ippolito, G.; Kasolo, F.; Zumla, A.; et al. Lujo Viral Hemorrhagic Fever: Considering Diagnostic Capacity and Preparedness in the Wake of Recent Ebola and Zika Virus Outbreaks. Rev. Med. Virol. 2016, 26, 446–454. [Google Scholar] [CrossRef]
- Grant, A.; Seregin, A.; Huang, C.; Kolokoltsova, O.; Brasier, A.; Peters, C.; Paessler, S. Junín Virus Pathogenesis and Virus Replication. Viruses 2012, 4, 2317–2339. [Google Scholar] [CrossRef]
- Reyna, R.A.; Littlefield, K.E.; Shehu, N.; Makishima, T.; Maruyama, J.; Paessler, S. The Importance of Lassa Fever and Its Disease Management in West Africa. Viruses 2024, 16, 266. [Google Scholar] [CrossRef]
- Brouillette, R.B.; Phillips, E.K.; Ayithan, N.; Maury, W. Differences in Glycoprotein Complex Receptor Binding Site Accessibility Prompt Poor Cross-Reactivity of Neutralizing Antibodies between Closely Related Arenaviruses. J. Virol. 2017, 91, e01454-16. [Google Scholar] [CrossRef]
- Russier, M.; Pannetier, D.; Baize, S. Immune Responses and Lassa Virus Infection. Viruses 2012, 4, 2766–2785. [Google Scholar] [CrossRef] [PubMed]
- Nunberg, J.H.; York, J. The Curious Case of Arenavirus Entry, and Its Inhibition. Viruses 2012, 4, 83–101. [Google Scholar] [CrossRef] [PubMed]
- Agnihothram, S.S.; Dancho, B.; Grant, K.W.; Grimes, M.L.; Lyles, D.S.; Nunberg, J.H. Assembly of Arenavirus Envelope Glycoprotein GPC in Detergent-Soluble Membrane Microdomains. J. Virol. 2009, 83, 9890–9900. [Google Scholar] [CrossRef] [PubMed]
- York, J.; Nunberg, J.H. Role of the Stable Signal Peptide of Junín Arenavirus Envelope Glycoprotein in PH-Dependent Membrane Fusion. J. Virol. 2006, 80, 7775–7780. [Google Scholar] [CrossRef]
- Hulseberg, C.E.; Fénéant, L.; Szymańska, K.M.; White, J.M. Lamp1 Increases the Efficiency of Lassa Virus Infection by Promoting Fusion in Less Acidic Endosomal Compartments. mBio 2018, 9, e01818-17. [Google Scholar] [CrossRef]
- York, J.; Dai, D.; Amberg, S.M.; Nunberg, J.H. PH-Induced Activation of Arenavirus Membrane Fusion Is Antagonized by Small-Molecule Inhibitors. J. Virol. 2008, 82, 10932–10939. [Google Scholar] [CrossRef]
- Parodi, A.S.; Greenway, D.J.; Rugeiro, H.R.; Frigerio, M.; de la Barrera, J.M.; Mettler, N.; Garzon, F.; Boxaca, M.; Guerrero, L.; Nota, N. Sobre La Etiologia Del Brote Epidemico de Junin. Dia Med. 1958, 30, 2300–2301. [Google Scholar]
- Kumar, S.; Yadav, D.; Singh, D.; Shakya, K.; Rathi, B. Poonam Recent Developments on Junin Virus, a Causative Agent for Argentine Haemorrhagic Fever. Rev. Med. Virol. 2023, 33, e2419. [Google Scholar] [CrossRef]
- Garcia, M.I.; Zampetti, A.; Asencio, M.D.; Leone, C.S.; Gutierrez, M.; Mindlin, P.E. Fiebre Hemorrágica Argentina: Comunicación De Dos Casos En Zona No Endémica. Medicina 2023, 83, 129–132. [Google Scholar]
- Carballal, G.; Videla, C.M.; Merani, M.S. Epidemiology of Argentine Hemorrhagic Fever. Eur. J. Epidemiol. 1988, 4, 259–274. [Google Scholar] [CrossRef]
- Mills, J.N.; Ellis, B.A.; Childs, J.E.; McKee, K.T., Jr.; Maiztegui, J.I.; Peters, C.J.; Ksiazek, T.G.; Jahrling, P.B. Prevalence of Infection with Junin Virus in Rodent Populations in the Epidemic Area of Argentine Hemorrhagic Fever. Am. J. Trop. Med. Hyg. 1994, 51, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Vitullo, A.D.; Hodara, V.L.; Merani, M.S. Effect of Persistent Infection with Junin Virus on Growth and Reproduction of Its Natural Reservoir. Am. J. Trop. Med. Hyg. 1987, 37, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Peters, C.J.; Jahrling, P.B.; Liu, C.T.; McKee, K.T., Jr.; Barrera Oro, J.G. Experimental Studies of Arenaviral Hemorrhagic Fevers. Curr. Top. Microbiol. Immunol. 1987, 134, 7–68. [Google Scholar]
- Briggiler, A.; Enria, D.; Feuillade, M.R.; Maiztegui, J.I. Contagio interhumano e infección inaperente por virus Junín en matrimonios del area endémica de fiebre hemorrágica argentina. Medicina 1987, 47, 565. [Google Scholar]
- Briggiler, A.; Enria, D.; Feuillade, M.R.; Maiztegui, J. Contagio interhumano e infección clínica con virus Junín en matrimonios residentes en el área endemica de fiebre hemorrágica argentina. Medicina 1987, 47, 565. [Google Scholar]
- Schwind, V. Viral Hemorrhagic Fever Attack: Arenaviruses. In Ciottone’s Disaster Medicine, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 754–756. [Google Scholar]
- Johnson, D.M.; Jokinen, J.D.; Wang, M.; Pfeffer, T.; Tretyakova, I.; Carrion, R.; Griffiths, A.; Pushko, P.; Lukashevich, I.S. Bivalent Junin & Machupo Experimental Vaccine Based on Alphavirus RNA Replicon Vector. Vaccine 2020, 38, 2949–2959. [Google Scholar] [CrossRef] [PubMed]
- Maiztegui, J.I.; Mckee, K.T.; Barrera Oro, J.G.; Harrison, L.H.; Gibbs, P.H.; Feuillade, M.R.; Enria, D.A.; Briggiler, A.M.; Levis, S.C.; Ambrosio, A.M.; et al. Protective Efficacy of a Live Attenuated Vaccine against Argentine Hemorrhagic Fever. J. Infect. Dis. 1998, 177, 277–283. [Google Scholar] [CrossRef]
- Enria, D.A.; Briggiler, A.M.; Sánchez, Z. Treatment of Argentine Hemorrhagic Fever. Antivir. Res. 2008, 78, 132–139. [Google Scholar] [CrossRef]
- Enria, D.A.; Briggiler, A.M.; Levis, S.; Vallejos, D.; Maiztegui, J.I.; Canonico, P.G. Preliminary Report: Tolerance and Antiviral Effect of Ribavirin in Patients with Argentine Hemorrhagic Fever. Antivir. Res. 1987, 7, 353–359. [Google Scholar] [CrossRef]
- Maiztegui, J.I.; Fernandez, N.J.; de Damilano, A.J. Efficacy of Immune Plasma in Treatment of Argentine Hemorrhagic Fever and Association Between Treatment and a Late Neurological Syndrome. Lancet 1979, 314, 1216–1217. [Google Scholar] [CrossRef]
- Frigerio, M.J.; Rondinone, S.N.; Laguens, R.P.; Calello, M.A.; Cabeza Meckert, P.; Colillas, O.; Weissenbacher, M.C. Infección de Primates Del Nuevo Mundo Con Virus Junín: III. Samiri Sciureus. Medicina 1982, 42, 519–525. [Google Scholar] [PubMed]
- Samoilovich, S.R.; Rondinone, S.N.; Laguens, R.P.; Colillas, O.; Frigerio, M.J.; Weissenbacher, M.C. Infection of New World Primates with Junín Virus. IV. Aotus Trivirgatus. Rev. Argent. Microbiol. 1983, 15, 219–222. [Google Scholar]
- Weissenbacher, M.C.; Callelo, M.A.; Colillas, O.J.; Golfera, H.; Rondinone, S.N.; Frigerio, M.J. Infección De Primates Del Nuevo Mundo Con Virus Junín: I. Alouatta Caraya. Medicina 1978, 38, 529–536. [Google Scholar] [PubMed]
- Weissenbacher, M.C.; De Guerrero, L.B.; Boxaca, M.C. Experimental Biology and Pathogenesis of Junin Virus Infection in Animals and Man. Bull. World Health Organ. 1975, 52, 507–515. [Google Scholar]
- Boxaca, M.; De Guerrero, L.B.; Savy, V.L. The Occurrence of Virus, Interferon, and Circulating Antibodies in Mice after Experimental Infection with Junin Virus. Arch. Gesamte Virusforsch. 1973, 40, 10–20. [Google Scholar] [CrossRef]
- Medeot, S.I.; Contigiani, M.S.; Brandan, E.R.; Sabattini, M.S. Neurovirulence of Wild and Laboratory Junin Virus Strains in Animal Hosts. J. Med. Virol. 1990, 32, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Campetella, O.E.; Galassi, N.V.; Sanjuan, N.; Barrios, H.A. Susceptible Adult Murine Model for Junin Virus. J. Med. Virol. 1988, 26, 443–451. [Google Scholar] [CrossRef]
- Besuschio, S.C.; Weissenbacher, M.C.; Schmunis, G.A. Different Histopathological Response to Arenovirus Infection in Thymectomized Mice. Arch. Gesamte Virusforsch. 1973, 40, 21–28. [Google Scholar] [CrossRef]
- Weissenbacher, M.C.; Calello, M.A.; Merani, S.; Oubiña, J.R.; Laguens, R.P.; Montoro, L.; Carballal, G. Induction of Junin Virus Persistence in Adult Athymic Mice. Intervirology 1986, 25, 210–215. [Google Scholar] [CrossRef]
- Kolokoltsova, O.A.; Yun, N.E.; Poussard, A.L.; Smith, J.K.; Smith, J.N.; Salazar, M.; Walker, A.; Tseng, C.-T.K.; Aronson, J.F.; Paessler, S. Mice Lacking Alpha/Beta and Gamma Interferon Receptors Are Susceptible to Junin Virus Infection. J. Virol. 2010, 84, 13063–13067. [Google Scholar] [CrossRef]
- Hickerson, B.T.; Sefing, E.J.; Bailey, K.W.; Van Wettere, A.J.; Penichet, M.L.; Gowen, B.B. Type I Interferon Underlies Severe Disease Associated with Junín Virus Infection in Mice. Elife 2020, 9, e55352. [Google Scholar] [CrossRef]
- Schmuñis, G.; Weissenbacher, M.; Parodi, A.S. Tolerance to Junin Virus in Thymectomized Mice. Arch. Gesamte Virusforsch. 1967, 21, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Nejamkis, M.R.; Weissenbacher, M.C.; Calello, M.A. Infeccion Experimental Con Virus Junin En La Rata. Medicina 1977, 37, 121–127. [Google Scholar]
- Lerman, G.D.; Blejer, J.L.; Carballal, G.; Nejamkis, M.R. Junin Virus Access to CNS by Extraneural Rat Inoculation. J. Med. Virol. 1986, 19, 71–77. [Google Scholar] [CrossRef]
- Blejer, J.L. Infeccion Experimental De La Rata Con Virus Junin. Ph.D. Dissertation, Universidad de Buenos Aires, Buenos Aires, Argentina, 1985. [Google Scholar]
- Remesar, M.C.; Blejer, J.L.; Lerman, G.D.; Dejean, C.; Nejamkis, M.R. Protección contra la encefalitis producida en ratas por una cepa patógena de virus Junín por inoculación periférica con una cepa atenuada. Rev. Argent. Microbiol. 1989, 21, 120–126. [Google Scholar] [PubMed]
- Weissenbacher, M.C.; Lascano, E.F.; Avila, M.M.; Berria, M.I. Chronic Neurologic Disease in Junín Virus-Infected Rats. J. Med. Virol. 1986, 20, 57–65. [Google Scholar] [CrossRef]
- Contigiani, M.S.; Sabattini, M.S. Características de la infección del criceto por virus Junín. Rev. Argent. Microbiol. 1983, 15, 41–45. [Google Scholar]
- Bruno-Lobo, G.G.; Bruno-Lobo, M.; Johnson, K.M.; Webb, P.A.; De Paola, D. Pathogenesis of Junin Virus Infection in the Infant Hamster. An. Microbiol 1968, 15, 11–33. [Google Scholar]
- Samoilovich, S.R.; Carballal, G.; Weissenbacher, M.C. Protection Against a Pathogenic Strain of Junin Virus by Mucosal Infection with an Attenuated Strain. Am. J. Trop. Med. Hyg. 1983, 32, 825–828. [Google Scholar] [CrossRef]
- Nota, N.R.; Frigerio, M.J.; De Guerrero, L.B.; Nejamkis, M.R. Estudio Hematologica En Cobayos Infectados Con Virus Junin Cepa XJ y Cepa XJ Clon 3. Medicina 1969, 29, 171–174. [Google Scholar]
- Molinas, F.C.; Paz, R.A.; Rimoldi, M.T.; De Bracco, M.M.E. Studies of Blood Coagulation and Pathology in Experimental Infection of Guinea Pigs with Junin Virus. J. Infect. Dis. 1978, 137, 740–746. [Google Scholar] [CrossRef] [PubMed]
- Gomez, M.M.; Boxaca, M.C. Efecto de La Infección Experimental de Cobayas Preñadas Con Virus Junín. Rev. Argent. Microbiol. 1981, 13, 35–40. [Google Scholar]
- de Guerrero, L.B.; Boxaca, M.C.; Malumbres, E.; Gomez, M. de la M. Pathogenesis of Attenuated Junin Virus in the Guinea Pig Model. J. Med. Virol. 1985, 15, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Boxaca, M.C.; de Guerrero, L.B.; Elsner, B.; Avagnina, A.; De Las Mercedes Gomez, M.; Lopez, S. Efecto de La Inoculacion Intracerebral Del Virus Junin En El Cobayo. Medicina 1982, 42, 284–294. [Google Scholar] [PubMed]
- Kenyon, R.H.; Mckee, K.T.; Maiztegui, J.L.; Green, D.E.; Peters, C.J. Heterogeneity of Junin Virus Strains. Med. Microbiol. Lmmunol 1986, 175, 169–172. [Google Scholar] [CrossRef]
- McKee, K.T., Jr.; Green, D.E.; Mahlandt, B.G.; Bagley, L.R.; Lyerly, W.H., Jr.; Peters, C.J.; Eddy, G.A. Infection of Cebus Monkeys with Junin Virus. Medicina 1985, 45, 144–152. [Google Scholar]
- de Guerrero, L.B.; Boxaca, M.; Weissenbacher, M.; Frigerio, M.J. Infeccion Experimental Del Cobayo Con Virus Junin. Medicina 1977, 37, 271–278. [Google Scholar]
- Yun, N.E.; Linde, N.S.; Dziuba, N.; Zacks, M.A.; Smith, J.N.; Smith, J.K.; Aronson, J.F.; Chumakova, O.V.; Lander, H.M.; Peters, C.J.; et al. Pathogenesis of XJ and Romero Strains of Junin Virus in Two Strains of Guinea Pigs. Am. J. Trop. Med. Hyg. 2008, 79, 275–282. [Google Scholar] [CrossRef]
- Kenyon, R.H.; Green, D.E.; Maiztegui, J.I.; Peters, C.J. Viral Strain Dependent Differences in Experimental Argentine Hemorrhagic Fever (Junin Virus) Infection of Guinea Pigs. Intervirology 1988, 29, 133–143. [Google Scholar] [CrossRef]
- Sorvillo, T.E.; Cross, R.W.; Johnson, D.M.; Dobias, N.S.; Fenton, K.A.; Mire, C.E.; Geisbert, T.W. Single Dose rVSVΔG-JUNVGP Vaccine Protects Guinea Pigs against Lethal Junin Virus Challenge. NPJ Vaccines 2021, 6, 96. [Google Scholar] [CrossRef]
- Gowen, B.B.; Hickerson, B.T.; York, J.; Westover, J.B.; Sefing, E.J.; Bailey, K.W.; Wandersee, L.; Nunberg, J.H. Second-Generation Live-Attenuated Candid#1 Vaccine Virus Resists Reversion and Protects against Lethal Junín Virus Infection in Guinea Pigs. J. Virol. 2021, 95, e0039721. [Google Scholar] [CrossRef] [PubMed]
- Zeitlin, L.; Geisbert, J.B.; Deer, D.J.; Fenton, K.A.; Bohorov, O.; Bohorova, N.; Goodman, C.; Kim, D.; Hiatt, A.; Pauly, M.H.; et al. Monoclonal Antibody Therapy for Junin Virus Infection. Proc. Natl. Acad. Sci. USA 2016, 113, 4458–4463. [Google Scholar] [CrossRef] [PubMed]
- Seregin, A.V.; Yun, N.E.; Poussard, A.L.; Peng, B.H.; Smith, J.K.; Smith, J.N.; Salazar, M.; Paessler, S. TC83 Replicon Vectored Vaccine Provides Protection against Junin Virus in Guinea Pigs. Vaccine 2010, 28, 4713–4718. [Google Scholar] [CrossRef]
- Salazar, M.; Yun, N.E.; Poussard, A.L.; Smith, J.N.; Smith, J.K.; Kolokoltsova, O.A.; Patterson, M.J.; Linde, J.; Paessler, S. Effect of Ribavirin on Junin Virus Infection in Guinea Pigs. Zoonoses Public Health 2012, 59, 278–285. [Google Scholar] [CrossRef]
- Gowen, B.B.; Westover, J.B.; Sefing, E.J.; Van Wettere, A.J.; Bailey, K.W.; Wandersee, L.; Komeno, T.; Furuta, Y. Enhanced Protection against Experimental Junin Virus Infection through the Use of a Modified Favipiravir Loading Dose Strategy. Antivir. Res. 2017, 145, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Weissenbacher, M.C.; Calello, M.A.; Rondinone, S.N.; Travi, B.; Frigerio, M.J. Infección de Primates Del Nuevo Mundo Con Virus Junín: II. Callithrix Jacchus. Rev. Med. 1980, 40, 21–30. [Google Scholar]
- Gonzalez, P.H.; Laguens, R.P.; Frigerio, M.J.; Calello, M.A.; Weissenbacher, M.C. Junin Virus Infection of Callithrix Jacchus: Pathologic Features. Am. J. Trop. Med. Hyg. 1983, 32, 417–423. [Google Scholar] [CrossRef]
- Weissenbacher, M.C.; Calello, M.A.; Colillas, O.J.; Rondinone, S.N.; Frigerio, M.J. Argentine Hemorrhagic Fever: A Primate Model. Intervirology 1979, 11, 363–365. [Google Scholar] [CrossRef]
- Frigerio, M.J.; Rondinone, S.N.; Calello, M.A.; Golfera, H.; Weissenbacher, M.C. Infección de Callithrix jacchus con virus Junín: Nuevo modelo experimental en Fiebre Hemorrágica Argentina. Medicina 1978, 38, 603–604. [Google Scholar]
- Avila, M.M.; Sarnoilovich, S.R.; Laguens, R.P.; Merani, M.S.; Weissenbacher, M.C. Protection of Junín Virus-infected Marmosets by Passive Administration of Immune Serum: Association with Late Neurologic Signs. J. Med. Virol. 1987, 21, 67–74. [Google Scholar] [CrossRef]
- Weissenbacher, M.C.; Calello, M.A.; Merani, M.S.; Rodriguez, M.; McCormick, J.B. Therapeutic Effect of the Antiviral Agent Ribavirin in Junín Virus Infection of Primates. J. Med. Virol. 1986, 20, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Weissenbacher, M.C.; Coto, C.E.; Calello, M.A.; Rondinone, S.N.; Damonte, E.B.; Frigerio, M.J. Cross-Protection in Nonhuman Primates Against Argentine Hemorrhagic Fever. Infect. Immun. 1982, 35, 425–430. [Google Scholar] [CrossRef]
- Carballal, G.; Cossio, P.M.; Oubina, J.R.; De La Vega, M.T.; Nagle, C.; Casanova, M.B.; Gonzalez, P.H.; Arana, R.M. Infeccion Experimental De Un Primate Sudamericano, El Cebus Sp., Con La Cepa XJ De Virus Junin. Medicina 1983, 43, 639–646. [Google Scholar]
- Zeitlin, L.; Cross, R.W.; Geisbert, J.B.; Borisevich, V.; Agans, K.N.; Prasad, A.N.; Enterlein, S.; Javad Aman, M.; Bornholdt, Z.A.; Brennan, M.B.; et al. Therapy for Argentine Hemorrhagic Fever in Nonhuman Primates with a Humanized Monoclonal Antibody. Proc. Natl. Acad. Sci. USA 2021, 118, e2023332118. [Google Scholar] [CrossRef]
- McKee, K.T., Jr.; Mahlandt, B.G.; Maiztegui, J.I.; Green, D.E.; Peters, C.J. Virus-Specific Factors in Experimental Argentine Hemorrhagic Fever in Rhesus Macaques. J. Med. Virol. 1987, 22, 99–111. [Google Scholar] [CrossRef]
- Kenyon, R.H.; Mckee, K.T., Jr.; Zack, P.M.; Rippy, M.K. Aerosol Infection of Rhesus Macaques with Junin Virus. Intervirology 1992, 33, 23–31. [Google Scholar] [PubMed]
- Green, D.E.; Mahlandt, B.G.; McKee, K.T., Jr. Experimental Argentine Hemorrhagic Fever in Rhesus Macaques: Virus-Specific Variations in Pathology. J. Med. Virol. 1987, 22, 113–133. [Google Scholar] [CrossRef]
- McKee, K.T., Jr.; Mahlandt, B.G.; Maiztegui, J.I.; Eddy, G.A.; Peters, C.J. Experimental Argentine Hemorrhagic Fever in Rhesus Macaques: Viral Strain-Dependent Clinical Response. J. Infect. Dis. 1985, 152, 218–221. [Google Scholar] [CrossRef] [PubMed]
- McKee, K.T., Jr.; Barrera Oro, J.G.; Kuehne, A.I.; Spisso, J.A.; Mahlandt, B.G. Safety and Immunogenicity of a Live-Attenuated Junin (Argentine Hemorrhagic Fever) Vaccine in Rhesus Macaques. Am. J. Trop. Med. Hyg. 1993, 48, 403–411. [Google Scholar] [CrossRef]
- BOLIVIA EPIDEMIC IS BELIEVED OVER; Virus Vector Was Found in Time, Scientists Report. The New York Times, 8 November 1964; p. 60.
- Webb, P.A. Properties of Machupo Virus. Am. J. Trop. Med. Hyg. 1965, 14, 799–802. [Google Scholar] [CrossRef]
- Kilgore, P.E.; Peters, C.J.; Mills, J.N.; Rollin, P.E.; Armstrong, L.; Khan, A.S.; Ksiazek, T.G. Prospects for the Control of Bolivian Hemorrhagic Fever. Emerg. Infect. Dis. 1995, 1, 97–100. [Google Scholar] [CrossRef]
- Johnson, K.M.; Mackenzie, R.B.; Webb, P.A.; Kuns, M.L. Chronic Infection of Rodents by Machupo Virus. Science 1965, 150, 1618–1619. [Google Scholar] [CrossRef]
- Peters, C.J.; Kuehne, R.W.; Mercado, R.R.; Le Bow, R.H.; Spertzel, R.O.; Webb, P.A. Hemorrhagic Fever in Cochabamba, Bolivia, 1971. Am. J. Epidemiol. 1974, 99, 425–433. [Google Scholar] [CrossRef]
- Rodriguez-Morales, A.J.; Castañeda-Hernández, D.M. Organisms of Concern but Not Foodborne or Confirmed Foodborne: Bolivian Hemorrhagic Fever Virus (Machupo Virus). In Encyclopedia of Food Safety; Elsevier: Amsterdam, The Netherlands, 2014; Volume 2, pp. 237–240. ISBN 9780123786128. [Google Scholar]
- Webb, P.A.; Johnson, K.M.; Mackenzie, R.B.; Kuns, M.L. Some Characteristics of Machupo Virus, Causative Agent of Bolivian Hemorrhagic Fever. Am. J. Trop. Med. Hyg. 1967, 16, 531–538. [Google Scholar] [CrossRef]
- Ignat’ev, G.M.; Tverdokhlebov, A.V.; Kaliberov, S.A.; Pereboeva, L.A.; Patrusheva, I.V.; Kashentseva, E.A. The Immunity Indices in the Infection of Mice of Different Strains with the Machupo Virus. Vopr. Virusol. 1993, 38, 167–170. [Google Scholar]
- Patterson, M.; Seregin, A.; Huang, C.; Kolokoltsova, O.; Smith, J.; Miller, M.; Smith, J.; Yun, N.; Poussard, A.; Grant, A.; et al. Rescue of a Recombinant Machupo Virus from Cloned CDNAs and In Vivo Characterization in Interferon (Aβ/γ) Receptor Double Knockout Mice. J. Virol. 2014, 88, 1914–1923. [Google Scholar] [CrossRef]
- Bradfute, S.B.; Stuthman, K.S.; Shurtleff, A.C.; Bavari, S. A STAT-1 Knockout Mouse Model for Machupo Virus Pathogenesis. Virol. J. 2011, 8, 300. [Google Scholar] [CrossRef]
- Johnson, K.M.; Wiebenga, N.H.; Mackenzie, R.B.; Kuns, M.L.; Tauraso, N.M.; Shelokov, A.; Webb, P.A.; Justines, G.; Beye, H.K. Virus Isolations from Human Cases of Hemorrhagic Fever in Bolivia. Proc. Soc. Exper. Biol. Med. 1965, 118, 113–118. [Google Scholar] [CrossRef]
- Terrell, T.G.; Stookey, J.L.; Spertzel, R.O.; Kuehne, R.W. Comparative Histopathology of Two Strains of Bolivian Hemorrhagic Fever Virus Infections in Suckling Hamsters. Am. J. Trop. Med. Hyg. 1973, 22, 814–818. [Google Scholar] [CrossRef]
- Bell, T.M.; Bunton, T.E.; Shaia, C.I.; Raymond, J.W.; Honnold, S.P.; Donnelly, G.C.; Shamblin, J.D.; Wilkinson, E.R.; Cashman, K.A. Pathogenesis of Bolivian Hemorrhagic Fever in Guinea Pigs. Vet. Pathol. 2016, 53, 190–199. [Google Scholar] [CrossRef]
- Webb, P.A.; Justines, G.; Johnson, K.M. Infection of Wild and Laboratory Animals with Machupo and Latino Viruses. Bull. World Health Organ. 1975, 52, 493–499. [Google Scholar]
- Mantlo, E.K.; Maruyama, J.; Manning, J.T.; Wanninger, T.G.; Huang, C.; Smith, J.N.; Patterson, M.; Paessler, S.; Koma, T. Machupo Virus with Mutations in the Transmembrane Domain and Glycosylation Sites of the Glycoprotein Is Attenuated and Immunogenic in Animal Models of Bolivian Hemorrhagic Fever. J. Virol. 2022, 96, e0020922. [Google Scholar] [CrossRef]
- Wagner, F.S.; Eddy, G.A.; Brand, O.M. The African Green Monkey as an Alternate Primate Host for Studying Machupo Virus Infection. Am. J. Trop. Med. Hyg. 1977, 26, 159–162. [Google Scholar] [CrossRef]
- Mcleod, C.G.; Stookey, J.L.; White, J.D.; Eddy, G.A.; Fry, G.A. Pathology of Bolivian Hemorrhagic Fever in the African Green Monkey. Am. J. Trop.Med. Hyg. 1978, 27, 822–826. [Google Scholar] [CrossRef]
- Eddy, G.A.; Scott, S.K.; Wagner, F.S.; Brand, O.M. Pathogenesis of Machupo Virus Infection in Primates. Bull. World Health Organ. 1975, 52, 517–521. [Google Scholar]
- Bell, T.M.; Shaia, C.I.; Bunton, T.E.; Robinson, C.G.; Wilkinson, E.R.; Hensley, L.E.; Cashman, K.A. Pathology of Experimental Machupo Virus Infection, Chicava Strain, in Cynomolgus Macaques (Macaca fascicularis) by Intramuscular and Aerosol Exposure. Vet. Pathol. 2015, 52, 26–37. [Google Scholar] [CrossRef]
- Lafoux, B.; Baillet, N.; Picard, C.; Fourcaud, G.; Borges-Cardoso, V.; Reynard, S.; Journeaux, A.; Germain, C.; Perthame, E.; Mateo, M.; et al. Hemostasis Defects Underlying the Hemorrhagic Syndrome Caused by Mammarenaviruses in a Cynomolgus Macaque Model. Blood 2023, 142, 2092–2104. [Google Scholar] [CrossRef]
- Reynard, S.; Carnec, X.; Picard, C.; Borges-Cardoso, V.; Journeaux, A.; Mateo, M.; Germain, C.; Hortion, J.; Albrecht, L.; Perthame, E.; et al. A MOPEVAC Multivalent Vaccine Induces Sterile Protection against New World Arenaviruses in Non-Human Primates. Nat. Microbiol. 2023, 8, 64–76. [Google Scholar] [CrossRef]
- McLeod, C.G., Jr.; Stookey, J.L.; Eddy, G.A.; Scott, S.K. Pathology of Chronic Bolivian Hemorrhagic Fever in the Rhesus Monkey. Am. J. Pathol. 1977, 211–218. [Google Scholar]
- KastelIo, M.D.; Eddy, G.A.; Kuehne, R.W. A Rhesus Monkey Model for the Study of Bolivian Hemorrhagic Fever. J. Infect. Dis. 1976, 133, 57–62. [Google Scholar] [CrossRef]
- Terrell, T.G.; Stookey, J.L.; Eddy, G.A.; Kastello, M.D. Pathology of Bolivian Hemorrhagic Fever in the Rhesus Monkey. Am. J. Pathol. 1973, 84, 477–488. [Google Scholar]
- Scott, S.K.; Hickman, R.L.; Lang, C.M.; Eddy, G.A.; Hilmas, D.E.; Spertzel, R.O. Studies of the Coagulation System and Blood Pressure During Experimental Bolivian Hemorrhagic Fever in Rhesus Monkeys. Am. J. Trop. Med. Hyg. 1978, 27, 1232–1239. [Google Scholar] [CrossRef] [PubMed]
- Stephen, E.L.; Jones, D.E.; Peters, C.J.; Eddy, G.A.; Loizeaux, P.S.; Jahrling, P.B. Ribavirin Treatment of Toga-, Arena- and Bunyavirus Infections in Subhuman Primates and Other Laboratory Animal Species; Academic Press Inc.: New York, NY, USA, 1980. [Google Scholar]
- Eddy, G.A.; Wagner, F.S.; Scott, S.K.; Mahlandt, B.J. Protection of Monkeys against Machupo Virus by the Passive Administration of Bolivian Haemorrhagic Fever Immunoglobulin (Human Origin). Bull. World Health Organ. 1975, 52, 723–727. [Google Scholar]
- Salas, R.; Pacheco, M.E.; Ramos, B.; Taibo, M.E.; Jaimes, E.; Vasquez, C.; Querales, J.; de Manzione, N.; Godoy, O.; Betancourt, A.; et al. Venezuelan Haemorrhagic Fever. Lancet 1991, 338, 1033–1036. [Google Scholar] [CrossRef]
- De Manzione, N.; Salas, R.A.; Paredes, H.; Godoy, O.; Rojas, L.; Araoz, F.; Fulhorst, C.F.; Ksiazek, T.G.; Mills, J.N.; Ellis, B.A.; et al. Venezuelan Hemorrhagic Fever: Clinical and Epidemiological Studies of 165 Cases. Clin. Infect. Dis. 1998, 26, 308–313. [Google Scholar] [CrossRef]
- Fulhorst, C.F.; Cajimat, M.N.B.; Milazzo, M.L.; Paredes, H.; de Manzione, N.M.C.; Salas, R.A.; Rollin, P.E.; Ksiazek, T.G. Genetic Diversity between and within the Arenavirus Species Indigenous to Western Venezuela. Virology 2008, 378, 205–213. [Google Scholar] [CrossRef]
- Álvarez, E. Difusión Del Conocimiento de La Fiebre Hemorrágica Venezolana (Fhv) En Los Ámbitos Académicos, Profesionales y Culturales Del País. Obs. Del. Conoc. 2021, 6, 12–31. [Google Scholar]
- Rodríguez-Morales, A.J.; Bonilla-Aldana, D.K.; Risquez, A.; Paniz-Mondolfi, A.; Suárez, J.A. Should We Be Concerned about Venezuelan Hemorrhagic Fever?—A Reflection on Its Current Situation in Venezuela and Potential Impact in Latin America amid the Migration Crisis. New Microbes New Infect. 2021, 44, 100945. [Google Scholar] [CrossRef] [PubMed]
- Tesh, R.; Wilson, M.L.; De Manzione, N.M.C.; Tovar, D.; Ksiazek, T.G.; Peters, C.J. Field Studies on the Epidemiology of Venezuelan Hemorrhagic Fever: Implication of the Cotton Rat Sigmodon Alstoni as the Probable Rodent Reservoir. Am. J. Trop. Med. Hyg. 1993, 49, 227–235. [Google Scholar] [CrossRef]
- Fulhorst, C.F.; Ksiazek, T.G.; Peters, C.J.; Tesh, R.B. Experimental Infection of the Cane Mouse Zygodontomys brevicauda (Family Muridae) with Guanarito Virus (Arenaviridae), the Etiologic Agent of Venezuelan Hemorrhagic Fever. J. Infect. Dis. 1999, 180, 966–969. [Google Scholar] [CrossRef]
- Tesh, R.B.; Jahrling, P.B.; Salas, R.; Shope, R.E. Description of Guanarito Virus (Arenaviridae: Arenavirus), The Etiologic Agent of Venezuelan Hemorrhagic Fever. Am. J. Trop. Med. Hyg. 1994, 50, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Hall, W.C.; Geisbert, T.W.; Huggins, J.W.; Jahrling, P.B. Experimental Infection of Guinea Pigs with Venezuelan Hemorrhagic Fever Virus (Guanarito): A Model of Human Disease. Am. J. Trop. Med. Hyg. 1996, 55, 81–88. [Google Scholar] [CrossRef]
- Cline, C.; Zeng, X.; Bell, T.M.; Shaia, C.; Facemire, P.; Williams, J.; Davis, N.; Babka, A.; Picado, E.; Fitzpatrick, C.; et al. Temporal Changes in Pathology and Viral RNA Distribution in Guinea Pigs Following Separate Infection with Two New World Arenaviruses. PLoS Negl. Trop. Dis. 2023, 17, e0011620C. [Google Scholar] [CrossRef] [PubMed]
- Golden, J.W.; Maes, P.; Kwilas, S.A.; Ballantyne, J.; Hooper, J.W. Glycoprotein-Specific Antibodies Produced by DNA Vaccination Protect Guinea Pigs from Lethal Argentine and Venezuelan Hemorrhagic Fever. J. Virol. 2016, 90, 3515–3529. [Google Scholar] [CrossRef] [PubMed]
- Loayza Mafayle, R.; Morales-Betoulle, M.E.; Romero, C.; Cossaboom, C.M.; Whitmer, S.; Alvarez Aguilera, C.E.; Avila Ardaya, C.; Cruz Zambrana, M.; Dávalos Anajia, A.; Mendoza Loayza, N.; et al. Chapare Hemorrhagic Fever and Virus Detection in Rodents in Bolivia in 2019. N. Engl. J. Med. 2022, 386, 2283–2294. [Google Scholar] [CrossRef]
- Escalera-Antezana, J.P.; Rodriguez-Villena, O.J.; Arancibia-Alba, A.W.; Alvarado-Arnez, L.E.; Bonilla-Aldana, D.K.; Rodríguez-Morales, A.J. Clinical Features of Fatal Cases of Chapare Virus Hemorrhagic Fever Originating from Rural La Paz, Bolivia, 2019: A Cluster Analysis. Travel Med. Infect. Dis. 2020, 36, 101589. [Google Scholar] [CrossRef]
- Johnson, D.M.; Fenton, K.A.; Dobias, N.; Geisbert, T.W.; Cross, R.W. Natural History of Chapare Virus Infection in Strain 13 Guinea Pigs. J. Infect. Dis. 2025, 231, e867–e872. [Google Scholar] [CrossRef]
- Johnson, D.M.; Woolsey, C.; Agans, K.N.; Borisevich, V.; Prasad, A.N.; Deer, D.J.; Dobias, N.S.; Fenton, K.A.; Cross, R.W.; Geisbert, T.W. Pathogenesis of Chapare Virus in Cynomolgus Macaques. EMI Anim. Amp Environ. 2025. [Google Scholar] [CrossRef]
- Lisieux Coimbra, T.M.; Nassar, E.S.; Burattini, M.N.; Terezinha Madia de Souza, L.; Ferreira, I.B.; Rocco, I.M.; A Travassos da Rosa, A.P.; C Vasconcelos, P.F.; Pinheiro, F.P.; LeDuc, J.W.; et al. New Arenavirus Isolated in Brazil. Lancet 1994, 343, 391–392. [Google Scholar] [CrossRef]
- Ellwanger, J.H.; Chies, J.A.B. Keeping Track of Hidden Dangers—The Short History of the Sabiá Virus. Rev. Soc. Bras. Med. Trop. 2017, 50, 3–8. [Google Scholar] [CrossRef]
- Nastri, A.C.; Duarte-Neto, A.N.; Casadio, L.V.B.; de Souza, W.M.; Claro, I.M.; Manuli, E.R.; Selegatto, G.; Salomão, M.C.; Fialkovitz, G.; Taborda, M.; et al. Understanding Sabiá Virus Infections (Brazilian mammarenavirus). Travel Med. Infect. Dis. 2022, 48, 102351. [Google Scholar] [CrossRef] [PubMed]
- Barry, M.; Russi, M.; Armstrong, L.; Geller, D.; Tesh, R.; Dembry, L.; Gonzalez, J.P.; Khan, A.S.; Peters, C.J. Brief Report: Treatment of a Laboratory-Acquired Sabia Virus Infection. N. Engl. J. Med. 1995, 333, 294–296. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alvarado, A.V.; Cross, R.W.; Geisbert, T.W.; Woolsey, C. Animal Models of Pathogenic New World Arenaviruses. Microorganisms 2025, 13, 1358. https://doi.org/10.3390/microorganisms13061358
Alvarado AV, Cross RW, Geisbert TW, Woolsey C. Animal Models of Pathogenic New World Arenaviruses. Microorganisms. 2025; 13(6):1358. https://doi.org/10.3390/microorganisms13061358
Chicago/Turabian StyleAlvarado, Alexander V., Robert W. Cross, Thomas W. Geisbert, and Courtney Woolsey. 2025. "Animal Models of Pathogenic New World Arenaviruses" Microorganisms 13, no. 6: 1358. https://doi.org/10.3390/microorganisms13061358
APA StyleAlvarado, A. V., Cross, R. W., Geisbert, T. W., & Woolsey, C. (2025). Animal Models of Pathogenic New World Arenaviruses. Microorganisms, 13(6), 1358. https://doi.org/10.3390/microorganisms13061358







