Early Response of Rhizosphere Microbial Community Network Characteristics to Thinning Intensity in Pinus massoniana Plantations
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Sample Collection
2.3. DNA Extraction and Sequencing
2.4. Bioinformatic Analysis
2.5. Statistical Analysis
3. Results
3.1. Diversity of Rhizosphere Microbial Community
3.2. Thinning-Sensitive Microbes
3.3. Co-Occurrence Networks
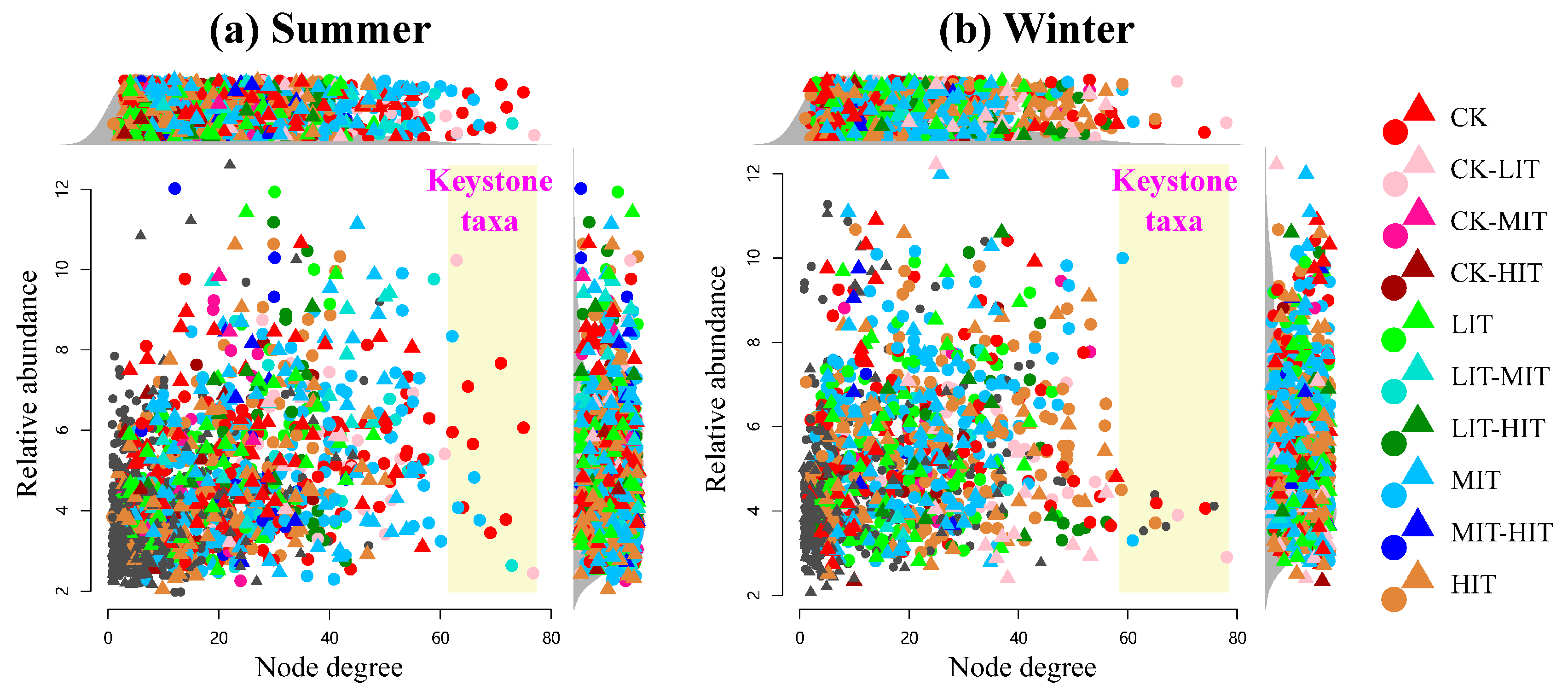
3.4. Keystone Taxa in Meta Co-Occurrence Networks
4. Discussion
4.1. Effects of Thinning on Microbial Diversities and Compositions
4.2. Effects of Thinning on Microbial Co-Occurrence Network
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Edwards, J.; Johnson, C.; Santos-Medellín, C.; Lurie, E.; Podishetty, N.K.; Bhatnagar, S.; Eisen, J.A.; Sundaresan, V.; Jeffery, L.D. Structure, variation, and assembly of the root-associated microbiomes of rice. Proc. Natl. Acad. Sci. USA 2015, 112, E911–E920. [Google Scholar] [CrossRef] [PubMed]
- Qu, Q.; Zhang, Z.; Peijnenburg, W.; Liu, W.; Lu, T.; Hu, B.; Chen, J.; Chen, J.; Lin, Z.; Qian, H. Rhizosphere Microbiome Assembly and Its Impact on Plant Growth. J. Agric. Food Chem. 2020, 68, 5024–5038. [Google Scholar] [CrossRef]
- York, L.M.; Carminati, A.; Mooney, S.J.; Ritz, K.; Bennett, M.J. The holistic rhizosphere: Integrating zones, processes, and semantics in the soil influenced by roots. J. Exp. Bot. 2016, 67, 3629–3643. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.F.; Chaparro, J.M.; Reardon, K.F.; Zhang, R.; Shen, Q.; Vivanco, J.M. Rhizosphere interactions: Root exudates, microbes, and microbial communities. Botany 2014, 92, 267–275. [Google Scholar] [CrossRef]
- Jacoby, R.; Peukert, M.; Succurro, A.; Koprivova, A.; Kopriva, S. The Role of Soil Microorganisms in Plant Mineral Nutrition—Current Knowledge and Future Directions. Front. Plant Sci. 2017, 8, 1617. [Google Scholar] [CrossRef]
- Kwaśna, H. Natural shifts in communities of rhizosphere fungi of common oak after felling. Plant Soil 2004, 264, 209–218. [Google Scholar] [CrossRef]
- Liu, S.; Yin, H.; Li, X.; Li, X.; Fan, C.; Chen, G.; Feng, M.; Chen, Y. Short-Term Thinning Influences the Rhizosphere Fungal Community Assembly of Pinus massoniana by Altering the Understory Vegetation Diversity. Front. Microbiol. 2021, 12, 620309. [Google Scholar] [CrossRef]
- Ye, Y.; Sun, X.; Zhao, J.; Chen, X.; Wang, M.; Li, J.; Guan, Q. Thinning alters the network patterns and keystone taxa of rhizosphere soil microbial communities in Chinese fir plantation. Appl. Soil Ecol. 2023, 189, 104956. [Google Scholar] [CrossRef]
- Olesinski, J.; Lavigne, M.B.; Kershaw, J.A.; Krasowski, M.J. Fine-root dynamics change during stand development and in response to thinning in balsam fir (Abies balsamea L. Mill.) forests. For. Ecol. Manag. 2012, 286, 48–58. [Google Scholar] [CrossRef]
- Wang, D.; Olatunji, O.A.; Xiao, J. Thinning increased fine root production, biomass, turnover rate and understory vegetation yield in a Chinese fir plantation. For. Ecol. Manag. 2019, 440, 92–100. [Google Scholar] [CrossRef]
- Philippot, L.; Raaijmakers, J.M.; Lemanceau, P.; Van Der Putten, W.H. Going back to the roots: The microbial ecology of the rhizosphere. Nat. Rev. Microbiol. 2013, 11, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, H.Y.H.; Chang, S.X. Meta-analysis shows that plant mixtures increase soil phosphorus availability and plant productivity in diverse ecosystems. Nat. Ecol. Evol. 2022, 6, 1112–1121. [Google Scholar] [CrossRef]
- Hartman, K.; van der Heijden, M.G.A.; Wittwer, R.A.; Banerjee, S.; Walser, J.C.; Schlaeppi, K. Cropping practices manipulate abundance patterns of root and soil microbiome members paving the way to smart farming. Microbiome 2018, 6, 14. [Google Scholar] [CrossRef]
- Wang, W.; Yang, Y.; Li, J.; Bu, P.; Lu, A.; Wang, H.; He, W.; Santos Bermudez, R.; Feng, J. Consecutive Fertilization-Promoted Soil Nutrient Availability and Altered Rhizosphere Bacterial and Bulk Fungal Community Composition. Forests 2024, 15, 514. [Google Scholar] [CrossRef]
- Dang, P.; Gao, Y.; Liu, J.; Yu, S.; Zhao, Z. Effects of thinning intensity on understory vegetation and soil microbial communities of a mature Chinese pine plantation in the Loess Plateau. Sci. Total Environ. 2018, 630, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Paré, D.; Martineau, C.; Yin, X.; Rodríguez-Rodríguez, J.C.; Gagné, P.; Bergeron, Y. Response of the soil microbial communities to forest ground cover manipulation in a boreal forest. For. Ecol. Manag. 2024, 553, 121615. [Google Scholar] [CrossRef]
- Kang, H.; Xue, Y.; Cui, Y.; Moorhead, D.L.; Lambers, H.; Wang, D. Nutrient limitation mediates soil microbial community structure and stability in forest restoration. Sci. Total Environ. 2024, 935, 173266. [Google Scholar] [CrossRef]
- Yang, Y.; Geng, Y.; Zhou, H.; Zhao, G.; Wang, L. Effects of gaps in the forest canopy on soil microbial communities and enzyme activity in a Chinese pine forest. Pedobiologia 2017, 61, 51–60. [Google Scholar] [CrossRef]
- Guseva, K.; Darcy, S.; Simon, E.; Alteio, L.V.; Montesinos-Navarro, A.; Kaiser, C. From diversity to complexity: Microbial networks in soils. Soil Biol. Biochem. 2022, 169, 108604. [Google Scholar] [CrossRef]
- Matchado, M.S.; Lauber, M.; Reitmeier, S.; Kacprowski, T.; Baumbach, J.; Haller, D.; List, M. Network analysis methods for studying microbial communities: A mini review. Comput. Struct. Biotechnol. 2021, 19, 2687–2698. [Google Scholar] [CrossRef]
- Pan, Z.; Chen, Y.; Zhou, M.; McAllister, T.A.; Guan, L.L. Microbial interaction-driven community differences as revealed by network analysis. Comput. Struct. Biotechnol. 2021, 19, 6000–6008. [Google Scholar] [CrossRef] [PubMed]
- Kang, P.; Pan, Y.; Yang, P.; Hu, J.; Zhao, T.; Zhang, Y.; Ding, X.; Yan, X. A comparison of microbial composition under three tree ecosystems using the stochastic process and network complexity approaches. Front. Microbiol. 2022, 13, 1018077. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Yang, L.; Gao, X.; Guo, S.; Wang, R. Changes in fungal diversity and key-stone taxa along soil-eroding catena. Appl. Soil Ecol. 2023, 188, 104924. [Google Scholar] [CrossRef]
- Oberholster, T.; Vikram, S.; Cowan, D.; Valverde, A. Key microbial taxa in the rhizosphere of sorghum and sunflower grown in crop rotation. Sci. Total Environ. 2018, 624, 530–539. [Google Scholar] [CrossRef]
- Yang, Z.; Zhu, Q.; Zhang, Y.; Jiang, P.; Wang, Y.; Fei, J.; Rong, X.; Peng, J.; Wei, X.; Luo, G. Soil carbon storage and accessibility drive microbial carbon use efficiency by regulating microbial diversity and key taxa in intercropping ecosystems. Biol. Fertil. Soils 2024, 60, 437–453. [Google Scholar] [CrossRef]
- Yang, P.; Yu, S.; Cheng, L.; Ning, K. Meta-network: Optimized species-species network analysis for microbial communities. BMC Genom. 2019, 20, 187. [Google Scholar] [CrossRef]
- Li, J.; Fan, M.; Wei, Z.; Zhang, K.; Ma, X.; Shangguan, Z. Broad environmental adaptation of abundant microbial taxa in Robinia pseudoacacia forests during long-term vegetation restoration. Environ. Res. 2024, 242, 117720. [Google Scholar] [CrossRef]
- Yuan, Q.; Wang, P.; Wang, X.; Hu, B.; Liu, S.; Ma, J. Abundant microbial communities act as more sensitive bio-indicators for ecological evaluation of copper mine contamination than rare taxa in river sediments. Environ. Pollut. 2022, 305, 119310. [Google Scholar] [CrossRef]
- Wang, P.; Wang, X.; Nie, J.; Wang, Y.; Zang, H.; Peixoto, L.; Yang, Y.; Zeng, Z. Manure Application Increases Soil Bacterial and Fungal Network Complexity and Alters Keystone Taxa. J. Soil Sci. Plant Nut. 2022, 22, 607–618. [Google Scholar] [CrossRef]
- Xue, M.; Guo, Z.; Gu, X.; Gao, H.; Weng, S.; Zhou, J.; Gu, D.; Lu, H.; Zhou, X. Rare rather than abundant microbial communities drive the effects of long-term greenhouse cultivation on ecosystem functions in subtropical agricultural soils. Sci. Total Environ. 2020, 706, 136004. [Google Scholar] [CrossRef]
- Zheng, H.; Yang, T.; Bao, Y.; He, P.; Yang, K.; Mei, X.; Wei, Z.; Xu, Y.; Shen, Q.; Banerjee, S. Network analysis and subsequent culturing reveal keystone taxa involved in microbial litter decomposition dynamics. Soil Biol. Biochem. 2021, 157, 108230. [Google Scholar] [CrossRef]
- Li, X.; Kang, W.; Liu, S.; Yin, H.; Lyu, Q.; Su, Y.; Liu, J.; Liu, J.; Fan, C.; Chen, G.; et al. Diversity of Ectomycorrhizal Fungal Communities in Four Types of Stands in Pinus massoniana Plantation in the West of China. Forests 2021, 12, 719. [Google Scholar] [CrossRef]
- Zhao, Z.; Chao, S.; Zhao, Z.; Jing, M. Climate Change Threatens the Habitat of Pinus massoniana in China. Forests 2024, 15, 323. [Google Scholar] [CrossRef]
- Deng, C.; Zhang, S.; Lu, Y.; Froese, R.E.; Xu, X.; Zeng, J.; Ming, A.; Liu, X.; Xie, Y.; Li, Q. Thinning effects on forest evolution in Masson pine (Pinus massoniana Lamb.) conversion from pure plantations into mixed forests. Forest Ecol. Manag. 2020, 477, 118503. [Google Scholar] [CrossRef]
- Lei, L.; Xiao, W.; Zeng, L.; Frey, B.; Huang, Z.; Zhu, J.; Cheng, R.; Li, M.H. Effects of thinning intensity and understory removal on soil microbial community in Pinus massoniana plantations of subtropical China. Appl. Soil Ecol. 2021, 167, 104055. [Google Scholar] [CrossRef]
- Shen, Y.; Cheng, R.; Xiao, W.; Yang, S. Effects of understory removal and thinning on soil aggregation, and organic carbon distribution in Pinus massoniana plantations in the three Gorges Reservoir area. Ecol. Indic. 2021, 123, 107323. [Google Scholar] [CrossRef]
- Shen, Y.; Wang, N.; Cheng, R.; Xiao, W.; Yang, S.; Guo, Y. Short-Term Effects of Low Intensity Thinning on the Fine Root Dynamics of Pinus massoniana Plantations in the Three Gorges Reservoir Area, China. Forests 2017, 8, 428. [Google Scholar] [CrossRef]
- Zhao, J.; Sun, X.; Wang, D.; Wang, M.; Li, J.; Wang, J.; Guan, Q. Responses of Fine Root Morphological and Chemical Traits among Branch Orders to Forest Thinning in Pinus massoniana Plantations. Forests 2024, 15, 495. [Google Scholar] [CrossRef]
- Gottel, N.R.; Castro, H.F.; Kerley, M.; Yang, Z.; Pelletier, D.A.; Podar, M.; Karpinets, T.; Uberbacher, E.; Tuskan, G.A.; Vilgalys, R.; et al. Distinct Microbial Communities within the Endosphere and Rhizosphere of Populus deltoides Roots across Contrasting Soil Types. Appl. Environ. Microb. 2011, 77, 5934–5944. [Google Scholar] [CrossRef]
- Bakker, P.A.H.M.; Pieterse, C.M.J.; de Jonge, R.; Berendsen, R.L. The Soil-Borne Legacy. Cell 2018, 172, 1178–1180. [Google Scholar] [CrossRef]
- Liu, K.L.; Chen, B.Y.; Zhang, B.; Wang, R.H.; Wang, C.S. Understory vegetation diversity, soil properties and microbial community response to different thinning intensities in Cryptomeria japonica var. sinensis plantations. Front. Microbiol. 2023, 14, 1117384. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.L.; Wang, D.; Chen, X.; Wang, J.; Diao, J.J.; Zhang, J.Y.; Guan, Q.W. Soil microbial functional diversity and biomass as affected by different thinning intensities in a Chinese fir plantation. Appl. Soil Ecol. 2015, 92, 35–44. [Google Scholar] [CrossRef]
- Cheng, X.; Xing, W.; Yuan, H.; Yu, M. Long-Term Thinning Does not Significantly Affect Soil Water-Stable Aggregates and Diversity of Bacteria and Fungi in Chinese Fir (Cunninghamia lanceolata) Plantations in Eastern China. Forests 2018, 9, 687. [Google Scholar] [CrossRef]
- Zhao, J.; Ye, Y.; Sun, X.; Shi, L.; Chen, X.; Guan, Q. Root exudation patterns of Chinese fir after thinning relating to root characteristics and soil conditions. For. Ecol. Manag. 2023, 541, 121068. [Google Scholar] [CrossRef]
- Sarmento, H.; Gasol, J.M. Use of phytoplankton-derived dissolved organic carbon by different types of bacterioplankton. Environ. Microbiol. 2012, 14, 2348–2360. [Google Scholar] [CrossRef]
- Liu, X.; Cong, J.; Lu, H.; Xue, Y.; Wang, X.; Li, D.; Zhang, Y. Community structure and elevational distribution pattern of soil Actinobacteria in alpine grasslands. Acta Ecol. Sin. 2017, 37, 213–218. [Google Scholar] [CrossRef]
- Barbhuiya, A.R.; Arunachalam, A.; Pandey, H.N.; Khan, M.L.; Arunachalam, K. Fine root dynamics in undisturbed and disturbed stands of a tropical wet evergreen forest in northeast India. Trop. Ecol. 2012, 53, 69–79. [Google Scholar] [CrossRef]
- Ma, C.; Zhang, W.; Wu, M.; Xue, Y.; Ma, L.; Zhou, J. Effect of aboveground intervention on fine root mass, production, and turnover rate in a Chinese cork oak (Quercus variabilis Blume) forest. Plant Soil 2013, 368, 201–214. [Google Scholar] [CrossRef]
- Žifčáková, L.; Větrovský, T.; Lombard, V.; Henrissat, B.; Howe, A.; Baldrian, P. Feed in summer, rest in winter: Microbial carbon utilization in forest topsoil. Microbiome 2017, 5, 122. [Google Scholar] [CrossRef]
- Qiu, L.; Zhang, Q.; Zhu, H.; Reich, P.B.; Banerjee, S.; van der Heijden, M.G.A.; Sadowsky, M.J.; Ishii, S.; Jia, X.; Shao, M.; et al. Erosion reduces soil microbial diversity, network complexity and multifunctionality. ISME J. 2021, 15, 2474–2489. [Google Scholar] [CrossRef]
- Chao, H.; Cai, A.; Heimburger, B.; Wu, Y.; Zhao, D.; Sun, M.; Hu, F. Keystone taxa enhance the stability of soil bacterial communities and multifunctionality under steelworks disturbance. J. Environ. Manag. 2024, 356, 120664. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yu, H.; Yu, Y.; Huang, J.; Zhou, Z.; Zeng, J.; Chen, P.; Xiao, F.; He, Z.; Yan, Q. Ecological stability of microbial communities in Lake Donghu regulated by keystone taxa. Ecol. Indic. 2022, 136, 108695. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, M.; Yang, Z.; Cong, M.; Zhu, X.; Jia, H. Soil Microbial Community Response to Nitrogen Application on a Swamp Meadow in the Arid Region of Central Asia. Front. Microbiol. 2021, 12, 797306. [Google Scholar] [CrossRef] [PubMed]
- Lu, R. Soil Agrochemical Analysis Methods; China Agricultural Science and Technology Press: Beijing, China, 2000. [Google Scholar]
- Stanford, G.; Carter, J.N.; Simpson, E.C.; Schwaninger, D.E. Nitrate determination by a modified conway microdiffusion method. J. AOAC Int. 1973, 56, 1365–1368. [Google Scholar] [CrossRef]
- Ren, Z.; Qu, X.; Peng, W.; Yu, Y.; Zhang, M. Functional properties of bacterial communities in water and sediment of the eutrophic river-lake system of Poyang Lake, China. PeerJ 2019, 7, e7318. [Google Scholar] [CrossRef]
- Bao, S.D. Soil and Agricultural Chemistry Analysis; China Agricultural Press: Beijing, China, 2000. [Google Scholar]




| Characteristics | Summer | Winter | ||||||
|---|---|---|---|---|---|---|---|---|
| CK | LIT | MIT | HIT | CK | LIT | MIT | HIT | |
| Stand density (tree 600 m−2) | 92 | 81 | 63 | 45 | / | / | / | / |
| Diamater at breast height (cm) | 16.1 ± 2.0 | 16.8 ± 1.6 | 17.5 ± 2.2 | 18.2 ± 1.2 | / | / | / | / |
| Soil water content (mg g−1) | 309.21 ± 45.88 | 238.63 ± 20.15 | 364.34 ± 82.92 | 362.00 ± 63.50 | 245.33 ± 56.57 | 289.68 ± 13.10 | 304.51 ± 53.08 | 257.10 ± 79.40 |
| Soil bulk density (g cm−3) | 1.34 ± 0.14 | 1.52 ± 0.05 | 1.24 ± 0.04 | 1.28 ± 0.21 | 1.17 ± 0.09 | 1.37 ± 0.13 | 1.24 ± 0.18 | 1.29 ± 0.10 |
| pH | 4.98 ± 0.02 | 4.88 ± 0.01 | 4.47 ± 0.03 | 4.87 ± 0.04 | 4.73 ± 0.02 | 4.93 ± 0.02 | 4.64 ± 0.05 | 4.96 ± 0.03 |
| Soil temperature (°C) | 23.73 ± 0.06 | 23.77 ± 0.06 | 24.47 ± 0.06 | 24.43 ± 0.06 | 10.83 ± 0.06 | 11.13 ± 0.06 | 11.20 ± 0.10 | 11.60 ± 0.10 |
| Soil organic carbon (g kg−1) | 8.40 ± 0.37 | 12.39 ± 0.69 | 16.57 ± 6.22 | 9.97 ± 0.69 | 6.73 ± 1.83 | 6.85 ± 0.33 | 8.83 ± 0.32 | 4.97 ± 0.83 |
| Total nitrogen (g kg−1) | 0.78 ± 0.05 | 1.26 ± 0.18 | 0.85 ± 0.07 | 0.68 ± 0.03 | 0.98 ± 0.01 | 0.75 ± 0.03 | 0.83 ± 0.04 | 0.58 ± 0.04 |
| Total phosphorus (g kg−1) | 0.10 ± 0.05 | 0.11 ± 0.01 | 0.12 ± 0.04 | 0.11 ± 0.06 | 0.08 ± 0.05 | 0.06 ± 0.01 | 0.10 ± 0.02 | 0.11 ± 0.05 |
| Total potassium (g kg−1) | 21.10 ± 4.47 | 19.90 ± 0.86 | 16.51 ± 2.01 | 16.79 ± 0.87 | 15.64 ± 0.42 | 16.63 ± 1.51 | 22.34 ± 6.24 | 17.04 ± 1.41 |
| Group | α-Diversity Index | H | p |
|---|---|---|---|
| Season | Bacterial Chao index | 11.603 | <0.001 *** |
| Bacterial Pielou index | 12.813 | <0.001 *** | |
| Fungi Chao index | 11.213 | <0.001 *** | |
| Fungi Pielou index | 0.030 | 0.862 | |
| Thinning intensity | Bacterial Chao index | 5.913 | 0.116 |
| Bacterial Pielou index | 3.233 | 0.357 | |
| Fungi Chao index | 4.540 | 0.209 | |
| Fungi Pielou index | 0.613 | 0.893 |
| Season | Number of Nodes | Number of Edges | Average Connectivity | Number of tsOTUs | Number of Keystones OTUs | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| B | F | B-B | F-F | B-F | B | F | B | F | ||
| Summer | 988 | 471 | 6415 | 1581 | 5132 | 18.00 | 645 | 336 | 15 | 0 |
| Winter | 810 | 307 | 5769 | 985 | 3343 | 18.08 | 531 | 218 | 12 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Yin, H.; Su, Y.; Li, X.; Fan, C. Early Response of Rhizosphere Microbial Community Network Characteristics to Thinning Intensity in Pinus massoniana Plantations. Microorganisms 2025, 13, 1357. https://doi.org/10.3390/microorganisms13061357
Liu S, Yin H, Su Y, Li X, Fan C. Early Response of Rhizosphere Microbial Community Network Characteristics to Thinning Intensity in Pinus massoniana Plantations. Microorganisms. 2025; 13(6):1357. https://doi.org/10.3390/microorganisms13061357
Chicago/Turabian StyleLiu, Size, Haifeng Yin, Yu Su, Xianwei Li, and Chuan Fan. 2025. "Early Response of Rhizosphere Microbial Community Network Characteristics to Thinning Intensity in Pinus massoniana Plantations" Microorganisms 13, no. 6: 1357. https://doi.org/10.3390/microorganisms13061357
APA StyleLiu, S., Yin, H., Su, Y., Li, X., & Fan, C. (2025). Early Response of Rhizosphere Microbial Community Network Characteristics to Thinning Intensity in Pinus massoniana Plantations. Microorganisms, 13(6), 1357. https://doi.org/10.3390/microorganisms13061357







