Abstract
Aquaculture play a vital role in enhancing human nutrition by producing commercially valuable fish, with gilthead seabream (Sparus aurata) being a key species in the Mediterranean region. In seabream larviculture, disinfection is commonly used to control pathogens and prevent microbial imbalances. However, this process may also remove beneficial microbiota that contribute to ecosystem stability. This study aims to investigate the impact of tank disinfection operations on the bacterial communities associated with seabream larvae and their rearing water in a commercial hatchery using 16S rRNA amplicon sequencing. For further comparison, the bacterial communities present in eggs and feed were also analyzed for comparison. Results showed that the use of different disinfectants significantly altered the bacterial composition of the larvae, while the duration of the dry period had no measurable effect. Across all larval samples, the phylum Pseudomonadota dominated, with members of the genus Psychrobacter consistently detected regardless of disinfection treatment. This suggests that Psychrobacter may be transmitted from eggs or acquired through the feed, mainly rotifers and Artemia nauplii. In contrast, the bacterial communities in the rearing water were more diverse and showed only minor differences in relative abundance across disinfection methods.
1. Introduction
Gilthead seabream (Sparus aurata) is the leading species in Mediterranean aquaculture due to its high market value and adaptability to farming conditions. Greece, a major global producer, accounts for 20% of international production and contributed 58% of the 210,247 tonnes of seabream and seabass produced in 2023 [1]. Seabream alone makes up 54% of Greece’s national seafood output [1]. Over the past 20 years, seabream production has grown in quantity and improved in quality, making a significant contribution to addressing the challenges related to nutrition and food security [2,3,4]. Larviculture, the process of growing fish larvae to a size appropriate for transfer to bigger rearing systems, is a crucial and challenging stage of fish farming [5,6,7]. Despite the advances in adult fish production, larviculture remains a major bottleneck, as it establishes the foundation for the entire production cycle. Infectious disease outbreaks during this phase present a significant economic risk and can threaten the stability of the whole system [8,9]. During this phase, the larvae interact with feed and discarded waste, creating conditions that promote the growth of beneficial, neutral, and potentially harmful microbes [10,11,12]. Beneficial symbiotic bacteria are critical to fish health, development, and survival, as they contribute to vital processes, including digestion and protection against pathogens [13,14,15]. Like humans and other animals, fish also host potentially pathogenic and non-pathogenic commensal bacteria. An imbalance in their microbiota can result in the emergence of pathogenic species and increase the risk of disease [16,17].
Maintaining a healthy microbiome is essential for the overall quality and productivity of fish larviculture [18]. Disinfection is usually the initial step in the treatment of the rearing tanks prior to egg introduction. This step aims to eliminate bacteria, viruses, and other microorganisms that are likely to cause diseases and affect the health of the reared fish. Therefore, it is crucial to disinfect the rearing tanks regularly, following the safety and hygiene standards applicable in the aquaculture industry [19].
A variety of disinfectants are frequently employed in aquaculture, which can be categorized based on their mode of application into synthetic chemical compounds and physical methods. Chlorination, the primary process used in chemical disinfection, is a widely used technique that may eradicate a variety of bacterial species, viruses, and parasites [20]. Chlorine works on bacteria by a reversible process of cell membrane penetration [21,22] and is especially effective in inactivating antibiotic-resistant pathogens [23]. However, it may cause controlled disinfection by-products (DBPs) to develop, which may be detrimental to fish [24,25,26]. After chlorination, it is crucial to neutralize and remove residual chlorine to prevent toxicity to aquatic organisms (especially fish eggs and larvae). Neutralization with sodium thiosulfate is the most common method in aquaculture. After neutralization, a thorough rinse with clean water removes any chemical residue. On the other hand, hydrogen peroxide (H2O2, HP) is a low-risk compound that breaks down relatively quickly into oxygen and water without leaving harmful disinfection by-products [27,28]. By generating hydroxyl radicals, it is a very effective chemical disinfectant used for fish, water, and feed [29,30,31,32,33]. Another commonly used chemical in aquaculture systems is peracetic acid (PAA). It is thought to be very effective against pathogens, especially bacteria, and environmentally neutral because it hydrolyses quickly to H2O2 and acetic acid and breaks down into biodegradable residues [34]. It can be utilized to enhance fish tank water quality in terms of reduced ammonium levels without disturbing microbial populations [35]. Peroxymonosulfate (PMS) is an oxidative chemical disinfectant that can inactivate non-enveloped viruses [21]. It can be used under controlled concentrations to disinfect the external surfaces of fish from parasites or eliminate bacterial and fungal pathogens [36]. The typical duration for the mentioned disinfectants is 5–15 min for eggs, 15–30 min for tanks, and up to 1 h for water treatments. Among physical disinfection methods, UV-C radiation displays the least negative effects on the fish microbiome. However, depending on the duration of its application, it can have an impact on phytoplankton, which is used as a background during the larval rearing phase [26,37,38,39].
Hence, the selection of an appropriate disinfectant is contingent upon several factors: (1) the type of aquaculture, since freshwater environments have different disinfection requirements than marine ones; (2) the species of fish, since many of them have varying sensitivity levels to particular disinfectants; (3) targeted disinfection, in which the disinfectant is effective against a particular contaminant or disease; and (4) the environmental effects, which must be considered to determine whether certain disinfectants can be environmentally harmful [40].
Disinfection in larviculture is a widely used but blunt tool, often implemented with limited understanding of its long-term effects on microbial ecology. Previous studies have focused on studying the dynamics of S. aurata larval-associated bacterial communities according to water treatment, developmental stages, and potential disease associations [41,42,43,44]. However, there remains a notable gap in understanding the specific impact of tank disinfection practices on both larval and rearing water microbiomes. To address this gap, this study aimed to examine the dynamics of the bacterial communities in both S. aurata larvae and rearing water from tanks subjected to different disinfection methods within an industrial production facility. The analysis of the bacterial communities was performed using high-throughput Illumina (San Diego, CA, USA) and ONT MinION (Oxford, UK) sequencing of 16S rRNA gene amplicons. To better understand the impact of the different types of disinfection on the larval and rearing water microbiome, we also examined the bacterial communities present in the provided feed. Additionally, fish eggs were also sampled and compared to the larvae to examine the evolution of the bacterial communities across developmental stages. Understanding the impact of different tank disinfection operations on the microbiome of seabream larvae and their environment could potentially lead to the adaptation of novel disinfection strategies by the aquaculture industry, reduce severe pathogen outbreaks, and eventually enhance production yields.
2. Materials and Methods
2.1. Experimental Setup and Sample Collection
The experiment was carried out under commercial farming conditions of S. aurata between January and June 2020 in a commercial hatchery (Vonitsa, Western Greece). This company is registered (registration number GGN 5200700699992) for aquaculture production in Greece and has secured a GLOBAL G.A.P quality certification. Three different disinfection operations were applied to the larval rearing tanks. After 24 h chlorination period, the tanks underwent one of the three disinfection treatments: (1) nebulization-based disinfection using a commercial product formulated with PAA (1–2.5%), and hydrogen peroxide (H2O2, HP) (10–20%) (Dis1), (2) disinfection using a commercial product formulated with potassium peroxymonosulfate (PMS) (21.41%) and sodium chloride (NaCl, SC) (1.5%) followed by a one-week dry period (Dis2), or a 50-day dry period (Dis3) (Table 1). After disinfecting tanks (10–30 min) with commercial products, thorough cleaning and rinsing with clean water are essential to ensure no harmful residues remain before rearing aquatic organisms.

Table 1.
Larval and water samples used in the 16S rRNA amplicon sequencing survey.
Fertilized eggs were derived from broodstock, disinfected, maintained at the same aquaculture facility, and placed in disinfected rearing tanks with a density of around 100 eggs/L. Eggs were surface disinfected, using iodophors, the most used, effective, and relatively gentle approach. Disinfection does not penetrate the egg interior; only surface pathogens are targeted. The hatching and breeding were carried out in a continuous water flow-through using natural sea water (treated with mechanical filtration and UV light sterilization) [45]. The feeding regime was as follows: microalgae (Chlorella) were added from 1- to 8-days post-hatching to support the early microbial environment; rotifers were introduced as the primary live feed from 9- to 14-dph; and Artemia were introduced at 15-dph. Larvae and their rearing water were collected at various time points: 3-, 8-, 11-, 14-, and 18-dph by the company and were provided post-mortem for analysis. Due to the small size of larvae, the analysis was conducted on the whole body. Samples of larvae in their surrounding water were collected from each tank using a 5 mL sterile plastic pipette to minimize cross-contamination and transferred to sterile containers. To prepare each larval sample, 1.5 mL of water containing larvae was centrifuged at 7000× g for 1 min. After the removal of the supernatant, the resulting pelleted material was subjected to DNA extraction. Samples of rearing water (1 L) were also collected at each time point in sterile flasks. These samples were filtered through 0.22 μm pore-sized membrane filters, which were used for DNA extraction. Three replicates were prepared for each larval and water sample. To investigate the contribution of different factors on the initial establishment of the larval bacteriome, samples of eggs collected 1–3 days before hatching (dph) (n = 36) as well as feed samples, including Chlorella (n = 14), rotifers (n = 13), and Artemia nauplii (n = 7), were also collected and underwent a similar process to the larval samples.
2.2. DNA Extraction, and 16S rRNA Gene Amplification
Total DNA extraction from all the samples, larvae, water filters, eggs, and feed were performed following a modified CTAB protocol [46]. The quality of DNA preparations and the concentration of double-stranded DNA were estimated using a Q5000 micro-volume UV Vis spectrophotometer (Quawell Technology, San Jose, CA, USA). DNA samples were preserved in 1.5 mL Eppendorf tubes at −20 °C until further use.
Polymerase chain reaction (PCR) amplification of the V3–V4 region of the 16S rRNA gene (~460 bp) was performed on the larvae, rearing water, and feed DNA samples using barcoded fusion primers U341F–MiSeq 5′-CCTACGGGRSGCAGCAG-3′ and 805R-MiSeq 5′-GACTACHVGGGTATCTAATCC-3′ [47]. On the other hand, PCR amplification of the whole region of the 16S rRNA gene (~1500 bp) was performed on the egg samples using fusion primers 27F 5′-AGRGTTTGATCMTGGCTCAG-3′ and 1492R 5′-GGTTACCTTGTTACGACTT-3′. The PCR reactions based on both sets of primers were performed in 25 μL reactions containing KAPA Taq Buffer (10×) at a final concentration of 1×, dNTP mix solution at 200 μM each, forward and reverse primer solution at 0.4 μM, 0.5 U of KAPA Taq DNA polymerase (5 U/μL), ≤250 ng from the template DNA solution, and sterile deionized water. The amplification process involved initial denaturation for 5 min at 95 °C and 35 cycles of denaturation for 30 s at 95 °C, annealing for 30 s at 54 °C, and extension for 30 s/500 bp at 72 °C, followed by 5 min of final extension step at 72 °C. Both positive and negative controls were present in parallel. To examine the presence and size of the amplified fragments, PCR products were electrophoresed on 1.5% (w/v) agarose gels in TAE buffer (1×) (40 mM Tris-acetate, 1 mM EDTA). Bio-Rad’s Gel DocTM XR+ was used to visualize the amplified products of around 550 bp and 1450 bp for V3–V4 and 16S regions, respectively. Positive PCR products of the correct size were then purified using polyethylene glycol (20% PEG, 2.5 M NaCl) [48].
2.3. Libraries Preparation and Sequencing
2.3.1. Illumina MiSeq Sequencing of 16S rRNA Amplicons (V3–V4 Region)
To incorporate the indexes and the Illumina adaptors in the larvae, rearing water, and feed samples, a second PCR was carried out in a final volume of 50 μL. Each reaction contained 10 μL of KAPA Taq Buffer (10×), 0.4 μL of dNTPs solution (25 mM each), 5 μL of the forward/reverse indexing primer (10 μM), 0.2 μL of KAPA Taq DNA Polymerase (5 U/μL), and 2 μL of the diluted purified PCR product (10 ng/μL) and sterile deionized water. The temperature profile used for the amplification was as follows: 3 min of initial denaturation at 95 °C and 8 cycles of denaturation at 95 °C for 30 s, 30 s of annealing at 55 °C, and 30 s of extension at 72 °C, and finally, 5 min of final extension at 72 °C. The resulting amplicons were purified using the NucleoMag NGS Clean-up and Size Selection kit (Macherey-Nagel, Düren, Germany) based on the manufacturer’s instructions. Using a Quawell Q5000 micro-volume UV-Vis spectrophotometer, indexed amplicons from different samples were quantified and pooled in equimolar ratio (8 nM). High-throughput amplicon sequencing was performed using a 2 × 300 bp paired-end kit on an Illumina MiSeq platform by Macrogen (Seoul, Republic of Korea).
2.3.2. ONT MinION Sequencing of 16S rRNA Amplicons (V1–V9 Region)
Library preparation for the amplified full 16S region in egg samples was performed using the Native Barcoding Kit 96 V14 (SQK-NBD114.96, ONT, Oxford, UK) following the manufacturer’s recommendation. Briefly, 200 ng of the purified full-length 16S purified PCR product of each sample was used as starting material for the library preparation. The final library with approximately 35 fmol of barcoded DNA was loaded into a MinION R10.4.1 flowcell (FLO-MIN114, ONT, Oxford, UK). Sequencing was carried out for 48 h on a MinION Mk1B device (ONT, Oxford, UK). The sequencing device, data acquisition, and real-time basecalling were monitored via the MinKNOW software (version 23.11.5, Dorado v7.2.13) with the high accuracy option using default settings.
2.4. Bioinformatic Data Processing
2.4.1. Illumina Sequencing Data
Raw sequencing reads were demultiplexed, trimmed of Illumina adapters, and converted to FASTQ using the Illumina standard algorithm. A combination of USEARCH version 11 [49] and QIIME2 distribution 2019.1 [50] was used for the analysis and processing of the resultant sequences. Using the fastq_mergepairs command, the forward and reverse reads from each sample were assembled into paired-end fragments and merged into a single Fastq file. Fragments with less than 400 bp length were excluded from the analysis. The fastq_filter command was used to enhance the constructed sequence quality, while the fastx_uniques tool was used to eliminate duplicate sequences. Unique paired-end fragments were then clustered into operational taxonomic units (OTUs) with cluster_otus command based on 97% OTU clustering using the UPARSE algorithm [51]. Using the uncross command, cross-talk errors were identified and eliminated using the UNCROSS2 algorithm [52]. Using the otutab_trim command, rare OTUs (less than 0.1% of all sequences in all samples) were discarded. Using the BLAST+ algorithm [53] as implemented in QIIME2, taxonomy was assigned to the representative sequences of the discovered OTUs based on the 16S rRNA gene SILVA 138.2 release database [54]. The phylogenetic tree was constructed using the FastTree algorithm [55] and rooted using midpoint-root approach as implemented in QIIME2.
2.4.2. Nanopore Sequencing Data
The adapter and barcode sequences were trimmed using Porechop v0.2.4 [56]. The reads were filtered by size and quality using NanoFilt (v.2.8.0-1) [57], retaining 1200–1800 bp sequences with a mean quality score > 8. Filtered reads from different samples were then concatenated into a single Fastq file. Subsequent analyses were performed using a modified NanoClust pipeline [58]. Briefly, unsupervised read clustering was performed with UMAP v0.5.7 [59], and HDBSCAN v0.8.40 [60]. A representative read was then selected from each cluster and polished using racon v1.4.3 [61] and medaka v1.12.0 [62] based on the remaining reads of the same cluster. Taxonomic classification of polished sequences was performed against the SILVA database, release 138 [63], using the classify-consensus-blast feature as implemented in the QIIME2 pipeline [50].
2.5. Bacterial Composition and Diversity Analysis
Alpha diversity indices, as well as indices depicting the population structure, were calculated using the vegan R package [64] based on a normalized OTU table at a depth of 5000 sequences/sample (ACE, Chao1, Shannon, and Simpson). The OTU count table was used to calculate the good’s coverage. Pairwise ANOVA was used to identify significant differences in alpha diversity indices between the different groups.
The diversity across samples (beta-diversity) was estimated based on Generalized UniFrac distance using the GUniFrac R package [65]. Overall community structure differences and similarities were presented using constrained ordination approaches (Canonical analysis of principal coordinates, CAP) based on 999 permutations, and unconstrained ordination approaches (Principal coordinates analyses, PCoA). Metaxplore (http://metaxplore.eu/, accessed on 1 February 2025) was used to conduct and illustrate CAP, PCoA analyses, and PERMANOVA tests [66]. Statistical significance was described by a p-value of equal to or less than 0.05.
3. Results
3.1. Dataset Overview
Sequencing of the hypervariable regions V3–V4 of the 16S rRNA gene resulted in 7,409,362 raw reads. Following quality filtering and eliminating chimeric sequences, 5,538,269 high-quality reads were grouped into OTUs, with an average of 19,850 reads/sample. With a mean coverage estimate of 0.98, the microbial communities were well sampled. After removing OTUs with an abundance of less than 0.1% across all samples, 90 OTUs were classified into seven phyla, nine classes, and 71 genera (Table S1). Pseudomonadota was the most abundant taxonomic group in all the studied samples (average 73.2 ± 1.5%), followed by Bacteroidota and Cyanobacteriota, representing around 12.0 ± 1.0% and 10.1 ± 0.8% of the total bacterial community. At the class level, Pseudomonadota was represented by Gammaproteobacteria and Alphaproteobacteria (45.1 ± 2.4% and 28.12 ± 1.55%, respectively), Bacteroidota with Bacteroidia (12.1 ± 1.0%), and Cyanobacteriota with Cyanobacteriia (10.1 ± 0.85%). Various taxa were detected at the genus level, with Psychrobacter, Chlorella (the chloroplast of the eukaryotic microalgae), and Pseudophaeobacter being the most dominant, followed by NS3a-marine-group. Each of the remaining genera showed less than 4% average relative abundance across all samples.
Regarding the eggs samples, the sequencing of the full 16S rRNA gene resulted in a total of 876,146 raw reads. After quality filtering, 269,864 high-quality reads were obtained with an average of 7496 reads/sample. After removing the clusters with an abundance of less than 0.1% across all samples, 137 clusters were categorized into eight phyla, 10 classes, and 74 genera (Table S1). Pseudomonadota was the most abundant taxonomic group in egg samples (66.50 ± 4.10%), followed by Bacillota, which represented 22.9 ± 4.6% of the total bacterial community. At the class level, Pseudomonadota was represented by Gammaproteobacteria and Alphaproteobacteria (61.7 ± 2.8% and 4.8 ± 0.7%, respectively) and Bacillota with Clostridia and Bacilli (16.9 ± 2.9% and 6.0 ± 0.8%). Various taxa were detected at the genus level, with Psychrobacter (33.6 ± 3.4%) being the most dominant, followed by Vibrio (15.6 ± 2.7%). Each of the remaining genera showed less than 10% relative abundance.
3.2. The Diversity of the Bacterial Communities Based on the Disinfection Method
Overall, larval samples exhibited lower species richness and diversity compared to their rearing water (Figure 1). Larval samples within the three types of disinfection showed, to some extent, similar bacterial richness. However, samples reared in tanks disinfected with the Dis1 method exhibited significantly lower bacterial diversity compared to those reared in tanks disinfected with the Dis2 and Dis3 methods. On the other hand, overall, no significant differences were observed in the richness and the diversity within the rearing water samples, except for the ACE index, which showed a difference between Dis1 and Dis3 (Figure 1).
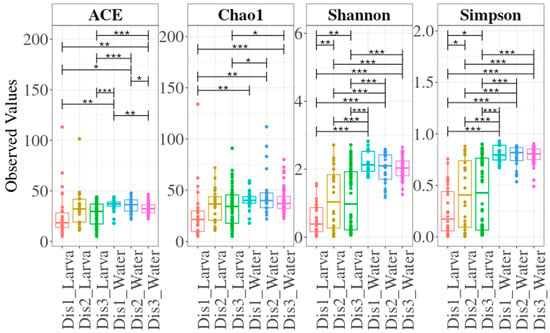
Figure 1.
Species richness and diversity indices for larval and water samples based on the disinfection method. Boxes represent the interquartile range, the line within the boxes is the median, and the dots represent samples. Lines with asterisks above the boxes denote significant differences between compared pairs of samples. Asterisks denote different p-value ranges (***: p ≤ 0.001; **: 0.001 < p ≤ 0.01; *: 0.01 < p ≤ 0.05).
As evident from the CAP and PCoA, along with PERMANOVA analysis, the bacterial profile of the larvae reared in tanks disinfected with the Dis1 method showed a significant difference compared to those reared in tanks disinfected with the Dis2 and Dis3 methods (PERMANOVA, p < 0.05; Figure 2 and Figure S1). However, the change in the dry period from 1 week (Dis2) to 50 days (Dis3) did not affect the bacterial community of larvae (PERMANOVA, p = 0.465). On the other hand, the disinfection method affected, to some extent, the bacterial profile of the rearing water (PERMANOVA, p < 0.05; Figure 2).
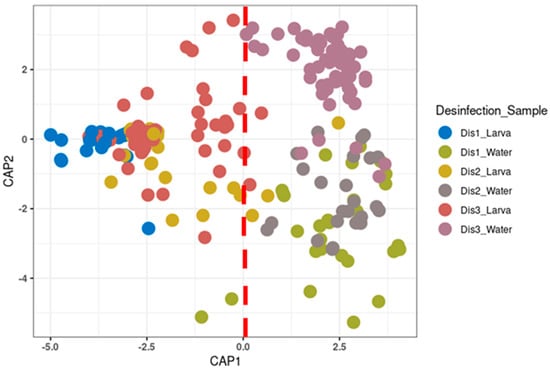
Figure 2.
Canonical analysis of principal coordinates (CAP) of beta-diversity based on GUnifrac distance between each type of disinfection at different components of the larviculture (p-value: 0.001).
3.3. Taxonomic Composition of Bacterial Communities Based on the Type of Disinfection
The bacterial community of larval samples was characterized mainly by the presence of four phyla: Pseudomonadota, Bacillota, Bacteroidota, and Cyanobacteriota (Figure 3A). Pseudomonadota was the dominant phylum in larvae, representing 90% of the bacterial community. In larvae from Dis1 tanks, the most abundant OTUs belonged to Gammaproteobacteria (89.4 ± 2.3%), followed by Bacilli (8.2 ± 2.2%) (Figure 3B). Larvae reared in Dis2 and Dis3 tanks shared similar bacterial communities, characterized by a decrease in Gammaproteobacteria (62.6 ± 4.5%, and 59.2% ± 5.0%, respectively) and an increase in Alphaproteobacteria (28.1 ± 3.7%, and 27.6 ± 3.5%, respectively) and Bacteroidia (7.9 ± 1.6%, and 11.2 ± 2.1%, respectively) (Figure 3B). At the genus level, larvae reared in Dis1 tanks showed a dominance of Psychrobacter (81.8 ± 2.8%) (Figure 4). For Dis2 and Dis3 tanks, larvae showed a lower abundance of Psychrobacter compared to those in Dis1 tanks (54.0 ± 5.3% and 55.4 ± 5.3%, respectively), followed by Pseudophaeobacter (15.0 ± 2.3% and 10.2 ± 1.7%, respectively). The relative abundance of each of the remaining genera was less than 10% (Figure 4, Table S1).
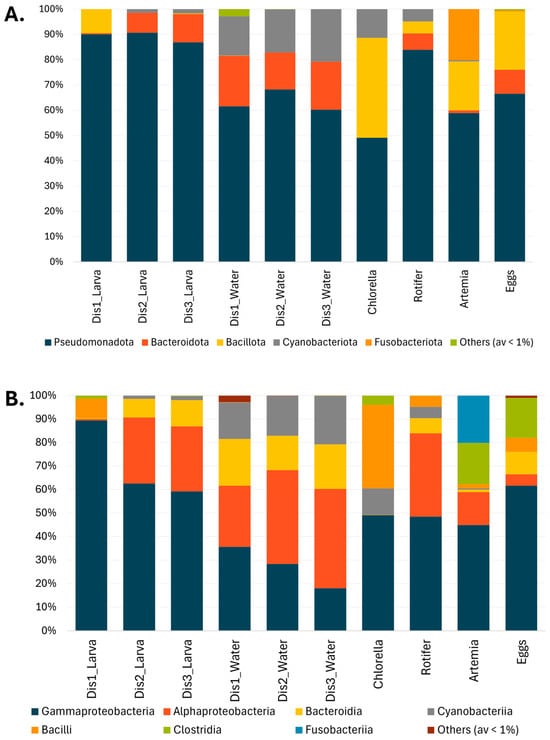
Figure 3.
The microbial composition associated with the three different types of disinfection, Dis1, Dis2, and Dis3, in S. aurata larvae, rearing water, feed, and eggs (A) at the phylum level, and (B) class level.
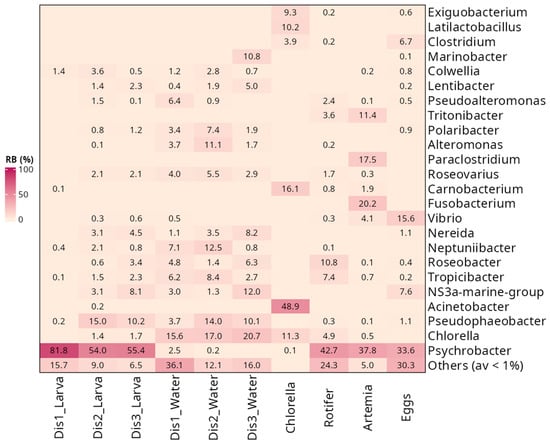
Figure 4.
Comparison of microbial composition associated with three types of disinfection, Dis1, Dis2, and Dis3, in S. aurata larvae, rearing water, and feed from all larval feeding stages at the genus level.
At a higher taxonomic level, similarities were observed in the bacterial community of the rearing water. At the phylum level, Pseudomonadota were dominant regardless of the disinfection method used (61.6 ± 3.2%, 68.3 ± 2.3%, and 60.2 ± 2.5% for Dis1, Dis2, and Dis3, respectively) (Figure 3A). At the class level, the predominance of Gammaproteobacteria (35.7 ± 3.9%) over Alphaproteobacteria (25.9 ± 1.4%) was observed in rearing water from tanks disinfected with Dis1, followed by Bacteroidia and Cyanobacteriia (19.9 ± 2.9% and 15.6 ± 2.1%, respectively) (Figure 3B). Water from the Dis2 and Dis3 tanks showed a decrease in Gammaproteobacteria (28.4 ± 3.9% and 18.1 ± 3.8%, respectively) along with an increase in Alphaproteobacteria (39.9 ± 2.6% and 42.2 ± 2.1%, respectively). They also contained Bacteroidia (14.6 ± 2.6% and 19.0 ± 1.6%, respectively) and Cyanobacteriia (17.0 ± 1.6%, and 20.7 ± 1.8%, respectively) at comparable values to Dis1 (Figure 3B). At the genus level, diverse communities dominated by Chlorella were found in rearing water regardless of the disinfection technique (15.6 ± 2.1%, 17.0 ± 1.6%, and 20.7 ± 1.8% in Dis1, Dis2, and Dis3, respectively). Water from Dis2 tanks was also characterized by increased density of Pseudophaeobacter and Neptuniibacter (14.0 ± 1.4% and 12.5 ± 1.5%, respectively). On the other hand, the rearing water from Dis3 tanks showed increased numbers of NS3a marine group and Pseudophaeobacter (12.0 ± 1.6% and 10.1 ± 1.3%, respectively) (Figure 4).
The bacterial taxa present in the larval feeds were found to be diverse. Samples from all the larval feeding stages were included in the survey, including Chlorella-based feed, rotifers, and Artemia nauplii. Chlorella-based feed was dominated by members of Pseudomonadota, mainly by Acinetobacter (48.9 ± 4.4%), followed by Bacillota, namely Carnobacterium (16.1 ± 2.9%), and Latilactobacillus (10.2 ± 3.1%) (Figure 4, Table S1). Rotifers were also dominated by Pseudomonadota (80.3%), specifically by Psychrobacter (42.7 ± 5.7%), in addition to a minor community of Roseobacter (10.8 ± 2.5%) and Tropicibacter (7.4 ± 1.0%). Similarly, Artemia nauplii were characterized by the strong presence of Pseudomonadota, mainly by Psychrobacter (37.8 ± 4.6%), followed by Fusobacterium (20.2 ± 4.2%), a member of the phylum Fusobacteriota, and Paraclostridium (17.5 ± 3.7%).
Samples from eggs were also collected to examine whether any genera persisted or were lost from the egg to the larval stage. The eggs were also mainly dominated by members of the class Gammaproteobacteria, with more than 60% relative abundance (Figure 3B). More specifically, the most frequently observed genera were Psychrobacter (33.6 ± 3.4%) and Vibrio (15.6 ± 2.7%). Notably, Psychrobacter was still dominant even in the larval stage, while Vibrio had decreased dramatically (Figure 4).
4. Discussion
Farmed fish are in constant interaction with their microbiome, which plays a vital role in determining their overall health and well-being [14,67,68,69]. In many studies, it has been stated that the microbiome of farmed fish is shaped by multiple factors, including water quality and feed composition [15,70,71,72,73,74,75,76,77]. Another important factor is the disinfection protocols applied to the tanks into which the hatched eggs will be introduced to start the larval life cycle. In recent years, various studies have been carried out on the disinfection methods used in aquaculture. Usually, the effect of chemical disinfectant agents is examined either on fish larvae, on the rearing water, or even on their feed [78]. Multiple studies have concluded that the concentration of disinfectants and the duration of their application are crucial for their effectiveness in eliminating pathogens in aquaculture facilities [78,79].
Here, we examined the bacterial communities associated with seabream larvae and their associated rearing water after the application of three different disinfection techniques in the rearing tanks. We additionally assessed the bacterial communities of larval feed and eggs before they were placed in the disinfected tanks. The current analysis, which makes use of next-generation sequencing of 16S rRNA amplicons, shows that disinfectants have a significant impact on the bacterial communities of S. aurata larvae. Distinctive features are evident between the larval microbiome from Dis1 versus Dis2, and Dis3, which have common characteristics. Furthermore, despite the differences observed between the bacterial community of larvae and the remaining vital components of the larviculture ecosystem, including the rearing water, feed, and eggs, similar traits were also recorded.
4.1. Type of Disinfection and Microbial Communities
The variation observed between the bacterial communities of the three studied larval groups suggests that the microbial composition was influenced by the type of disinfection that was applied to the tanks. Since the larval-associated microbiome is shaped by a variety of other factors, such as the rearing water and the provided live feed [12,80,81,82,83], we tried to ensure uniformity in all the tanks used in this study by standardizing the aforementioned factors. Despite the regular use of disinfectants in aquaculture [78,84], most studies focus mainly on adult fish, examining the effect of different dosages and their prolonged application in rearing water [35,79]. To the best of our knowledge, no studies have specifically investigated the impact of tank disinfection on the microbial community of fish larvae. This gap in research is especially relevant given the widespread use of disinfectants in this stage of aquaculture production.
Three main phyla were identified by the taxonomic classification of the microbiome of S. aurata larvae: Pseudomonadota, Bacteroidota, and Bacillota, with variations in the relative abundance across disinfection methods. In previous studies, Pseudomonadota also appeared to be the dominant phylum of the bacterial community found in both European seabass and seabream eggs and larvae [85,86]. The bacterial communities of the larvae in all types of disinfection were mainly dominated by members of Gammaproteobacteria, and more specifically, the genus Psychrobacter. This genus appears to be mostly part of the normal surface microbiota of fish skin, seaweeds, or algae, but it can also be found in the tissues of marine animals and sponges [87,88,89].
The commercial PAA-HP (Dis1) and PMS-SC (Dis2 and Dis3) mixtures are commonly used in aquaculture for water treatment, facility disinfection, and control of bacterial or parasitic infections during disease outbreaks [78,90,91,92]. They differ in the way they impact the water chemistry, which may affect the larviculture environment and therefore the overall health of larvae. PMS used in Dis2 and Dis3 produces highly reactive sulphate radicals (SO4−) and hydroxyl radicals (•OH), which target bacteria, fungal spores, and viruses [93,94,95]. As a gram-negative bacterium, Psychrobacter may be susceptible to these radicals, which can damage proteins, the cell wall, and the cell membrane [93]. This effect may explain the lower abundance of Psychrobacter in larval samples from rearing tanks disinfected with Dis2 and Dis3. The reduction in the presence of Psychrobacter may have led to the proliferation of other bacterial genera, thus increasing the recorded diversity in these tanks. Similarly, PAA-HP used in Dis1 also have strong antimicrobial properties. However, their action is often milder than that of potassium peroxymonosulfate, which was used in Dis2 and Dis3 tanks [96,97,98]. In this regard, the cultivable bacterial diversity of the shrimps abdominal cavity has been shown to remain unaffected by different doses of PAA in the rearing water [79]. The milder effect of PAA-HP potentially allows Psychrobacter to survive in higher abundance in larvae reared in tanks disinfected with Dis1 and dominate the community. However, since these compounds rarely exhibit specialized activity, it is more likely that the application of Dis1 resulted in the drastic reduction in bacterial diversity in the larvae, leaving behind a rather simple community composed mainly of Psychrobacter and the tolerant gram-positive genus Bacillus. Significant reduction in the bacterial diversity of the external surface of shrimps was previously recorded 29 days after the application of PAA (1 mg/L) in the rearing water [79]. In this scenario, the other two disinfection methods had a milder effect on the community of their larvae and eventually led to the formation of more diverse and possibly more resilient bacteriomes. These bacteriomes were characterized by the presence of multiple bacterial genera, including Pseudophaeobacter, Trobicibacter, NS3a marine group, Roseobacter, etc., that were generally absent or found with very low abundances in Dis1. This notion is also supported by the diversity of the microbiota of the water that resembled more the microbiota of Dis2 and Dis3 larvae. Regardless of the scenario, it seems that Psychrobacter is a rather persistent genus capable of retaining a strong presence in larvae, possibly assisted by its availability in the microbial pools of the eggs and the feed, mainly rotifer and Artemia.
4.2. Factors Influencing the Composition of the Larval Microbiota
The microbial community observed in rearing water was different from larvae, with higher richness and diversity in all the disinfection types. It was dominated by members of Cyanobacteriia and Alphaproteobacteria, such as the genera Chlorella (the chloroplast of the eukaryotic microalgae) and Tropicibacter, respectively. Despite the observed differences in the dominant genera, water samples and seabream larvae, especially Dis2 and Dis3 larvae, shared common characteristics, including the presence of Tropicibacter, NS3a marine group, Roseobacter, Neptuniibacter, Nereida, Polaribacter, Lentibacter, and Colwellia. The bacterial communities of the water seem to be affected by all the components of the tank ecosystem. They may receive bacteria from the disinfected tank walls, the eggs that are placed inside the tanks to hatch, the feed that is provided to the larvae, and ultimately from the developing larvae. For example, water samples seem to have received Chlorella from the larval feed (mostly Chlorella and rotifers), as well as Tropicibacter and Roseobacter from the rotifers. At the same time, water also affects the microbiome of larvae. During development, the larvae tend to acquire bacteria from the rearing water, such as Pseudophaeobacter, which is consistent with previous studies on S. aurata larvae [42,43]. Notably, water samples contained a very small amount of Psychrobacter, even though the bacterium was prevalent in feed, eggs, and larvae. This discrepancy is probably due to the fact that the identified Psychrobacter strains are either symbiotic or strongly associated with their hosts, even though free-living species are known to exist (e.g., in seawater) [99]. Another putative reason for the low density of Psychrobacter is its interaction with Chlorella in the water, as the eukaryotic microalgae are highly competitive with certain bacteria, such as Bacillus and Pseudomonas [100]. This interspecific competition could be further supported by the fact that the bacterium was not identified in the Chlorella-based feed. On the contrary, the possibility that this depletion could be attributed to disinfectant residues in the water that affected the proliferation of Psychrobacter seems rather low. Similarly, it is difficult to attribute the remaining observed differences between the microbiota of the water samples to the application of the three disinfection methods on the tanks. As mentioned previously, these communities are rather rich and diverse, and the observed variations in relative abundance could be most likely linked to the dynamic interactions among their components.
A detailed examination of the developmental stages from egg to larva allows for the estimation of which bacterial taxa are eliminated, and which persist throughout early growth. The bacterial profile of the eggs revealed the presence of the genera Clostridium, Pseudomonas, and Vibrio, known to include species pathogenic to fish [101,102]. In contrast, these genera were either absent or nearly undetectable in larvae. The discrepancy between the two developmental stages can also be influenced by the sequencing approach: the bacterial community in eggs was characterized using full-length 16S rRNA sequencing, whereas the larvae were analyzed only using the V3–V4 region. Since less abundant taxa can be masked when using Illumina V3–V4 sequencing, this limitation may have contributed to the observed differences [103]. On the other hand, Psychrobacter seems to persist from the eggs to the larval stage, where it emerges as the dominant genus in both developmental stages. This aligns with the findings identifying Psychrobacter as a common colonizer of the gastrointestinal tracts of finfish species [104]. Overall, it seems that the bacterial profile of the eggs influenced the bacterial communities of both larvae and water samples.
In larviculture systems, Chlorella, rotifers, and Artemia nauplii are frequently employed as feed organisms. They sustain the early growth and development of fish larvae and offer vital nutritional elements [105]. Both the current and previous studies have shown that there is a significant overlap between the larval microbiome and live feed [42,43,83]. Regarding Chlorella, its effect on the bacterial profile of the larvae was negligible since the observed dominant genera, such as Acinetobacter and Carnobacterium, were not present in the larvae samples. On the contrary, the bacterial profiles of rotifers and Artemia nauplii harbored common taxa with the larvae, as both categories were dominated by the genus Psychrobacter and contained Pseudophaeobacter, Tropicibacter, Roseobacter, Vibrio, and Pseudoalteromonas. The presence of Vibrio in rotifer and Artemia-based feed may indicate a source of potentially pathogenic strains, as several species have been implicated in causing disease in farmed fish [106]. In this regard, Vibrio was identified with very low density in Dis2 and Dis3 larvae. However, its presence in the larvae may also be linked to the bacterial pool of the eggs, which contained a high titer of Vibrio. Additionally, certain genera, such as Ruegeria, Paraclostridium, and Fusobacterium, were identified exclusively in these feed categories. It seems that these genera do not proliferate in the water or larvae, possibly due to competitive interactions with the remaining bacteria of the larviculture ecosystem, although an effect of disinfectant residues in the water cannot be excluded.
This study provides practical insights relevant to commercial hatcheries using similar disinfection protocols for seabream. However, due to its limited taxonomic, geographical, and methodological breadth, generalizability is constrained to other species, other systems, or different microbial endpoints (functional or virulence-related microbial properties may not follow the same trends as taxonomic composition). While the study has methodological and contextual limitations (no functional microbiome analysis, lack of host health, single-species and single site experiment), it makes a meaningful contribution to aquaculture microbiome research. It underscores the complex interplay between disinfection practices, microbial ecology, and hatchery outcomes. Future work building on this foundation—particularly with functional analyses—could lead to microbiome-informed hatchery management strategies that enhance larval health and performance while minimizing unnecessary interventions.
5. Conclusions
This study demonstrated that that S. aurata larvae reared in tanks treated with Dis1 were characterized by distinct bacterial communities compared to their counterparts reared in tanks treated with Dis2 and Dis3. Members of the class Gammaproteobacteria dominated the bacterial profiles of larvae, with most associations linked to the genus Psychrobacter, which was also abundant in egg samples. Water samples developed different bacterial communities devoid of Psychrobacter, despite the strong presence of the bacterium in most components of the larviculture ecosystem. Nonetheless, they shared multiple bacterial genera among them, but also with larvae from tanks subjected to Dis2 and Dis3. Moreover, S. aurata larvae exhibited an increased likelihood of obtaining bacteria from the supplied live feed, mainly rotifers and Artemia nauplii, including Psychrobacter, Tropicibacter, and Roseobacter. However, many prevalent genera that were identified in the feed did not establish in either the water or the larvae. Overall, these findings suggest that the microbial profiles of the larvae were influenced by both their interactions with the other components of the tank ecosystem, and by the specific disinfection methods applied. However, the functional contribution of key microbial taxa, such as Psychrobacter, to the host remains unclear. Future studies are required to evaluate the roles of these species in S. aurata performance and to assess the long-term effects of the different disinfection methods on the microbiota at later life stages of S. aurata.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/microorganisms13061359/s1, Figure S1: Principal Coordinate Analysis (PCoA) of beta-diversity based on GUnifrac distance between each type of disinfection at different components of the larviculture.; Table S1: Identified taxa in S. aurata larvae, rearing water, feeds, and eggs.
Author Contributions
Conceptualization, G.T. and P.S.; methodology, G.T. and P.S.; software, N.B.M.; validation, N.B.M., E.A., G.T. and P.S.; formal analysis, N.B.M. and E.A.; investigation, G.A., N.B.M. and K.T.; resources, K.T., G.T. and P.S.; data curation, E.A.; writing—original draft preparation, G.A.; writing—review and editing, N.B.M., E.A., E.D., K.T., G.K., G.T. and P.S.; visualization, G.A., N.B.M. and E.A.; supervision, G.K., G.T. and P.S.; project administration, E.D.; funding acquisition, G.T. and P.S. All authors have read and agreed to the published version of the manuscript.
Funding
The research leading to these results has received funding from the Hellenic Ministry of Rural Development and Food (2018–2021) from the project titled “Study of the genome and microbial communities in the development and production of aquacultured seabream and sea bass” (FishμBiome). P.S. was supported by the funding programme “MEDICUS”, of the University of Patras.
Institutional Review Board Statement
Rearing of gilthead sea bream embryos and larvae was performed under routine production conditions at Andromeda S.A. This company is registered (registration number GGN 5200700699992) for aquaculture production in Greece and holds a GLOBAL G.A.P quality certification, which ensures regular oversight by a certified Veterinary Doctor who verifies fish health and welfare. Animal sampling followed routine procedures, and samples were collected by a qualified member of staff from standard production cycles. The legislation and measures implemented by Andromeda S.A. comply with both Greek (PD 56/2013) and EU (Directive 63/2010) regulations, which protect animals kept for farming. Production and sampling were carried out by an experienced worker with the goal of minimizing unnecessary pain, suffering, or injury, and ensuring optimal larval survival. No live animals were utilized in any capacity during our study.
Informed Consent Statement
Not applicable.
Data Availability Statement
The NCBI Bioproject accession number for the raw sequencing data reported in this study is PRJNA1113936.
Acknowledgments
The authors are thankful to Andromeda S.A. for collecting the samples of the analyzed larviculture system.
Conflicts of Interest
Kosmas Toskas is employed by Avramar Aquaculture SA. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
References
- Hellenic Aquaculture Producers Organization. HAPO Aquaculture in Greece, Annual Report 2024; Hellenic Aquaculture Producers Organization: Attica, Greece, 2024. [Google Scholar]
- FAO. The State of World Fisheries and Aquaculture 2022; FAO: Rome, Italy, 2022; ISBN 978-92-5-136364-5. [Google Scholar]
- Freitas, J.; Vaz-Pires, P.; Câmara, J.S. From Aquaculture Production to Consumption: Freshness, Safety, Traceability and Authentication, the Four Pillars of Quality. Aquaculture 2020, 518, 734857. [Google Scholar] [CrossRef]
- Ottinger, M.; Clauss, K.; Kuenzer, C. Aquaculture: Relevance, Distribution, Impacts and Spatial Assessments—A Review. Ocean Coast. Manag. 2016, 119, 244–266. [Google Scholar] [CrossRef]
- Araujo, G.S.; da Silva, J.W.A.; Cotas, J.; Pereira, L. Fish Farming Techniques: Current Situation and Trends. J. Mar. Sci. Eng. 2022, 10, 1598. [Google Scholar] [CrossRef]
- Gopakumar, G.; Madhu, K.; Jayasankar, R.; Madhu, R.; Kizhakudan, J.K.; Josileen, J.; Ignatius, B.; Vijayagopal, P.; Joseph, S.; Mohamed, G.; et al. Live Feed Research for Larviculture of Marine Finfish and Shellfish. Mar. Fish. Inf. Serv. T E Ser. 2008, 197, 1–6. [Google Scholar]
- Shields, R.J. Larviculture of Marine Finfish in Europe. Aquaculture 2001, 200, 55–88. [Google Scholar] [CrossRef]
- Muniesa, A.; Basurco, B.; Aguilera, C.; Furones, D.; Reverté, C.; Sanjuan-Vilaplana, A.; Jansen, M.D.; Brun, E.; Tavornpanich, S. Mapping the Knowledge of the Main Diseases Affecting Sea Bass and Sea Bream in Mediterranean. Transbound. Emerg. Dis. 2020, 67, 1089–1100. [Google Scholar] [CrossRef]
- Subasinghe, R.; Bernoth, E.-M. Disease Control and Health Management: Aquaculture Development, Health and Wealth; FAO: Rome, Italy, 2000. [Google Scholar]
- Borges, N.; Keller-Costa, T.; Sanches-Fernandes, G.M.M.; Louvado, A.; Gomes, N.C.M.; Costa, R. Bacteriome Structure, Function, and Probiotics in Fish Larviculture: The Good, the Bad, and the Gaps. Annu. Rev. Anim. Biosci. 2021, 9, 423–452. [Google Scholar] [CrossRef]
- Savaş, S.; Kubilay, A.; Basmaz, N. Effect of Bacterial Load in Feeds on Intestinal Microflora of Seabream (Sparus aurata) Larvae and Juveniles. Isr. J. Aquac.—Bamidgeh 2005, 57, 3–9. [Google Scholar] [CrossRef]
- Vadstein, O.; Attramadal, K.J.K.; Bakke, I.; Forberg, T.; Olsen, Y.; Verdegem, M.; Giatsis, C.; Skjermo, J.; Aasen, I.M.; Gatesoupe, F.-J.; et al. Managing the Microbial Community of Marine Fish Larvae: A Holistic Perspective for Larviculture. Front. Microbiol. 2018, 9, 1820. [Google Scholar] [CrossRef]
- Banerjee, G.; Ray, A.K. Bacterial Symbiosis in the Fish Gut and Its Role in Health and Metabolism. Symbiosis 2017, 72, 1–11. [Google Scholar] [CrossRef]
- Luan, Y.; Li, M.; Zhou, W.; Yao, Y.; Yang, Y.; Zhang, Z.; Ringø, E.; Erik Olsen, R.; Liu Clarke, J.; Xie, S.; et al. The Fish Microbiota: Research Progress and Potential Applications. Engineering 2023, 29, 137–146. [Google Scholar] [CrossRef]
- Sehnal, L.; Brammer-Robbins, E.; Wormington, A.M.; Blaha, L.; Bisesi, J.; Larkin, I.; Martyniuk, C.J.; Simonin, M.; Adamovsky, O. Microbiome Composition and Function in Aquatic Vertebrates: Small Organisms Making Big Impacts on Aquatic Animal Health. Front. Microbiol. 2021, 12, 567408. [Google Scholar] [CrossRef] [PubMed]
- Brugman, S.; Ikeda-Ohtsubo, W.; Braber, S.; Folkerts, G.; Pieterse, C.M.; Bakker, P.A. A Comparative Review on Microbiota Manipulation: Lessons from Fish, Plants, Livestock, and Human Research. Front. Nutr. 2018, 5, 80. [Google Scholar] [CrossRef] [PubMed]
- Wynne, J.W.; Thakur, K.K.; Slinger, J.; Samsing, F.; Milligan, B.; Powell, J.F.F.; McKinnon, A.; Nekouei, O.; New, D.; Richmond, Z.; et al. Microbiome Profiling Reveals a Microbial Dysbiosis During a Natural Outbreak of Tenacibaculosis (Yellow Mouth) in Atlantic Salmon. Front. Microbiol. 2020, 11, 586387. [Google Scholar] [CrossRef] [PubMed]
- Assefa, A.; Abunna, F. Maintenance of Fish Health in Aquaculture: Review of Epidemiological Approaches for Prevention and Control of Infectious Disease of Fish. Vet. Med. Int. 2018, 2018, 5432497. [Google Scholar] [CrossRef]
- American Fisheries Society. Guide to Using Drugs, Biologics & Other Chemicals in Aquaculture; American Fisheries Society—Fish Culture Section: Bethesda, MD, USA, 2019. [Google Scholar]
- Russell Danner, G.; Merrill, P. Disinfectants, Disinfection, and Biosecurity in Aquaculture. In Aquaculture Biosecurity; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2005; pp. 91–128. ISBN 978-0-470-37685-0. [Google Scholar]
- Eleraky, N.Z.; Potgieter, L.N.D.; Kennedy, M.A. Virucidal Efficacy of Four New Disinfectants. J. Am. Anim. Hosp. Assoc. 2002, 38, 231–234. [Google Scholar] [CrossRef]
- Kelkar, V.P.; Rolsky, C.B.; Pant, A.; Green, M.D.; Tongay, S.; Halden, R.U. Chemical and Physical Changes of Microplastics during Sterilization by Chlorination. Water Res. 2019, 163, 114871. [Google Scholar] [CrossRef]
- Sanawar, H.; Xiong, Y.; Alam, A.; Croué, J.-P.; Hong, P.-Y. Chlorination or Monochloramination: Balancing the Regulated Trihalomethane Formation and Microbial Inactivation in Marine Aquaculture Waters. Aquaculture 2017, 480, 94–102. [Google Scholar] [CrossRef]
- Duan, Y.; Tang, Y.; Huang, J.; Zhang, J.; Lin, H.; Jiang, S.; Wang, R.; Wang, G. Changes in the Microbial Community of Litopenaeus vannamei Larvae and Rearing Water during Different Growth Stages after Disinfection Treatment of Hatchery Water. J. Microbiol. 2020, 58, 741–749. [Google Scholar] [CrossRef]
- Gallard, H.; von Gunten, U. Chlorination of Natural Organic Matter: Kinetics of Chlorination and of THM Formation. Water Res. 2002, 36, 65–74. [Google Scholar] [CrossRef]
- Maapea, A.D.; Vine, N.G.; Macey, B.M. Bacterial Microbiome of Dusky Kob Argyrosomus japonicus Eggs and Rearing Water and the Bacteriostatic Effect of Selected Disinfectants. Aquaculture 2021, 542, 736882. [Google Scholar] [CrossRef]
- Dawson, V.K.; Meinertz, J.R.; Schmidt, L.J.; Gingerich, W.H. A Simple Analytical Procedure to Replace HPLC for Monitoring Treatment Concentrations of Chloramine-T on Fish Culture Facilities. Aquaculture 2003, 217, 61–72. [Google Scholar] [CrossRef]
- Arvin, E.; Pedersen, L.-F. Hydrogen Peroxide Decomposition Kinetics in Aquaculture Water. Aquac. Eng. 2015, 64, 1–7. [Google Scholar] [CrossRef]
- Avendaño-Herrera, R.; Magariños, B.; Irgang, R.; Toranzo, A.E. Use of Hydrogen Peroxide against the Fish Pathogen Tenacibaculum maritimum and Its Effect on Infected Turbot (Scophthalmus maximus). Aquaculture 2006, 257, 104–110. [Google Scholar] [CrossRef]
- Bögner, D.; Bögner, M.; Schmachtl, F.; Bill, N.; Halfer, J.; Slater, M.J. Hydrogen Peroxide Oxygenation and Disinfection Capacity in Recirculating Aquaculture Systems. Aquac. Eng. 2021, 92, 102140. [Google Scholar] [CrossRef]
- Giménez-Papiol, G.; Padrós, F.; Roque, A.; Estévez, A.; Furones, D. Effects of a Peroxide-Based Commercial Product on Bacterial Load of Larval Rearing Water and on Larval Survival of Two Species of Sparidae under Intensive Culture: Preliminary Study. Aquac. Res. 2009, 40, 504–508. [Google Scholar] [CrossRef]
- Vallés, R.; Roque, A.; Caballero, A.; Estévez, A. Use of Ox-Aquaculture© for Disinfection of Live Prey and Meagre Larvae, Argyrosomus regius (Asso, 1801). Aquac. Res. 2015, 46, 413–419. [Google Scholar] [CrossRef]
- Rach, J.J.; Gaikowski, M.P.; Ramsay, R.T. Efficacy of Hydrogen Peroxide to Control Parasitic Infestations on Hatchery-Reared Fish. J. Aquat. Anim. Health 2000, 12, 267–273. [Google Scholar] [CrossRef]
- Kitis, M. Disinfection of Wastewater with Peracetic Acid: A Review. Environ. Int. 2004, 30, 47–55. [Google Scholar] [CrossRef]
- Suurnäkki, S.; Pulkkinen, J.T.; Lindholm-Lehto, P.C.; Tiirola, M.; Aalto, S.L. The Effect of Peracetic Acid on Microbial Community, Water Quality, Nitrification and Rainbow Trout (Oncorhynchus mykiss) Performance in Recirculating Aquaculture Systems. Aquaculture 2020, 516, 734534. [Google Scholar] [CrossRef]
- Francis-Floyd, R.; Klinger, R. Use of Potassium Permanganate to Control External Infections of Ornamental Fish; University of Florida: Florida, FL, USA, 2002. [Google Scholar]
- Attramadal, K.J.K.; Øien, J.V.; Kristensen, E.; Evjemo, J.O.; Kjørsvik, E.; Vadstein, O.; Bakke, I. UV Treatment in RAS Influences the Rearing Water Microbiota and Reduces the Survival of European Lobster Larvae (Homarus gammarus). Aquac. Eng. 2021, 94, 102176. [Google Scholar] [CrossRef]
- Attramadal, K.J.K.; Øie, G.; Størseth, T.R.; Alver, M.O.; Vadstein, O.; Olsen, Y. The Effects of Moderate Ozonation or High Intensity UV-Irradiation on the Microbial Environment in RAS for Marine Larvae. Aquaculture 2012, 330–333, 121–129. [Google Scholar] [CrossRef]
- Llewellyn, S.; Inpankaew, T.; Nery, S.V.; Gray, D.J.; Verweij, J.J.; Clements, A.C.A.; Gomes, S.J.; Traub, R.; McCarthy, J.S. Application of a Multiplex Quantitative PCR to Assess Prevalence and Intensity of Intestinal Parasite Infections in a Controlled Clinical Trial. PLoS Neglected Trop. Dis. 2016, 10, e0004380. [Google Scholar] [CrossRef] [PubMed]
- World Organization for Animal Health. Aquatic Code. Available online: https://www.woah.org/en/what-we-do/standards/codes-and-manuals/aquatic-code-online-access/ (accessed on 15 May 2024).
- Bel Mokhtar, N.; Apostolopoulou, G.; Koumoundouros, G.; Tzokas, K.; Toskas, K.; Gourzioti, E.; Stathopoulou, P.; Tsiamis, G. Bacterial Community Structures and Dynamics Associated with Rotated Positioning Syndrome in Gilthead Sea Bream (Sparus aurata) Larviculture. Front. Aquac. 2024, 2, 1270932. [Google Scholar] [CrossRef]
- Califano, G.; Castanho, S.; Soares, F.; Ribeiro, L.; Cox, C.J.; Mata, L.; Costa, R. Molecular Taxonomic Profiling of Bacterial Communities in a Gilthead Seabream (Sparus aurata) Hatchery. Front. Microbiol. 2017, 8, 204. [Google Scholar] [CrossRef]
- Nikouli, E.; Meziti, A.; Antonopoulou, E.; Mente, E.; Kormas, K.A. Host-Associated Bacterial Succession during the Early Embryonic Stages and First Feeding in Farmed Gilthead Sea Bream (Sparus aurata). Genes 2019, 10, 483. [Google Scholar] [CrossRef]
- Al-Ashhab, A.; Alexander-Shani, R.; Avrahami, Y.; Ehrlich, R.; Strem, R.I.; Meshner, S.; Shental, N.; Sharon, G. Sparus aurata and Lates calcarifer Skin Microbiota under Healthy and Diseased Conditions in UV and Non-UV Treated Water. Anim. Microbiome 2022, 4, 42. [Google Scholar] [CrossRef]
- Moretti, A.; Pedini Fernandez-Criado, M.; Vetillart, R. Manual on Hatchery Production of Seabass and Gilthead Seabream; FAO: Rome, Italy, 2005; Volume 2. [Google Scholar]
- Doyle, J.J.; Doyle, J.L. A Rapid DNA Isolation Procedure for Small Quantities of Fresh Leaf Tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of General 16S Ribosomal RNA Gene PCR Primers for Classical and Next-Generation Sequencing-Based Diversity Studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef]
- Hartley, J.; Bowen, H. PEG Precipitation for Selective Removal of Small DNA Fragments. Focus 1996, 18, 27. [Google Scholar]
- Edgar, R.C. Search and Clustering Orders of Magnitude Faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. UPARSE: Highly Accurate OTU Sequences from Microbial Amplicon Reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Edgar, R.C. UNCROSS2: Identification of Cross-Talk in 16S rRNA OTU Tables. bioRxiv 2018, 400762. [Google Scholar]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and Applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef]
- Pruesse, E.; Quast, C.; Knittel, K.; Fuchs, B.M.; Ludwig, W.; Peplies, J.; Glöckner, F.O. SILVA: A Comprehensive Online Resource for Quality Checked and Aligned Ribosomal RNA Sequence Data Compatible with ARB. Nucleic Acids Res. 2007, 35, 7188–7196. [Google Scholar] [CrossRef] [PubMed]
- Price, M.N.; Dehal, P.S.; Arkin, A.P. FastTree: Computing Large Minimum Evolution Trees with Profiles Instead of a Distance Matrix. Mol. Biol. Evol. 2009, 26, 1641–1650. [Google Scholar] [CrossRef]
- Wick, R.R.; Judd, L.M.; Gorrie, C.L.; Holt, K.E. Completing bacterial genome assemblies with multiplex MinION sequencing. Microb. Genom. 2017, 3, e000132. [Google Scholar] [CrossRef]
- De Coster, W.; D’Hert, S.; Schultz, D.T.; Cruts, M.; Van Broeckhoven, C. NanoPack: Visualizing and Processing Long-Read Sequencing Data. Bioinformatics 2018, 34, 2666–2669. [Google Scholar] [CrossRef]
- Rodríguez-Pérez, H.; Ciuffreda, L.; Flores, C. NanoCLUST: A Species-Level Analysis of 16S rRNA Nanopore Sequencing Data. Bioinformatics 2021, 37, 1600–1601. [Google Scholar] [CrossRef]
- McInnes, L.; Healy, J.; Melville, J. UMAP: Uniform Manifold Approximation and Projection for Dimension Reduction. arXiv 2018, arXiv:1802.03426. [Google Scholar]
- McInnes, L.; Healy, J.; Astels, S. Hdbscan: Hierarchical Density Based Clustering. J. Open Source Softw. 2017, 2, 205. [Google Scholar] [CrossRef]
- Vaser, R.; Sovic, I.; Nagarajan, N.; Sikic, M. Fast and Accurate de Novo Genome Assembly from Long Uncorrected Reads. Genome Res. 2017, 27, 737–746. [Google Scholar] [CrossRef]
- Medaka, Version 2.1.0. Sequence Correction Provided by ONT Research. Oxford Nanopore Technologies Ltd.: Oxford, UK, 2020. Available online: https://github.com/nanoporetech/medaka (accessed on 1 February 2025).
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA Ribosomal RNA Gene Database Project: Improved Data Processing and Web-Based Tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Oksanen, J.; Simpson, G.L.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Solymos, P.; Stevens, M.H.H.; Szoecs, E.; et al. Vegan: Community Ecology Package. 2020. Available online: https://CRAN.R-project.org/package=vegan (accessed on 1 February 2025).
- Chen, J.; Zhang, X.; Yang, L.; Zhang, L. GUniFrac: Generalized UniFrac Distances, Distance-Based Multivariate Methods and Feature-Based Univariate Methods for Microbiome Data Analysis. 2023. Available online: https://CRAN.R-project.org/package=GUniFrac (accessed on 1 February 2025).
- Bel Mokhtar, N.; Asimakis, E.; Galiatsatos, I.; Maurady, A.; Stathopoulou, P.; Tsiamis, G. Development of MetaXplore: An Interactive Tool for Targeted Metagenomic Analysis. Curr. Issues Mol. Biol. 2024, 46, 4803–4814. [Google Scholar] [CrossRef] [PubMed]
- de Bruijn, I.; Liu, Y.; Wiegertjes, G.F.; Raaijmakers, J.M. Exploring Fish Microbial Communities to Mitigate Emerging Diseases in Aquaculture. FEMS Microbiol. Ecol. 2018, 94, fix161. [Google Scholar] [CrossRef] [PubMed]
- Perry, W.B.; Lindsay, E.; Payne, C.J.; Brodie, C.; Kazlauskaite, R. The Role of the Gut Microbiome in Sustainable Teleost Aquaculture. Proc. R. Soc. B Biol. Sci. 2020, 287, 20200184. [Google Scholar] [CrossRef]
- Raulo, A.; Ruokolainen, L.; Lane, A.; Amato, K.; Knight, R.; Leigh, S.; Stumpf, R.; White, B.; Nelson, K.E.; Baden, A.L.; et al. Social Behaviour and Gut Microbiota in Red-Bellied Lemurs (Eulemur rubriventer): In Search of the Role of Immunity in the Evolution of Sociality. J. Anim. Ecol. 2018, 87, 388–399. [Google Scholar] [CrossRef]
- Karlsen, C.; Tzimorotas, D.; Robertsen, E.M.; Kirste, K.H.; Bogevik, A.S.; Rud, I. Feed Microbiome: Confounding Factor Affecting Fish Gut Microbiome Studies. ISME Commun. 2022, 2, 14. [Google Scholar] [CrossRef]
- Krotman, Y.; Yergaliyev, T.M.; Alexander Shani, R.; Avrahami, Y.; Szitenberg, A. Dissecting the Factors Shaping Fish Skin Microbiomes in a Heterogeneous Inland Water System. Microbiome 2020, 8, 9. [Google Scholar] [CrossRef]
- Nikouli, E.; Meziti, A.; Smeti, E.; Antonopoulou, E.; Mente, E.; Kormas, K. Gut Microbiota of Five Sympatrically Farmed Marine Fish Species in the Aegean Sea. Microb. Ecol. 2021, 81, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Quero, G.M.; Piredda, R.; Basili, M.; Maricchiolo, G.; Mirto, S.; Manini, E.; Seyfarth, A.M.; Candela, M.; Luna, G.M. Host-Associated and Environmental Microbiomes in an Open-Sea Mediterranean Gilthead Sea Bream Fish Farm. Microb. Ecol. 2022, 86, 1319–1330. [Google Scholar] [CrossRef] [PubMed]
- Rabelo-Ruiz, M.; Newman-Portela, A.M.; Peralta-Sánchez, J.M.; Martín-Platero, A.M.; del Mar Agraso, M.; Bermúdez, L.; Aguinaga, M.A.; Baños, A.; Maqueda, M.; Valdivia, E.; et al. Beneficial Shifts in the Gut Bacterial Community of Gilthead Seabream (Sparus aurata) Juveniles Supplemented with Allium-Derived Compound Propyl Propane Thiosulfonate (PTSO). Animals 2022, 12, 1821. [Google Scholar] [CrossRef]
- Roquigny, R.; Mougin, J.; Le Bris, C.; Bonnin-Jusserand, M.; Doyen, P.; Grard, T. Characterization of the Marine Aquaculture Microbiome: A Seasonal Survey in a Seabass Farm. Aquaculture 2021, 531, 735987. [Google Scholar] [CrossRef]
- Wang, A.R.; Ran, C.; Ringø, E.; Zhou, Z.G. Progress in Fish Gastrointestinal Microbiota Research. Rev. Aquac. 2018, 10, 626–640. [Google Scholar] [CrossRef]
- Zeng, A.; Tan, K.; Gong, P.; Lei, P.; Guo, Z.; Wang, S.; Gao, S.; Zhou, Y.; Shu, Y.; Zhou, X.; et al. Correlation of Microbiota in the Gut of Fish Species and Water. 3 Biotech 2020, 10, 472. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Ellakany, H.F.; Elbestawy, A.R.; Abaza, S.S.; Geneedy, A.M.; Khafaga, A.F.; Salem, H.M.; Abd El-Aziz, A.H.; Selim, S.; et al. Inhibition of Microbial Pathogens in Farmed Fish. Mar. Pollut. Bull. 2022, 183, 114003. [Google Scholar] [CrossRef]
- Teitge, F.; Peppler, C.; Steinhagen, D.; Jung-Schroers, V. Effect of Disinfection with Peracetic Acid on the Microbial Community of a Seawater Aquaculture Recirculation System for Pacific White Shrimp (Litopenaeus vannamei). J. Fish Dis. 2020, 43, 991–1017. [Google Scholar] [CrossRef]
- De Schryver, P.; Vadstein, O. Ecological Theory as a Foundation to Control Pathogenic Invasion in Aquaculture. ISME J. 2014, 8, 2360–2368. [Google Scholar] [CrossRef]
- Vestrum, R.I.; Attramadal, K.J.K.; Winge, P.; Li, K.; Olsen, Y.; Bones, A.M.; Vadstein, O.; Bakke, I. Rearing Water Treatment Induces Microbial Selection Influencing the Microbiota and Pathogen Associated Transcripts of Cod (Gadus morhua) Larvae. Front. Microbiol. 2018, 9, 851. [Google Scholar] [CrossRef]
- Ingerslev, H.-C.; von Gersdorff Jørgensen, L.; Lenz Strube, M.; Larsen, N.; Dalsgaard, I.; Boye, M.; Madsen, L. The Development of the Gut Microbiota in Rainbow Trout (Oncorhynchus mykiss) Is Affected by First Feeding and Diet Type. Aquaculture 2014, 424–425, 24–34. [Google Scholar] [CrossRef]
- Wilkes Walburn, J.; Wemheuer, B.; Thomas, T.; Copeland, E.; O’Connor, W.; Booth, M.; Fielder, S.; Egan, S. Diet and Diet-Associated Bacteria Shape Early Microbiome Development in Yellowtail Kingfish (Seriola lalandi). Microb. Biotechnol. 2019, 12, 275–288. [Google Scholar] [CrossRef] [PubMed]
- Wennberg, A.C.; Martins, S.E.; Furseth, K.; Tobiesen, A.E.D.; Hess-Erga, O.-K. Evaluation of Factors Influencing Disinfection Efficacy for Aquaculture; Norsk Institutt for Vannforskning: Oslo, Norway, 2022; ISBN 978-82-577-7537-7. [Google Scholar]
- Kormas, K.A.; Meziti, A.; Mente, E.; Frentzos, A. Dietary Differences Are Reflected on the Gut Prokaryotic Community Structure of Wild and Commercially Reared Sea Bream (Sparus aurata). MicrobiologyOpen 2014, 3, 718–728. [Google Scholar] [CrossRef] [PubMed]
- Najafpour, B.; Pinto, P.I.S.; Moutou, K.A.; Canario, A.V.M.; Power, D.M. Factors Driving Bacterial Microbiota of Eggs from Commercial Hatcheries of European Seabass and Gilthead Seabream. Microorganisms 2021, 9, 2275. [Google Scholar] [CrossRef]
- Bowman, J.P. The Genus Psychrobacter. In The Prokaryotes: A Handbook on the Biology of Bacteria Volume 6: Proteobacteria: Gamma Subclass; Dworkin, M., Falkow, S., Rosenberg, E., Schleifer, K.-H., Stackebrandt, E., Eds.; Springer: New York, NY, USA, 2006; pp. 920–930. ISBN 978-0-387-30746-6. [Google Scholar]
- Denner, E.B.; Mark, B.; Busse, H.J.; Turkiewicz, M.; Lubitz, W. Psychrobacter proteolyticus sp. nov., a Psychrotrophic, Halotolerant Bacterium Isolated from the Antarctic Krill Euphausia superba Dana, Excreting a Cold-Adapted Metalloprotease. Syst. Appl. Microbiol. 2001, 24, 44–53. [Google Scholar] [CrossRef]
- Pukall, R.; Kramer, I.; Rohde, M.; Stackebrandt, E. Microbial Diversity of Cultivatable Bacteria Associated with the North Sea Bryozoan Flustra foliacea. Syst. Appl. Microbiol. 2001, 24, 623–633. [Google Scholar] [CrossRef]
- Baldry, M.G.C. The Bactericidal, Fungicidal and Sporicidal Properties of Hydrogen Peroxide and Peracetic Acid. J. Appl. Bacteriol. 1983, 54, 417–423. [Google Scholar] [CrossRef]
- Niu, T.; Zhou, Z.; Ren, W.; Jiang, L.-M.; Li, B.; Wei, H.; Li, J.; Wang, L. Effects of Potassium Peroxymonosulfate on Disintegration of Waste Sludge and Properties of Extracellular Polymeric Substances. Int. Biodeterior. Biodegrad. 2016, 106, 170–177. [Google Scholar] [CrossRef]
- Pedersen, L.-F.; Pedersen, P.B.; Nielsen, J.L.; Nielsen, P.H. Peracetic Acid Degradation and Effects on Nitrification in Recirculating Aquaculture Systems. Aquaculture 2009, 296, 246–254. [Google Scholar] [CrossRef]
- Acosta, F.; Montero, D.; Izquierdo, M.; Galindo-Villegas, J. High-Level Biocidal Products Effectively Eradicate Pathogenic γ-Proteobacteria Biofilms from Aquaculture Facilities. Aquaculture 2021, 532, 736004. [Google Scholar] [CrossRef]
- Kunanusont, N.; Punyadarsaniya, D.; Jantafong, T.; Pojprasath, T.; Takehara, K.; Ruenphet, S. Bactericidal Efficacy of Potassium Peroxymonosulfate under Various Concentrations, Organic Material Conditions, Exposure Timing and Its Application on Various Surface Carriers. J. Vet. Med. Sci. 2020, 82, 320–324. [Google Scholar] [CrossRef]
- Wu, G.; Wang, J.; Wan, Q.; Cao, S.; Huang, T.; Lu, J.; Ma, J.; Wen, G. Kinetics and Mechanism of Sulfate Radical-and Hydroxyl Radical-Induced Disinfection of Bacteria and Fungal Spores by Transition Metal Ions-Activated Peroxymonosulfate. Water Res. 2023, 243, 120378. [Google Scholar] [CrossRef]
- Marchand, P.-A.; Phan, T.-M.; Straus, D.L.; Farmer, B.D.; Stüber, A.; Meinelt, T. Reduction of In Vitro Growth in Flavobacterium columnare and Saprolegnia parasitica by Products Containing Peracetic Acid. Aquac. Res. 2012, 43, 1861–1866. [Google Scholar] [CrossRef]
- Jakob, H.; Leininger, S.; Lehmann, T.; Jacobi, S.; Gutewort, S. Peroxo Compounds, Inorganic. In Ullmann’s Encyclopedia of Industrial Chemistry; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2007; ISBN 978-3-527-30673-2. [Google Scholar]
- Kiejza, D.; Kotowska, U.; Polińska, W.; Karpińska, J. Peracids—New Oxidants in Advanced Oxidation Processes: The Use of Peracetic Acid, Peroxymonosulfate, and Persulfate Salts in the Removal of Organic Micropollutants of Emerging Concern—A Review. Sci. Total Environ. 2021, 790, 148195. [Google Scholar] [CrossRef] [PubMed]
- Welter, D.K.; Ruaud, A.; Henseler, Z.M.; De Jong, H.N.; van Coeverden de Groot, P.; Michaux, J.; Gormezano, L.; Waters, J.L.; Youngblut, N.D.; Ley, R.E. Free-Living, Psychrotrophic Bacteria of the Genus Psychrobacter Are Descendants of Pathobionts. mSystems 2021, 6, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.; Wang, R.; Zhao, P.; Chen, R.; Zhou, W.; Tang, L.; Tang, X. Interaction between Chlorella vulgaris and Bacteria: Interference and Resource Competition. Acta Oceanol. Sin. 2014, 33, 135–140. [Google Scholar] [CrossRef]
- Frans, I.; Michiels, C.W.; Bossier, P.; Willems, K.A.; Lievens, B.; Rediers, H. Vibrio Anguillarum as a Fish Pathogen: Virulence Factors, Diagnosis and Prevention. J. Fish Dis. 2011, 34, 643–661. [Google Scholar] [CrossRef]
- Hussein, M.A.; El-tahlawy, A.S.; Abdelmoneim, H.M.; Abdallah, K.M.E.; Bayomi, R.M.E. Pseudomonas aeruginosa in Fish and Fish Products: A Review on the Incidence, Public Health Significance, Virulence Factors, Antimicrobial Resistance, and Biofilm Formation. J. Adv. Vet. Res. 2023, 13, 1464–1468. [Google Scholar]
- Toxqui-Rodríguez, S.; Naya-Català, F.; Sitjà-Bobadilla, A.; Piazzon, M.C.; Pérez-Sánchez, J. Fish Microbiomics: Strengths and Limitations of MinION Sequencing of Gilthead Sea Bream (Sparus aurata) Intestinal Microbiota. Aquaculture 2023, 569, 739388. [Google Scholar] [CrossRef]
- Wuertz, S.; Beça, F.; Kreuz, E.; Wanka, K.M.; Azeredo, R.; Machado, M.; Costas, B. Two Probiotic Candidates of the Genus Psychrobacter Modulate the Immune Response and Disease Resistance after Experimental Infection in Turbot (Scophthalmus maximus, Linnaeus 1758). Fishes 2023, 8, 144. [Google Scholar] [CrossRef]
- Lahnsteiner, F.; Lahnsteiner, E.; Duenser, A. Suitability of Different Live Feed for First Feeding of Freshwater Fish Larvae. Aquac. J. 2023, 3, 107–120. [Google Scholar] [CrossRef]
- Ina-Salwany, M.Y.; Al-saari, N.; Mohamad, A.; Mursidi, F.-A.; Mohd-Aris, A.; Amal, M.N.A.; Kasai, H.; Mino, S.; Sawabe, T.; Zamri-Saad, M. Vibriosis in Fish: A Review on Disease Development and Prevention. J. Aquat. Anim. Health 2019, 31, 3–22. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).