Microbial Community and Functional Analysis of Regionally Produced Traditional Korean Grain Vinegar
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Grain Vinegars According to Manufacturing Environment
2.2. Acidity
2.3. Microbial Community
2.4. Organic Acids
2.5. Free Amino Acids
2.6. Volatile Flavor Components
2.7. Statistical Analysis
3. Results
3.1. Analysis of Grain Vinegar According to Manufacturing Environment
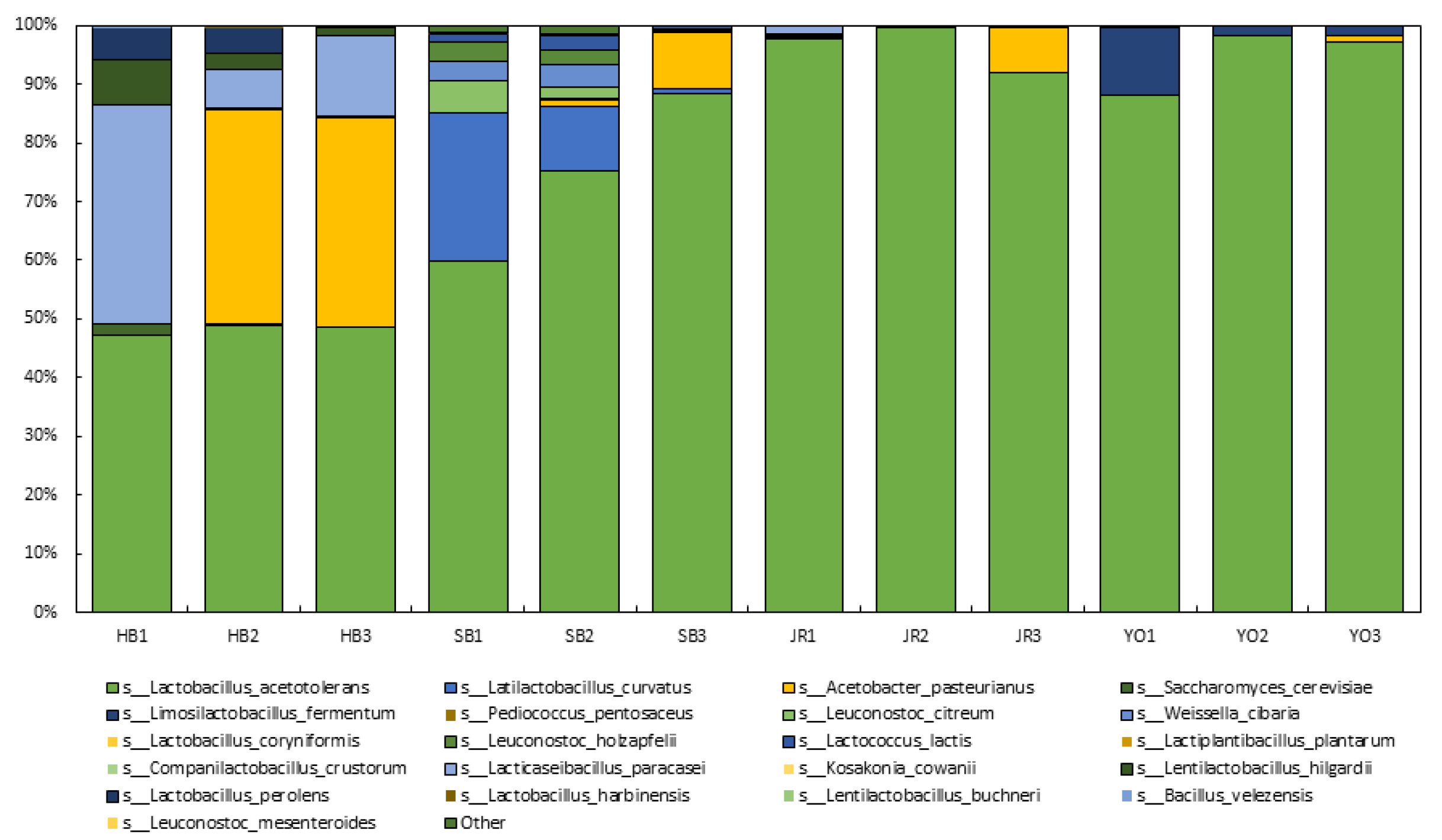
3.2. Microbial Community
3.2.1. Metagenome-Based Microbial Community Analysis
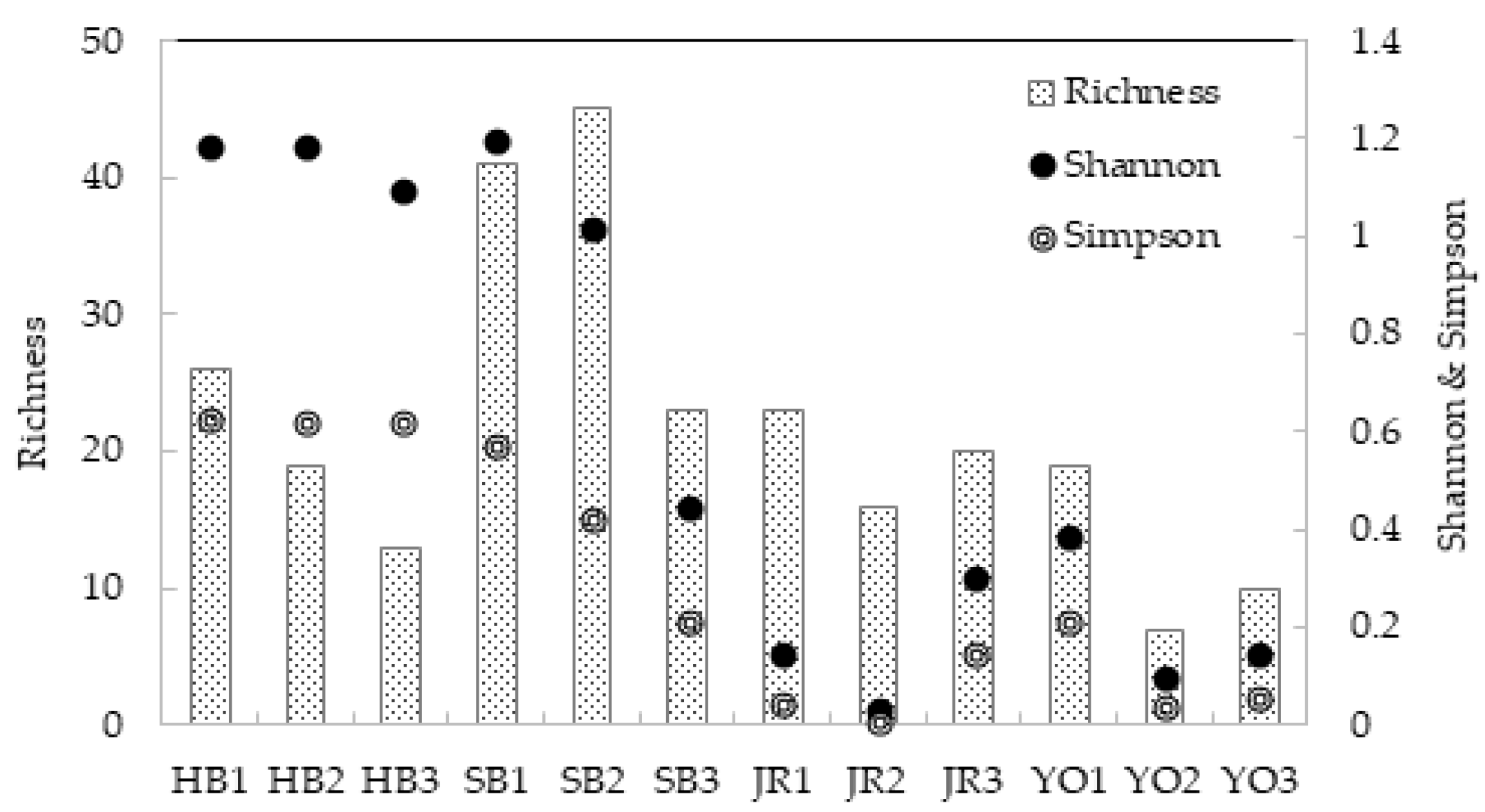
3.2.2. Diversity Analysis
3.3. Organic Acids
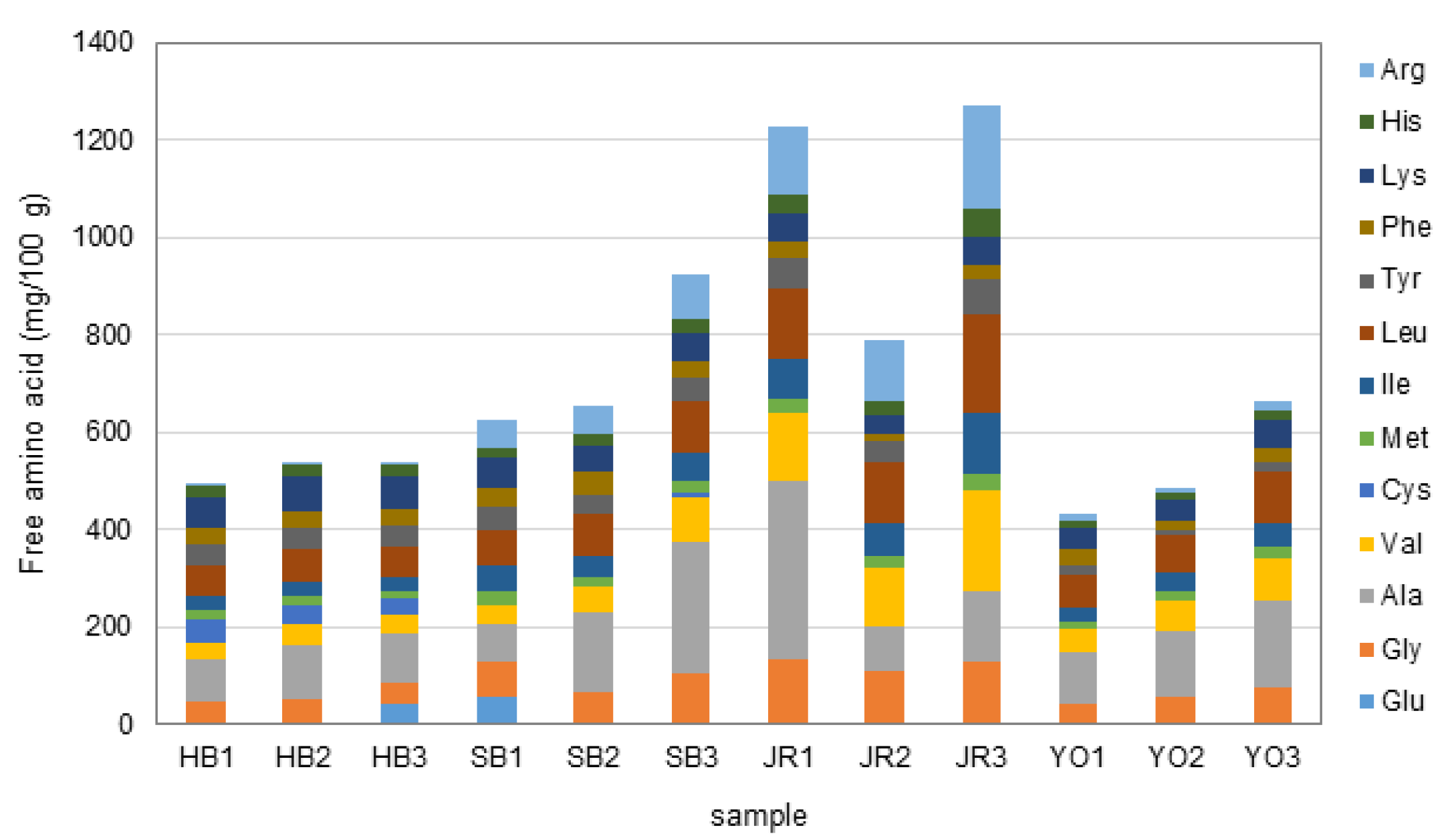
3.4. Free Amino Acids
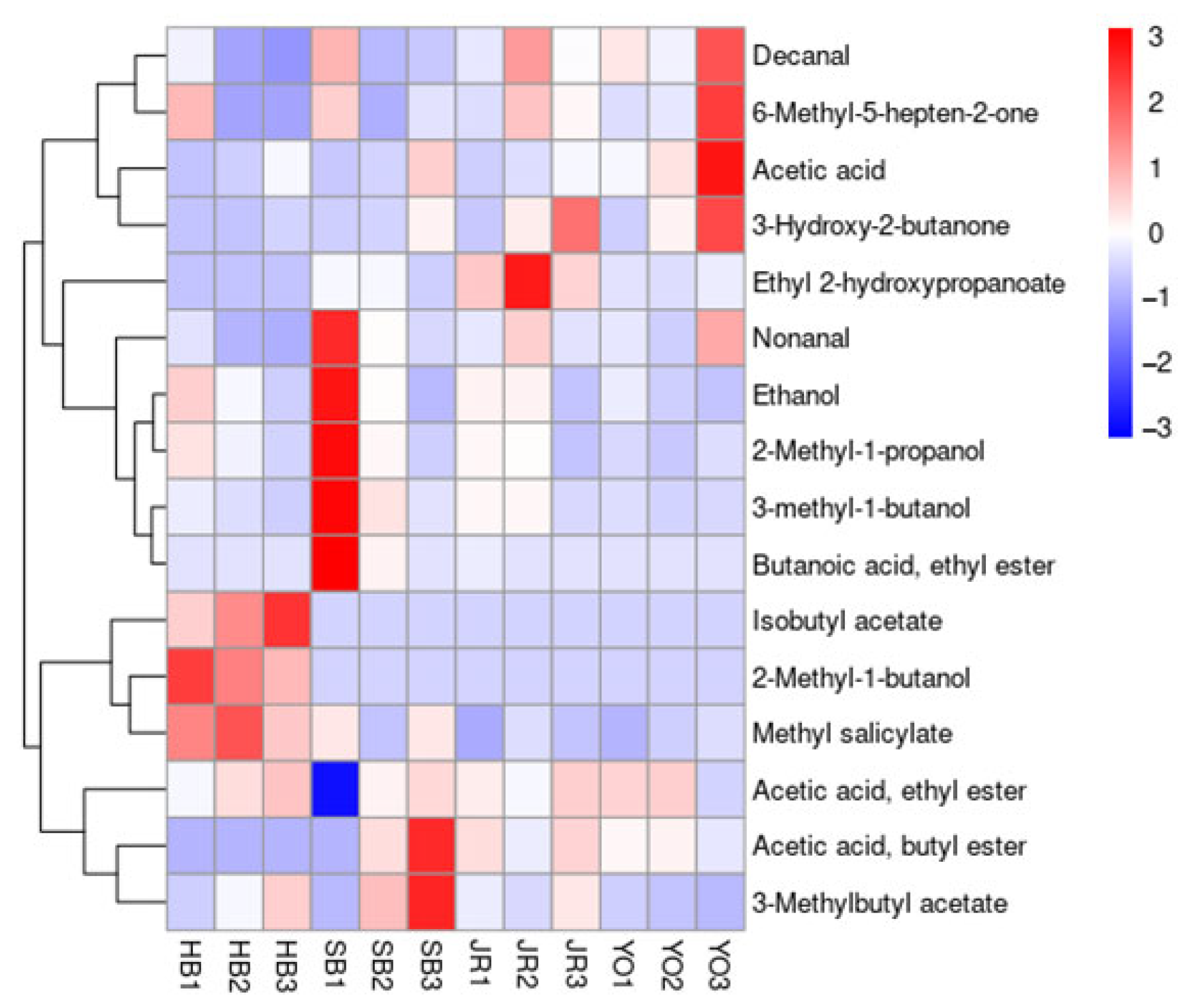
3.5. Volatile Flavor Components
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ubeda, C.; Callejon, R.M.; Hidalgo, C.; Torija, M.J.; Mas, A.; Troncoso, A.M.; Morales, M.L. Determination of major volatile compounds during the production of fruit vinegars by static headspace gas chromatography-mass spectrometry method. Food Res. Int. 2011, 44, 259–268. [Google Scholar] [CrossRef]
- Ren, M.; Wang, X.; Tian, C.; Li, X.; Zhang, B.; Song, X.; Zhang, J. Characterization of organic acids and phenolic compounds of cereal vinegars and fruit vinegars in china. J. Food Process. Preserv. 2007, 41, e12937. [Google Scholar] [CrossRef]
- Juan, J.R.C.; Isidoro, G.G.; Ines, M.S.D.; Teresa, G.M.; Juan, C.M. Latest trends in industrial vinegar production and he role of acetic acid bacteria: Classification, metabolism, and applications—A comprehensive review. Foods 2023, 12, 3705. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, P.; Feng, F.; Luo, L.X. Microbial diversity and their roles in the vinegar fermentation process. Appl. Microbiol. Biotechnol. 2015, 99, 4997–5024. [Google Scholar] [CrossRef]
- Mas, A.; Torija, M.J.; Garcia-Parrilla, M.D.C.; Troncoso, A.M. acetic acid bacteria and the production and quality of wine vinegar. Sci. World J. 2014, 2014, 394671. [Google Scholar] [CrossRef]
- Justyna, A.; Karolina, J.; Pawer, K.; Dominika, M.M.; Joanna, K.; Ewa, R.M.; Katarzyna, J.M. Analysis of Antioxidant Capacity and Antimicrobial Properties of Selected Polish Grape Vinegars Obtained by Spontaneous Fermentation. Molecules 2021, 26, 4727. [Google Scholar] [CrossRef]
- Song, N.E.; Cho, S.H.; Baik, S.H. Microbial community, and biochemical and physiological properties of Korean traditional black raspberry (Robus coreanus Miquel) vinegar. J. Sci. Food Agric. 2016, 96, 3723–3730. [Google Scholar] [CrossRef]
- Bouazza, A.; Bitam, A.; Amiali, M.; Nounihi, A.; Yargui, L.; Koceir, E.A. Effect of fruit vinegars on liver damage and oxidative stress in high-fat-fed rats. Pharm. Biol. 2016, 54, 260–265. [Google Scholar] [CrossRef]
- Budak, N.H.; Aykin, E.; Seydim, A.C.; Greene, A.K.; Guzel-Seydim, Z.B. Functional properties of vinegar. J. Food Sci. 2014, 79, 757–764. [Google Scholar] [CrossRef]
- Ai, M.; Qiu, X.; Huang, J.; Wu, C.; Jin, Y.; Zhou, R. Characterizing the microbial diversity and major metabolites of Sichuan bran vinegar augmented by Monascus purpureus. Int. J. Food Microbiol. 2019, 292, 83–90. [Google Scholar] [CrossRef]
- Park, E.H.; Choi, C.Y.; Kwon, H.J.; Kim, M.D. Literature review on type and manufacturing methods of Korean traditional vinegar. Food Sci. Ind. 2016, 49, 94–99. [Google Scholar]
- Han, E. Industrial technology and prospect of traditional vinegar. Food Tech. 1997, 10, 69–77. [Google Scholar]
- Greco, E.; Cervellati, R.; Litterio, M.L. Antioxidant capacity and total reducing power of balsamic and traditional balsamic vinegar from Modena and Reggio Emilia by conventional chemical assays. Food Sci. Technol. 2013, 48, 114–120. [Google Scholar] [CrossRef]
- Jang, Y.K.; Lee, M.Y.; Kim, H.Y.; Lee, S.; Yeo, S.H.; Beak, S.Y.; Lee, C.H. Comparison of traditional and commercial vinegars based on metabolite profiling and antioxidant activity. J. Microbiol. Biotechnol. 2015, 25, 217–226. [Google Scholar] [CrossRef]
- Chung, N.; Jo, Y.; Hao, Y.; Gu, S.Y.; Jeong, Y.J.; Kwon, J.H. Comparison of physicochemical properties and antioxidant activities of naturally-fermented commercial rice vinegars produced in Korea, China, and Japan. J. Korean Soc. Food Sci. Nutr. 2015, 44, 1799–1805. [Google Scholar] [CrossRef]
- Bolger, A.M.; Marc, L.; Bjoern, U. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Beghini, F.; Mclver, L.J.; Blanco-Miquez, A.; Dubois, L.; Asnicar, F.; Maharjan, S.; Mailyan, A.; Manghi, P.; Scholz, M.; Thomas, A.M.; et al. Intergrating taxonomic, functional, and strain-level profiling of diverse microbial communities with bioBakery 3. eLife 2021, 10, e65088. [Google Scholar] [CrossRef]
- Blanco-Miguez, A.; Beghini, F.; Cumbo, F.; Mclver, L.J.; Thompson, K.N.; Zolfo, N.; Manghi, P.; Dubols, L.; Huang, K.D.; Thomas, A.M.; et al. Extending and improving metagenomics taxonomic profiling with uncharacterized species using MetaPhlAn4. Nat. Biotechnol. 2022, 41, 1633–1644. [Google Scholar] [CrossRef]
- Choi, H.S.; Kim, E.J.; Kang, J.E.; Yeo, S.H.; Jeong, S.T.; Kim, C.W. Effect of organic acids addition to fermentation on the brewing characteristics of Soju distilled from rice. Korean J. Food Sci. Technol. 2015, 47, 579–585. [Google Scholar] [CrossRef]
- Zeng, F.; Ou, J.; Huang, Y.; Li, Q.; Xu, Q.; Liu, Z.; Yang, S. Determination of 21 free amino acids in fruit juice by HPLS using a modification of the 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate (AQC) method. Food Anal. Methods 2015, 8, 428–437. [Google Scholar] [CrossRef]
- Kong, H.; Kim, S.H.; Jeong, W.S.; Kim, S.Y.; Yeo, S.H. Microbiome and volatile metabolic profile of acetic acid fermentation using multiple starters for traditional grain vinegar. Fermentation 2023, 9, 423. [Google Scholar] [CrossRef]
- Jeong, Y.J.; Seo, J.H.; Jung, S.H.; Shin, S.R.; Kim, K.S. The quality comparison of uncleaned rice vinegar by two stages fermentation with commercial uncleaned rice vinegar. Korean J. Postharvest Sci. Technol. 1998, 5, 374–379. [Google Scholar]
- Woo, S.M.; Jo, Y.J.; Lee, S.W.; Kwon, J.H.; Yeo, S.H.; Jeong, Y.J. Quality comparison of static-culture and commercial brown rice vinegars. Korean J. Food Preserv. 2012, 19, 301–307. [Google Scholar] [CrossRef]
- Lea, B.M. Effects of the different types of container on the characteristics of sugarcane vinegar. Int. J. Educ. Res. 2016, 4, 235–248. [Google Scholar]
- Lee, S.H.; Jung, J.Y.; Jeon, C.O. Microbial successions and metabolite changes during fermentation of salted shrimp (Saeu-jeot) with different salt concentrations. PLoS ONE 2014, 9, e90115. [Google Scholar] [CrossRef]
- Jung, J.Y.; Lee, S.H.; Lee, H.J.; Seo, H.Y.; Park, W.S.; Jeon, C.O. Effects of Leuconostoc mesenteroides starter cultures on microbial communities and metabolites during kimchi fermentation. Int. J. Food Microb. 2011, 153, 378–387. [Google Scholar] [CrossRef]
- Merlino, J.; Pillay, K.; Rizzo, S.; Baskar, S.R.; Seed, D.; Siarakas, S.; Hettiarachchi, R.; Mckew, G.; Gray, T. Bacterial skin infection caused by a plant pathogen Kosakonia cowanii: Identification with the MALDI Biotyper Sirius one and susceptibility testing. Access Microbiol. 2025, 7, 000923. [Google Scholar] [CrossRef]
- Entani, E.; Masai, H.; Suzuki, K.I. Lactobacillus acetotolerans, a new species from fermented vinegar broth. Int. J. Syst. Bacteriol. 1986, 36, 544–549. [Google Scholar] [CrossRef]
- Ana, A.B.; Carolina, D.; Graciela, L.G.; Analia, G.A. Health-promoting properties of Lacticaseibacillus paracasei: A focus on kefir isolates and exopolysaccharide-producing strains. Foods 2021, 10, 2239. [Google Scholar]
- Pascal, D.; Julien, T.; Erica, B.D.S.; Emmanuelle, A. Changes to the microbiome of alfalfa during the growing season and after ensiling with Lentilactobacillus buchneri and Lentilactobacillus gilardii inoculant. J. Appl. Microbiol. 2022, 133, 2331–2347. [Google Scholar]
- Wang, X.; Li, D.; Meng, Z.; Kim, K.; Oh, S. Latilactobacillus curvatus Latilactobacillus curvatus BYB3 isolated from kimchi alleviates dextran sulfate sodium (DSS)-induced colitis in mice by inhibiting IL-6 and TNF-R1 production. J. Bicrobiol. Biotechnol. 2022, 32, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Shadi, P.; Armin, T.; Rohit, T.; Manyu, W.; Viviana, c.; Alessio, G. Limosilatobacillus fermentum ING8, a potential multifunctional non-starter strain with relevant technological properties and antimicrobial activity. Foods 2022, 11, 703. [Google Scholar]
- Xia, T.; Zhang, B.; Duan, W.; Zhang, J.; Wang, M. Nutrients and bioactive components from vinegar: A fermented and functional food. J. Funct. Foods 2019, 64, 103681. [Google Scholar] [CrossRef]
- Aguiar, A.; Nascimento, R.A.D.A.; Ferretti, L.P.; Goncalves, A.R. Determination of organic acids and ethanol in commercial vinegars. Braz. J. Food Technol. 2005, 5, 51–56. [Google Scholar]
- Zotta, T.; Parente, E.; Ricciardi, A. Aerobic metabolism in the genus Lactobacillus: Impact on stress response and potential applications in the food industry. J. Appl. Microbiol. 2017, 122, 857–869. [Google Scholar] [CrossRef]
- Bartowsky, E.J.; Henschke, P.A. The ‘buttery’ attribute of wine-diacetyl-desirabillity, spoilage and beyond. Int. J. Food Microbiol. 2004, 96, 235–252. [Google Scholar] [CrossRef]
- Liu, S.; Lou, Y.; Li, Y.; Zhao, Y.; Laaksonen, O.; Li, P.; Zhang, J.; Battino, M.; Yang, B.; Gu, Q. Aroma characteristics of volatile compounds brought by variations in microbes in winemaking. Food Chem. 2023, 420, 136075. [Google Scholar] [CrossRef]
- Lee, H.J.; Ahn, H.J.; Kang, C.S.; Choi, J.C.; Choi, H.J.; Lee, K.G.; Kim, J.I.; Kim, H.Y. Naturally occurring propionic acid in foods marketed in South Korea. Food Control 2010, 21, 217–220. [Google Scholar] [CrossRef]
- Gao, Y.; Jo, Y.; Chung, N.; Gu, S.Y.; Jeong, Y.J.; Kwon, J.H. Physicochemical qualities and flavor patterns of traditional Chinese vinegars manufactured by different fermentation methods and aging periods. Prev. Nutr. Food Sci. 2017, 22, 30–36. [Google Scholar] [CrossRef]
- Kong, Y.; Zhang, L.L.; Sun, Y.; Zhang, Y.Y.; Sun, B.G.; Chen, H.T. Determination of the free amino acid, organic acid, and nucleotide in commercial vinegars. J. Food Sci. 2017, 82, 1116–1123. [Google Scholar] [CrossRef]
- Lu, Q.; Shan, X.; Zeng, W.; Zhou, J. Production of pyruvic acid with Candida glabrata using self-fermenting spent yeast cell dry powder as a seed nitrogen source. Bioresour. Bioprocess. 2022, 9, 109. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kim, H.S. Rice-based gluten-free foods and technologies. Foods 2023, 12, 4110. [Google Scholar] [CrossRef] [PubMed]
- Hegde, S.R.; Thangalakshmi, S.; Singh, R. A review of gluten and sorghum as a gluten free substitute. Trends Hortic. 2023, 6, 2840. [Google Scholar] [CrossRef]
- Wu, G. Dietary protein intake and human health. Food Funct. 2016, 7, 1251–1265. [Google Scholar] [CrossRef]
- Zhao, G.; Kuang, G.; Li, J.; Hadiatullah, H.; Chen, Z.; Wang, X.; Yao, Y.; Pan, Z.H.; Wang, Y. Characterization of aldegydes and hydroxyl acids as the main contribution to the traditional Chinese rose vinegar by flavor and taste analyses. Food Res. Int. 2020, 129, 108879. [Google Scholar] [CrossRef]
- Chung, W.C.; Beak, H.H.; Shin, D.H. Changes in volatile compounds of persimmon vinegar during aging. Korean J. Food Sci. Technol. 2019, 51, 438–446. [Google Scholar]
- Wagner, M.; Heredia, J.Z.; Segura-Borrego, M.P.; Morales, M.L.; Camina, J.M.; Azcarate, S.M.; Callejon, R.M.; Rios-Reina, R. Identification of potential volatile markers for characterizing argentine wine vinegars based on their production process. Talata Open 2024, 10, 100370. [Google Scholar] [CrossRef]
- Liang, J.; Xie, J.; Hou, L.; Zhao, M.; Zhao, J.; Cheng, J.; Wang, S.; Sun, B.G. Aroma constituents in Shanxi aged vinegar before and after aging. J. Agric. Food Chem. 2016, 64, 7597–7605. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, A.; Zhou, Y.; Zhang, W.; Liang, K.; Roman-Camacho, J.J.; Zhou, J.; Song, J.; Zheng, Y.; Wang, M. Identification of aroma active compounds in Shanxi aged vinegar and tracing the source in the entire production process. Food Chem. X 2024, 24, 101918. [Google Scholar] [CrossRef]
- Xie, Z.; Koysomboon, C.; Zhang, H.; Lu, Z.; Zhang, X.; Chen, F. Vinegar volatile organic compounds: Analytical methods, constituents, and formation processes. Front. Microbiol. 2022, 13, 907883. [Google Scholar] [CrossRef]
- Huang, T.; Lu, Z.M.; Peng, M.Y.; Liu, Z.F.; Chai, L.J.; Zhang, X.J.; Shi, J.S.; Xu, Z.H. Combined effects of fermentation starters and environmental factors on the microbial community assembly and flavor formation of Zhenjiang aromatic vinegar. Food Res. Int. 2022, 152, 110900. [Google Scholar] [CrossRef]
| Type of Sample | Region | Raw Material | Fermenter | Temperature | Period (Days) | Sample Code | Acidity (%) |
|---|---|---|---|---|---|---|---|
| HB | Gangwon state | Brown rice (100%) | Earthenware Jar | 28–30 °C | 0 | HB1 | 1.60 ± 0.03 c 1 |
| 10 | HB2 | 3.16 ± 0.01 b | |||||
| 20 | HB3 | 4.87 ± 0.02 a | |||||
| SB | Gyeonggi-do | Barley (100%) | Glass | 30 °C | 0 | SB1 | 1.41 ± 0.02 b |
| 10 | SB2 | 2.51 ± 0.02 a | |||||
| 20 | SB3 | 6.83 ± 0.02 c | |||||
| JR | Jeonbuk state | Rice (100%) | Earthenware Jar | 30 °C | 0 | JR1 | 1.22 ± 0.02 a |
| 10 | JR2 | 2.52 ± 0.01 a | |||||
| 20 | JR3 | 5.43 ± 0.02 b | |||||
| YO | Gyeongsangbuk-do | Five-grain (brown rice (65%), barley (20%), sorghum (5%), millet (5%), and foxtail millet (5%)) | Stainless Steel | 30 °C | 0 | YO1 | 8.54 ± 0.02 d |
| 10 | YO2 | 9.53 ± 0.01 c | |||||
| 20 | YO3 | 14.67 ± 0.01 d |
| Sample | Organic Acid (mg/mL) | Total | |||||
|---|---|---|---|---|---|---|---|
| Citric Acid | Malic Acid | Succinic Acid | Lactic Acid | Acetic Acid | Propionic Acid | ||
| HB1 | N.D. 1 | N.D. | N.D. | 9.36 ± 0.80 abc | 1.66 ± 0.16 a | N.D. | 11.02 ± 0.96 |
| HB2 | N.D. | N.D. | N.D. | 3.53 ± 0.33 ab | 6.04 ± 0.56 ab | N.D. | 9.56 ± 0.89 |
| HB3 | N.D. | N.D. | N.D. | 0.15 ± 0.02 a | 13.59 ± 1.18 b | N.D. | 13.75 ± 1.20 |
| SB1 | 0.63 ± 0.02 c 2 | 0.15 ± 0.26 ab | 0.51 ± 0.00 ab | 16.5 ± 9.48 bc | 4.37 ± 3.35 a | 0.30 ± 0.22 a | 22.00 ± 13.33 |
| SB2 | 0.27 ± 0.31 ab | N.D. | 1.08 ± 0.54 bcd | 20.00 ± 12.64 c | 9.33 ± 1.19 ab | 3.55 ± 1.69 bc | 34.23 ± 16.37 |
| SB3 | 0.25 ± 0.30 ab | N.D. | 0.76 ± 0.41 bc | 10.23 ± 4.32 abc | 36.99 ± 11.83 d | 2.07 ± 3.58 abc | 50.30 ± 20.43 |
| JR1 | 0.64 ± 0.04 c | 0.32 ± 0.28 c | 1.35 ± 0.73 cd | 10.59 ± 9.41 abc | 2.53 ± 3.51 a | 0.18 ± 0.24 a | 15.61 ± 14.22 |
| JR2 | 0.44 ± 0.32 bc | N.D. | 1.48 ± 0.37 d | 12.75 ± 12.58 abc | 9.85 ± 0.87 ab | 2.57 ± 1.78 abc | 27.10 ± 15.91 |
| JR3 | 0.44 ± 0.32 bc | N.D. | 1.04 ± 0.45 bcd | 7.88 ± 4.18 abc | 42.99 ± 10.55 d | 4.38 ± 3.79 c | 56.72 ± 19.29 |
| YO1 | 1.48 ± 0.01 d | N.D. | 0.87 ± 0.01 bcd | 5.93 ± 0.09 ab | 25.88 ± 0.70 c | 0.95 ± 0.12 ab | 35.11 ± 0.92 |
| YO2 | 1.39 ± 0.00 d | N.D. | 0.86 ± 0.00 bcd | 5.96 ± 0.09 ab | 40.04 ± 0.34 d | N.D. | 48.25 ± 0.44 |
| YO3 | 1.45 ± 0.01 d | N.D. | 0.83 ± 0.00 bc | 4.48 ± 2.43 ab | 58.07 ± 0.31 e | N.D. | 64.84 ± 2.75 |
| Compound | Order Description | Type of Sample | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HB | SB | JR | YO | ||||||
| p-Value 1 | VIP 2 | p-Value | VIP | p-Value | VIP | p-Value | VIP | ||
| Alcohols | |||||||||
| Ethanol | Alcohol | 2.85 × 10−3 | 1.02 | 2.78 × 10−6 | 0.96 | 3.66 × 10−6 | 0.95 | 1.33 × 10−7 | 1.01 |
| 2-Methyl-1-propanol | Nail-polish-like, sweet | 2.01 × 10−2 | 0.89 | 3.25 × 10−6 | 0.97 | 3.09 × 10−7 | 1.01 | 2.06 × 10−7 | 1.02 |
| 2-Methyl-1-butanol | Sweet, fruity | 3.92 × 10−1 | 0.99 | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| 3-Methyl-1-butanol | Rancid, banana-like | 2.60 × 10−2 | 0.90 | 1.11 × 10−5 | 0.95 | 7.00 × 10−9 | 1.03 | 4.55 × 10−9 | 1.02 |
| Alehydes | |||||||||
| Nonanal | Sweet orange, rose | 5.83 × 10−2 | 1.02 | 2.59 × 10−1 | 1.05 | 1.21 × 10−2 | 0.68 | 2.36 × 10−1 | 0.73 |
| Decanal | Grass-like | 6.53 × 10−2 | 0.77 | 1.00 | 0.04 | 2.21 × 10−1 | 0.96 | 1.99 × 10−1 | 0.43 |
| Esters | |||||||||
| Acetic acid, ethyl ester | Sweet, soapy, grass-like | 7.39 × 10−6 | 1.16 | 4.53 × 10−6 | 1.08 | 4.36 × 10−9 | 1.01 | 2.05 × 10−8 | 1.30 |
| Acetic acid, butyl ester | Banana-like, appley, sweet | N.D. | N.D. | 5.91 × 10−5 | 1.01 | 8.94 × 10−4 | 0.89 | 1.52 × 10−3 | 1.21 |
| Isobutyl acetate | Pear, apple | 5.76 × 10−5 | 0.99 | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| Butanoic acid, ethyl ester | Fruity, pineapple-like | N.D. | N.D. | 2.50 × 10−7 | 0.91 | 6.79 × 10−7 | 1.56 | N.D. | N.D. |
| 3-Methylbutyl acetate | Banana-like | 6.04 × 10−5 | 0.99 | 8.61 × 10−7 | 1.04 | 4.81 × 10−8 | 0.89 | 2.59 × 10−5 | 1.24 |
| Methyl salicylate | Sweet, fruity | 7.38 × 10−2 | 1.33 | 7.41 × 10−2 | 0.72 | 2.92 × 10−3 | 1.20 | 8.17 × 10−4 | 1.12 |
| Acids | |||||||||
| Ethyl 2-hydroxypropanoate | Sweet, fruity | N.D. | N.D. | 5.29 × 10−5 | 1.16 | 3.34 × 10−7 | 0.65 | 1.55 × 10−2 | 0.84 |
| Acetic acid | Pungent | 6.34 × 10−3 | 0.98 | 1.55 × 10−7 | 1.14 | 7.20 × 10−8 | 0.95 | 2.24 × 10−7 | 1.02 |
| Ketones | |||||||||
| 3-Hydroxy-2-butanone | Buttery, creamy | 8.08 × 10−4 | 1.18 | 7.68 × 10−8 | 1.21 | 1.76 × 10−10 | 1.01 | 6.04 × 10−9 | 1.02 |
| 6-Methyl-5-hepten-2-one | Herby, grass-like | 5.02 × 10−1 | 0.54 | 3.10 × 10−1 | 1.18 | 2.39 × 10−1 | 0.89 | 2.01 × 10−1 | 0.68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.J.; Kim, S.H.; Gwon, H.-M.; Park, J. Microbial Community and Functional Analysis of Regionally Produced Traditional Korean Grain Vinegar. Microorganisms 2025, 13, 1308. https://doi.org/10.3390/microorganisms13061308
Lee SJ, Kim SH, Gwon H-M, Park J. Microbial Community and Functional Analysis of Regionally Produced Traditional Korean Grain Vinegar. Microorganisms. 2025; 13(6):1308. https://doi.org/10.3390/microorganisms13061308
Chicago/Turabian StyleLee, Su Jeong, Sun Hee Kim, Hee-Min Gwon, and Jinju Park. 2025. "Microbial Community and Functional Analysis of Regionally Produced Traditional Korean Grain Vinegar" Microorganisms 13, no. 6: 1308. https://doi.org/10.3390/microorganisms13061308
APA StyleLee, S. J., Kim, S. H., Gwon, H.-M., & Park, J. (2025). Microbial Community and Functional Analysis of Regionally Produced Traditional Korean Grain Vinegar. Microorganisms, 13(6), 1308. https://doi.org/10.3390/microorganisms13061308






