Host Serum Amyloid A1 Facilitates Streptococcus pneumoniae Adaptation to Acidic Stress Induced by Pneumococcal Anaerobic Metabolism
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Culture
2.2. Animal Infection Experiments
2.3. Quantification of SAA1 and CRP Using ELISA
2.4. Incubation of S. pneumoniae with THY Broth Containing 20% Serum In Vitro
2.5. AF488-Recombinant SAA1 Intake by S. pneumoniae Using Fluorescence Microscope
2.6. pH Measurement of Bacterial Culture Under Aerobic and Anaerobic Environments
2.7. Bacterial Growth Curve in Lethal Formic, Lactic and Acetic Acid Concentration
2.8. Statistical Analysis
2.9. Data Visualization
3. Results
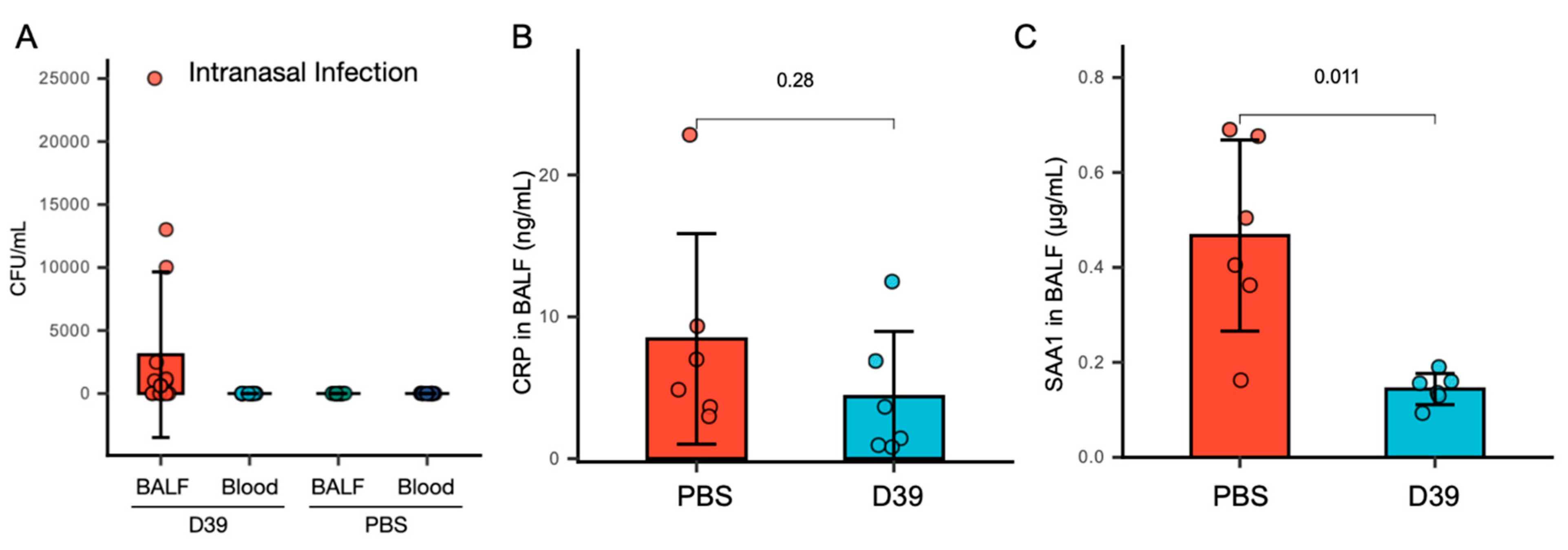
3.1. SAA1 in BALF Significantly Decreases, While CRP Levels Remain Unchanged Following Nasal Infection with S. pneumoniae
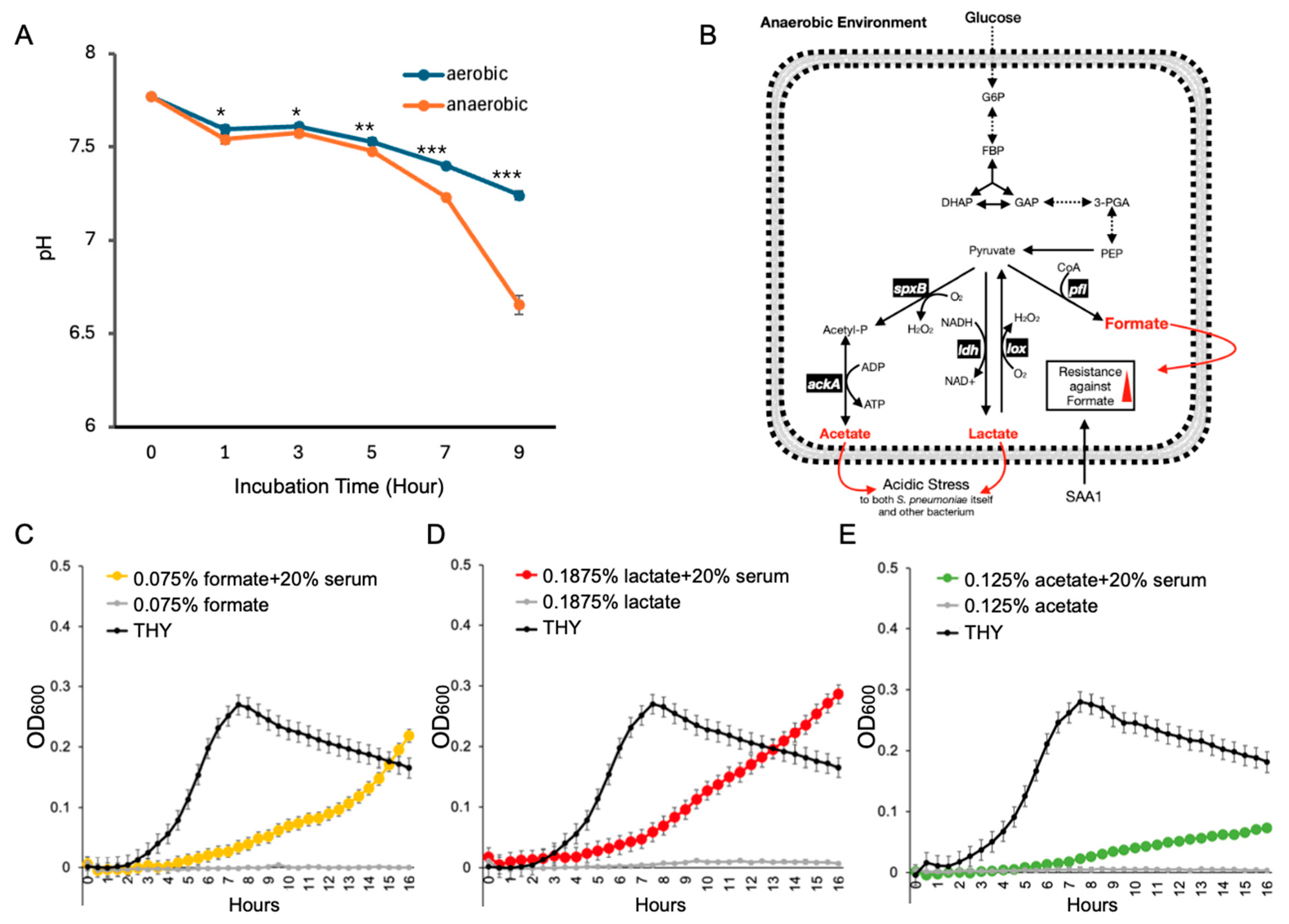
3.2. Serum Components Enhance S. pneumoniae Survival Under Acidic Stress Caused by Anaerobic Metabolism
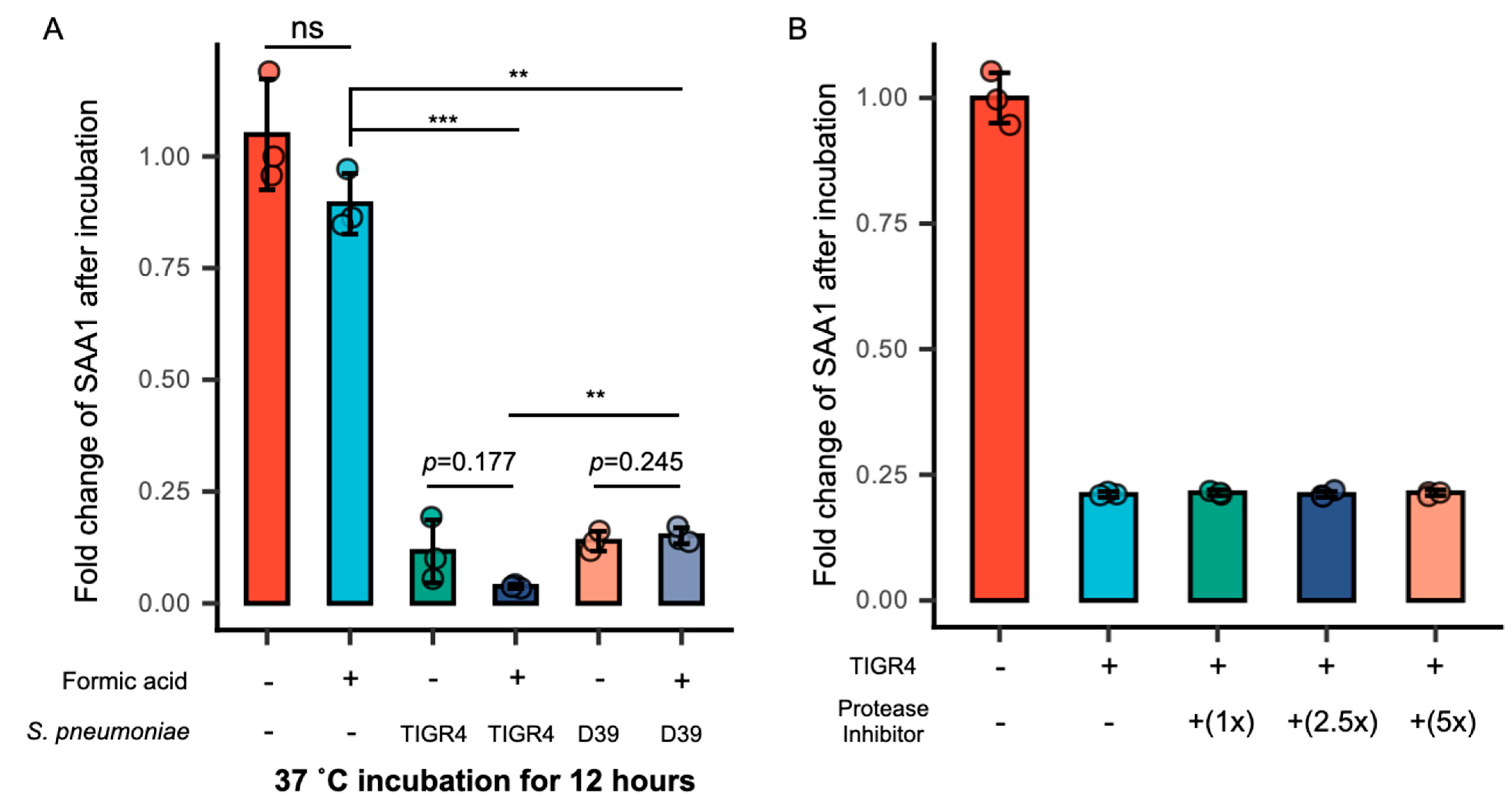
3.3. Formic Acid Promotes the Intake of SAA1 by S. pneumoniae
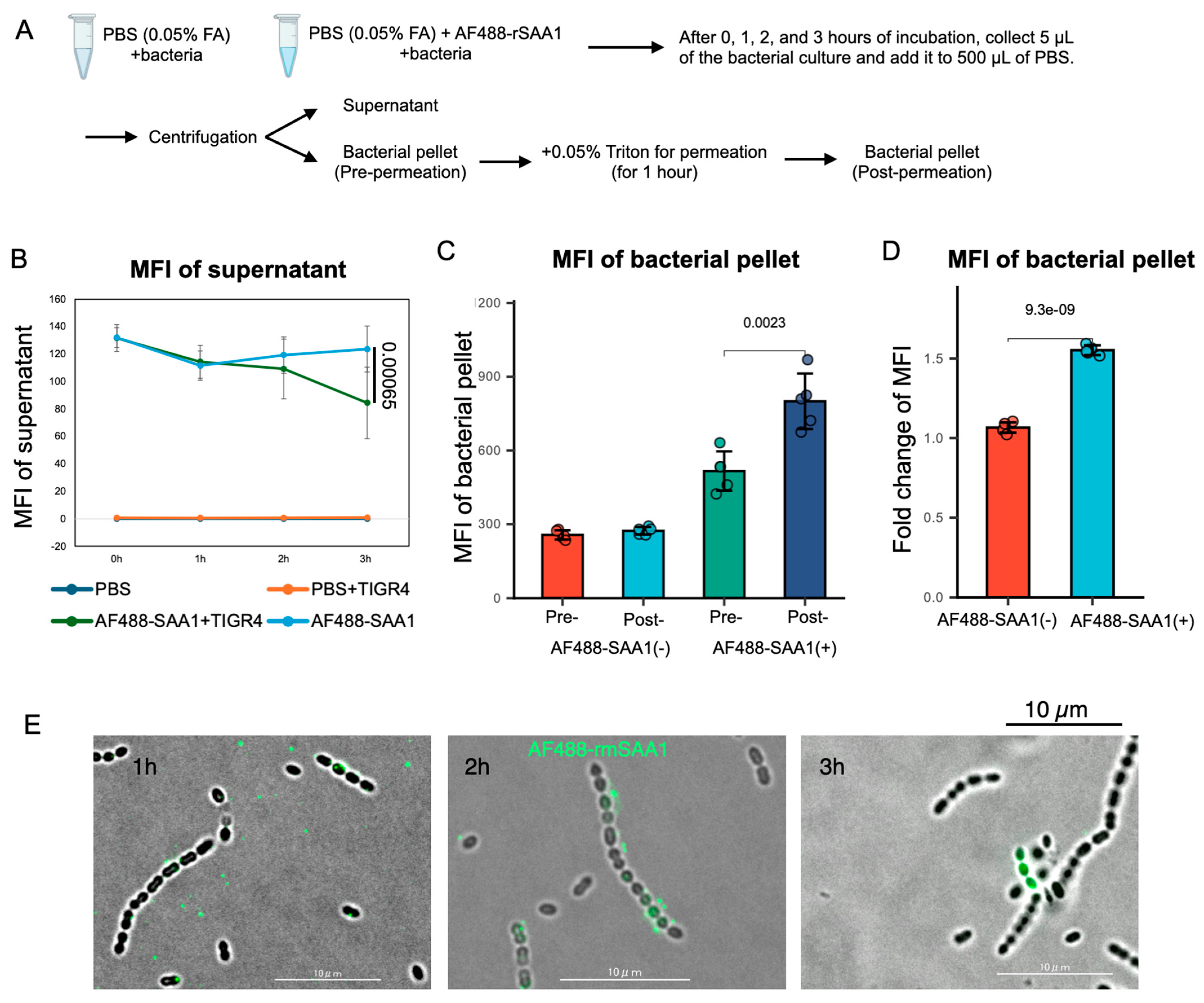
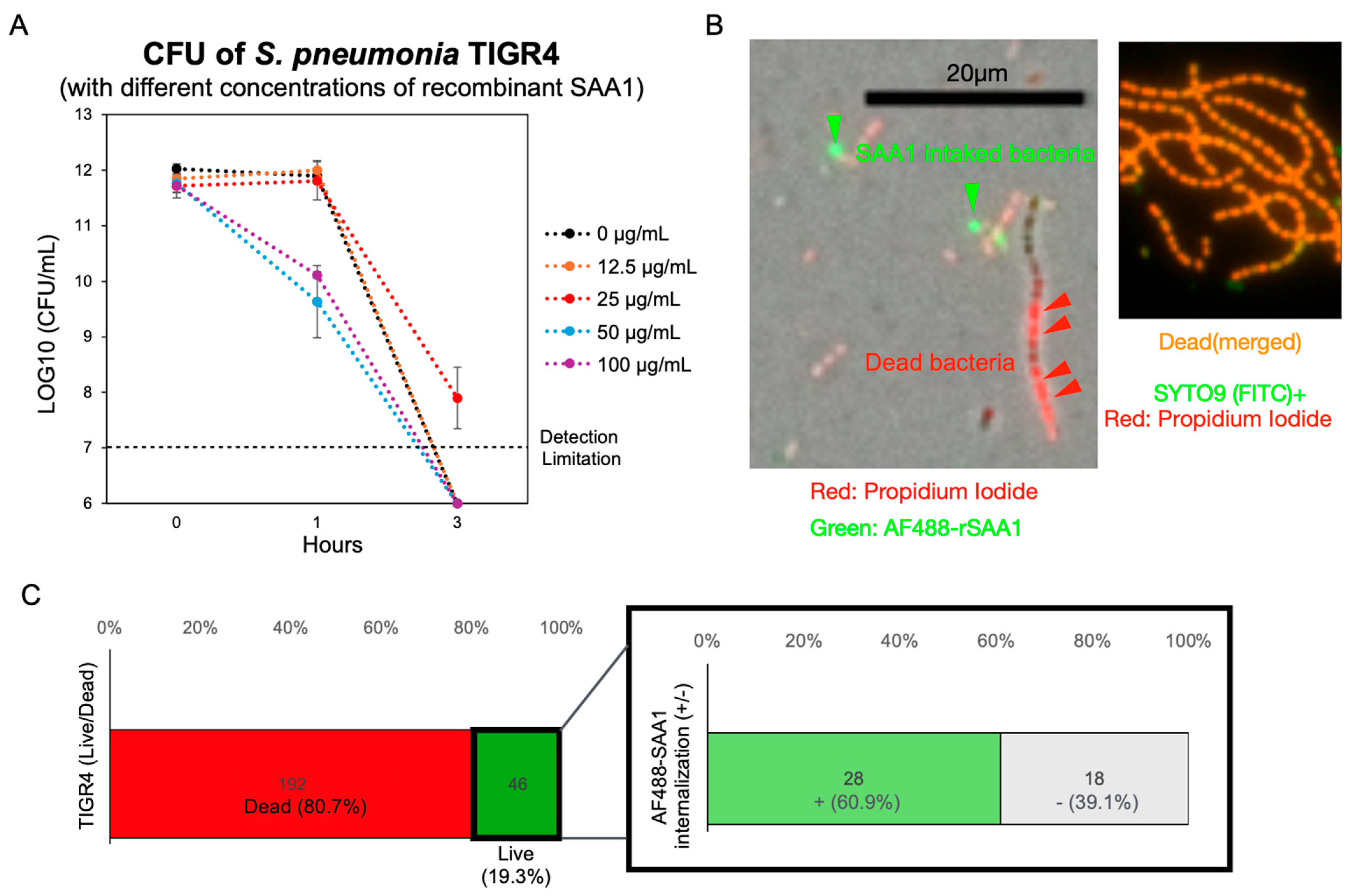
3.4. Recombinant SAA1 Enhances S. pneumoniae Tolerance to Formic Acid
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Austrian, R. Some aspects of the pneumococcal carrier state. J. Antimicrob. Chemother. 1986, 18 (Suppl. A), 35–45. [Google Scholar] [CrossRef]
- Musher, D.M. How contagious are common respiratory tract infections? N. Engl. J. Med. 2003, 348, 1256–1266. [Google Scholar] [CrossRef] [PubMed]
- Garvy, B.A.; Harmsen, A.G. The importance of neutrophils in resistance to pneumococcal pneumonia in adult and neonatal mice. Inflammation 1996, 20, 499–512. [Google Scholar] [CrossRef]
- Koppe, U.; Suttorp, N.; Opitz, B. Recognition of Streptococcus pneumoniae by the innate immune system. Cell. Microbiol. 2012, 14, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Craig, A.; Mai, J.; Cai, S.; Jeyaseelan, S. Neutrophil recruitment to the lungs during bacterial pneumonia. Infect. Immun. 2009, 77, 568–575. [Google Scholar] [CrossRef] [PubMed]
- McNamee, L.A.; Harmsen, A.G. Both influenza-induced neutrophil dysfunction and neutrophil-independent mechanisms contribute to increased susceptibility to a secondary Streptococcus pneumoniae infection. Infect. Immun. 2006, 74, 6707–6721. [Google Scholar] [CrossRef]
- Oyinloye, B.E.; Adenowo, A.F.; Kappo, A.P. Reactive oxygen species, apoptosis, antimicrobial peptides and human inflammatory diseases. Pharmaceuticals 2015, 8, 151–175. [Google Scholar] [CrossRef]
- Narasaraju, T.; Yang, E.; Samy, R.P.; Ng, H.H.; Poh, W.P.; Liew, A.-A.; Phoon, M.C.; van Rooijen, N.; Chow, V.T. Excessive neutrophils and neutrophil extracellular traps contribute to acute lung injury of influenza pneumonitis. Am. J. Pathol. 2011, 179, 199–210. [Google Scholar] [CrossRef]
- Ware, L.B.; Matthay, M.A. Acute pulmonary edema. N. Engl. J. Med. 2005, 353, 2788–2796. [Google Scholar] [CrossRef]
- Chapuy-Regaud, S.; Duthoit, F.; Malfroy-Mastrorillo, L.; Gourdon, P.; Lindley, N.D.; Trombe, M.-C. Competence regulation by oxygen availability and by Nox is not related to specific adjustment of central metabolism in Streptococcus pneumoniae. J. Bacteriol. 2001, 183, 2957–2962. [Google Scholar] [CrossRef]
- Yesilkaya, H.; Kadioglu, A.; Gingles, N.; Alexander, J.E.; Mitchell, T.J.; Andrew, P.W. Role of manganese-containing superoxide dismutase in oxidative stress and virulence of Streptococcus pneumoniae. Infect. Immun. 2000, 68, 2819–2826. [Google Scholar] [CrossRef]
- Hajaj, B.; Yesilkaya, H.; Benisty, R.; David, M.; Andrew, P.W.; Porat, N. Thiol peroxidase is an important component of Streptococcus pneumoniae in oxygenated environments. Infect Immun. 2012, 80, 4333–4343. [Google Scholar] [CrossRef]
- Pericone, C.D.; Park, S.; Imlay, J.A.; Weiser, J.N. Factors contributing to hydrogen peroxide resistance in Streptococcus pneumoniae include pyruvate oxidase (SpxB) and avoidance of the Fenton reaction. J. Bacteriol. 2003, 185, 681–690. [Google Scholar] [CrossRef]
- Pesakhov, S.; Benisty, R.; Sikron, N.; Cohen, Z.; Gomelsky, P.; Khozin-Goldberg, I.; Dagan, R.; Porat, N. Effect of hydrogen peroxide production and the Fenton reaction on membrane composition of Streptococcus pneumoniae. Biochim. Biophys. Acta. 2007, 1768, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Neijssel, O.M.; Snoep, J.L.; De Mattos, M.J.T. Regulation of energy source metabolism in streptococci. Soc. Appl. Bacteriol. Symp. Ser. 1997, 26, 12S–19S. [Google Scholar] [CrossRef] [PubMed]
- Yesilkaya, H.; Spissu, F.; Carvalho, S.M.; Terra, V.S.; Homer, K.A.; Benisty, R.; Porat, N.; Neves, A.R.; Andrew, P.W. Pyruvate formate lyase is required for pneumococcal fermentative metabolism and virulence. Infect. Immun. 2009, 77, 5418–5427. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Martín-Galiano, A.J.; de la Campa, A.G. High-efficiency genetic transformation of Streptococcus pneumoniae: Properties of the endA1 mutant. J. Bacteriol. 2005, 187, 7601–7610. [Google Scholar]
- Álvarez-Ortega, C.; de la Campa, A.G. RNA polymerase mutation in Streptococcus pneumoniae that causes overexpression of the F0F1 ATPase operon increases acid tolerance. J. Bacteriol. 2007, 189, 8564–8567. [Google Scholar]
- Giammarinaro, P.; Paton, J.C. Role of the response regulator CiaR of Streptococcus pneumoniae in acid tolerance response. J. Bacteriol. 2002, 184, 2823–2829. [Google Scholar]
- Cortes, P.R.; Piñas, G.E.; Cian, M.B.; Yandar, N.; Echenique, J. Stress-triggered signaling affecting survival or suicide of Streptococcus pneumoniae. Int. J. Med. Microbiol. 2015, 305, 157–169. [Google Scholar] [CrossRef]
- Martner, A.; Skovbjerg, S.; Paton, J.C.; Wold, A.E. Streptococcus pneumoniae autolysis prevents phagocytosis and production of phagocyte-activating cytokines. Infect. Immun. 2009, 77, 3826–3837. [Google Scholar] [CrossRef] [PubMed]
- Sung, C.K.; Li, H.; Claverys, J.P.; Morrison, D.A. An rpsL cassette, janus, for gene replacement through negative selection in Streptococcus pneumoniae. Appl. Environ. Microbiol. 2001, 67, 5190–5196. [Google Scholar] [CrossRef] [PubMed]
- Moscoso, M.; García, E.; López, R. Biofilm formation by Streptococcus pneumoniae: Role of extracellular DNA and proteins. J. Bacteriol. 2006, 188, 7785–7795. [Google Scholar] [CrossRef]
- Moshage, H. Cytokines and the hepatic acute phase response. J. Pathol. 1997, 181, 257–266. [Google Scholar] [CrossRef]
- Uhlar, C.M.; Whitehead, A.S. Serum amyloid A, the major vertebrate acute-phase reactant. Eur. J. Biochem. 1999, 265, 501–523. [Google Scholar] [CrossRef]
- Arnon, S.; Litmanovitz, I.; Regev, R.H.; Bauer, S.; Shainkin-Kestenbaum, R.; Dolfin, T. Serum amyloid A: An early and accurate marker of neonatal early-onset sepsis. J. Perinatol. 2007, 27, 297–302. [Google Scholar] [CrossRef]
- De Buck, M.; Gouwy, M.; Wang, J.M.; Van Snick, J.; Proost, P.; Struyf, S.; Van Damme, J. The cytokine-serum amyloid A-chemokine network. Cytokine Growth Factor Rev. 2016, 30, 55–69. [Google Scholar] [CrossRef]
- Abouelasrar Salama, S.; Gouwy, M.; Van Damme, J.; Struyf, S. The turning away of serum amyloid A biological activities and receptor usage. Immunology 2021, 163, 115–127. [Google Scholar] [CrossRef]
- Sproston, N.R.; Ashworth, J. Role of C-reactive protein at sites of inflammation and infection. Front. Immunol. 2018, 9, 754. [Google Scholar] [CrossRef]
- Holzer, T.J.; Edwards, K.M.; Gewurz, H.; Mold, C. Binding of C-reactive protein to the pneumococcal capsule or cell wall results in differential localization of C3 and stimulation of phagocytosis. J. Immunol. 1984, 133, 1424–1430. [Google Scholar] [CrossRef]
- Hari-Dass, R.; Shah, C.; Meyer, D.J.; Raynes, J.G. Serum amyloid A protein binds to outer membrane protein A of gram-negative bacteria. J. Biol. Chem. 2005, 280, 18562–18567. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Li, H.; Zhang, J.; Fan, H.; Jia, L.; Ma, W.; Ma, S.; Wang, S.; You, H.; Yin, Z.; et al. Serum amyloid A exhibits pH dependent antibacterial action and contributes to host defense against Staphylococcus aureus cutaneous infection. J. Biol. Chem. 2020, 295, 2570–2581. [Google Scholar] [CrossRef] [PubMed]
- Suresh, M.V.; Singh, S.K.; Ferguson, D.A.; Agrawal, A. Human C-reactive protein protects mice from Streptococcus pneumoniae infection without binding to pneumococcal C-polysaccharide. J. Immunol. 2007, 178, 1158–1163. [Google Scholar] [CrossRef]
- Varney, N.R.; Buckley, P. Neuroanatomy, parasympathetic nervous system. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023; Available online: https://www.ncbi.nlm.nih.gov/books/NBK539907/ (accessed on 31 October 2022).
- Wang, Z.; Rahkola, J.; Redzic, J.S.; Chi, Y.-C.; Tran, N.; Holyoak, T.; Zheng, H.; Janoff, E.; Eisenmesser, E. Mechanism and inhibition of Streptococcus pneumoniae IgA1 protease. Nat. Commun. 2020, 11, 6063. [Google Scholar] [CrossRef] [PubMed]
- Van Damme, J.; Struyf, S.; Proost, P.; Opdenakker, G.; Gouwy, M. Functional Interactions Between Recombinant Serum Amyloid A1 (SAA1) and Chemokines in Leukocyte Recruitment. Int. J. Mol. Sci. 2025, 26, 2258. [Google Scholar] [CrossRef]
- He, R.; Shepard, L.W.; Chen, J.; Pan, Z.K.; Ye, R.D. Serum amyloid A is an endogenous ligand that differentially induces IL-12 and IL-23. J. Immunol. 2006, 177, 4072–4079. [Google Scholar] [CrossRef]
- Ather, J.L.; Ckless, K.; Martin, R.; Foley, K.L.; Suratt, B.T.; E Boyson, J.; A Fitzgerald, K.; A Flavell, R.; Eisenbarth, S.C.; E Poynter, M. Serum amyloid A activates the NLRP3 inflammasome and promotes Th17 allergic asthma in mice. J. Clin. Investig. 2011, 121, 4424–4441. [Google Scholar] [CrossRef]
- Niemi, K.; Teirilä, L.; Lappalainen, J.; Rajamäki, K.; Baumann, M.H.; Öörni, K.; Wolff, H.; Kovanen, P.T.; Matikainen, S.; Eklund, K.K. Serum amyloid A activates the NLRP3 inflammasome via P2X7 receptor and a cathepsin B-sensitive pathway. J. Immunol. 2011, 186, 6119–6128. [Google Scholar] [CrossRef]
- Cheng, N.; He, R.; Tian, J.; Ye, P.P.; Ye, R.D. Cutting edge: TLR2 is a functional receptor for acute-phase serum amyloid A. J. Immunol. 2008, 181, 22–26. [Google Scholar] [CrossRef]
- Hatanaka, E.; Dermargos, A.; Armelin, H.A.; Curi, R.; Campa, A. Serum amyloid A induces NADPH-oxidase-dependent reactive oxygen species in neutrophils via a β2 integrin receptor-mediated mechanism. Biochem. Biophys. Res. Commun. 2008, 372, 339–344. [Google Scholar]
- Furlaneto, C.J.; Campa, A. A novel function of serum amyloid A: A potent stimulus for the release of TNF-α, IL-1β and IL-8 by human blood neutrophils. Biochem. Biophys. Res. Commun. 2000, 268, 405–408. [Google Scholar] [CrossRef] [PubMed]
- Baranova, I.N.; Vishnyakova, T.G.; Bocharov, A.V.; Kurlander, R.; Chen, Z.; Kimelman, M.L.; Remaley, A.T.; Csako, G.; Thomas, F.; Eggerman, T.L.; et al. Serum amyloid A binding to CLA-1/SR-BI: A new high-affinity interaction of an acute-phase protein with lipoprotein receptors. J. Biol. Chem. 2005, 280, 8031–8040. [Google Scholar] [CrossRef] [PubMed]
- Furlaneto-Maia, L.; Campa, A. Serum amyloid A induces degranulation of neutrophils and increases reactive oxygen species production. Mediators Inflamm. 2000, 9, 255–260. [Google Scholar]
- Badolato, R.; Wang, J.M.; Murphy, W.J.; Lloyd, A.R.; Michiel, D.F.; Bausserman, L.L.; Kelvin, D.J.; Oppenheim, J.J. Serum amyloid A is a chemoattractant: Induction of migration, adhesion and tissue infiltration of monocytes and polymorphonuclear leukocytes. J. Exp. Med. 1994, 180, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Bozinovski, S.; Hutchinson, A.; Thompson, M.; MacGregor, L.; Black, J.; Giannakis, E.; Karlsson, A.S.; Silvestrini, R.; Smallwood, D.; Vlahos, R.; et al. Serum amyloid A is a biomarker of acute exacerbations of chronic obstructive pulmonary disease. Front. Immunol. 2017, 8, 1001. [Google Scholar] [CrossRef]
- Alcorlo, M.; Abdullah, M.R.; Steil, L.; Sotomayor, F.; Oro, L.L.-D.; de Castro, S.; Velázquez, S.; Kohler, T.P.; Jiménez, E.; Medina, A.; et al. Molecular and structural basis of oligopeptide recognition by the Ami transporter system in pneumococci. PLoS Pathog. 2024, 20, e1011883. [Google Scholar] [CrossRef]
- Kuwahara, H.; Miyamoto, Y.; Akaike, T.; Kubota, T.; Sawa, T.; Okamoto, S.; Maeda, H. Helicobacter pylori urease suppresses bactericidal activity of peroxynitrite via carbon dioxide production. Infect. Immun. 2000, 68, 4378–4383. [Google Scholar] [CrossRef]
- Krishnamurthy, H.K.; Pereira, M.; Bosco, J.; George, J.; Jayaraman, V.; Krishna, K.; Wang, T.; Bei, K.; Rajasekaran, J.J. Gut commensals and their metabolites in health and disease. Front. Microbiol. 2023, 14, 1244293. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, W.; Ono, M.; Sumitomo, T.; Kobayashi, M.; Hirose, Y.; Kawabata, S. Host Serum Amyloid A1 Facilitates Streptococcus pneumoniae Adaptation to Acidic Stress Induced by Pneumococcal Anaerobic Metabolism. Microorganisms 2025, 13, 1309. https://doi.org/10.3390/microorganisms13061309
Gong W, Ono M, Sumitomo T, Kobayashi M, Hirose Y, Kawabata S. Host Serum Amyloid A1 Facilitates Streptococcus pneumoniae Adaptation to Acidic Stress Induced by Pneumococcal Anaerobic Metabolism. Microorganisms. 2025; 13(6):1309. https://doi.org/10.3390/microorganisms13061309
Chicago/Turabian StyleGong, Weichen, Masayuki Ono, Tomoko Sumitomo, Momoko Kobayashi, Yujiro Hirose, and Shigetada Kawabata. 2025. "Host Serum Amyloid A1 Facilitates Streptococcus pneumoniae Adaptation to Acidic Stress Induced by Pneumococcal Anaerobic Metabolism" Microorganisms 13, no. 6: 1309. https://doi.org/10.3390/microorganisms13061309
APA StyleGong, W., Ono, M., Sumitomo, T., Kobayashi, M., Hirose, Y., & Kawabata, S. (2025). Host Serum Amyloid A1 Facilitates Streptococcus pneumoniae Adaptation to Acidic Stress Induced by Pneumococcal Anaerobic Metabolism. Microorganisms, 13(6), 1309. https://doi.org/10.3390/microorganisms13061309







