Bacillus tropicus YJ33 and Medicago sativa L. Synergistically Enhance Soil Aggregate Stability in Saline–Alkali Environments
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Collection of Plant and Soil Samples
2.3. Soil Aggregate Fractionation
2.4. Determination of Relevant Indexes of Plant and Soil Samples
2.5. Determination of Soil Enzyme Activity
2.6. Statistical Analysis
3. Results
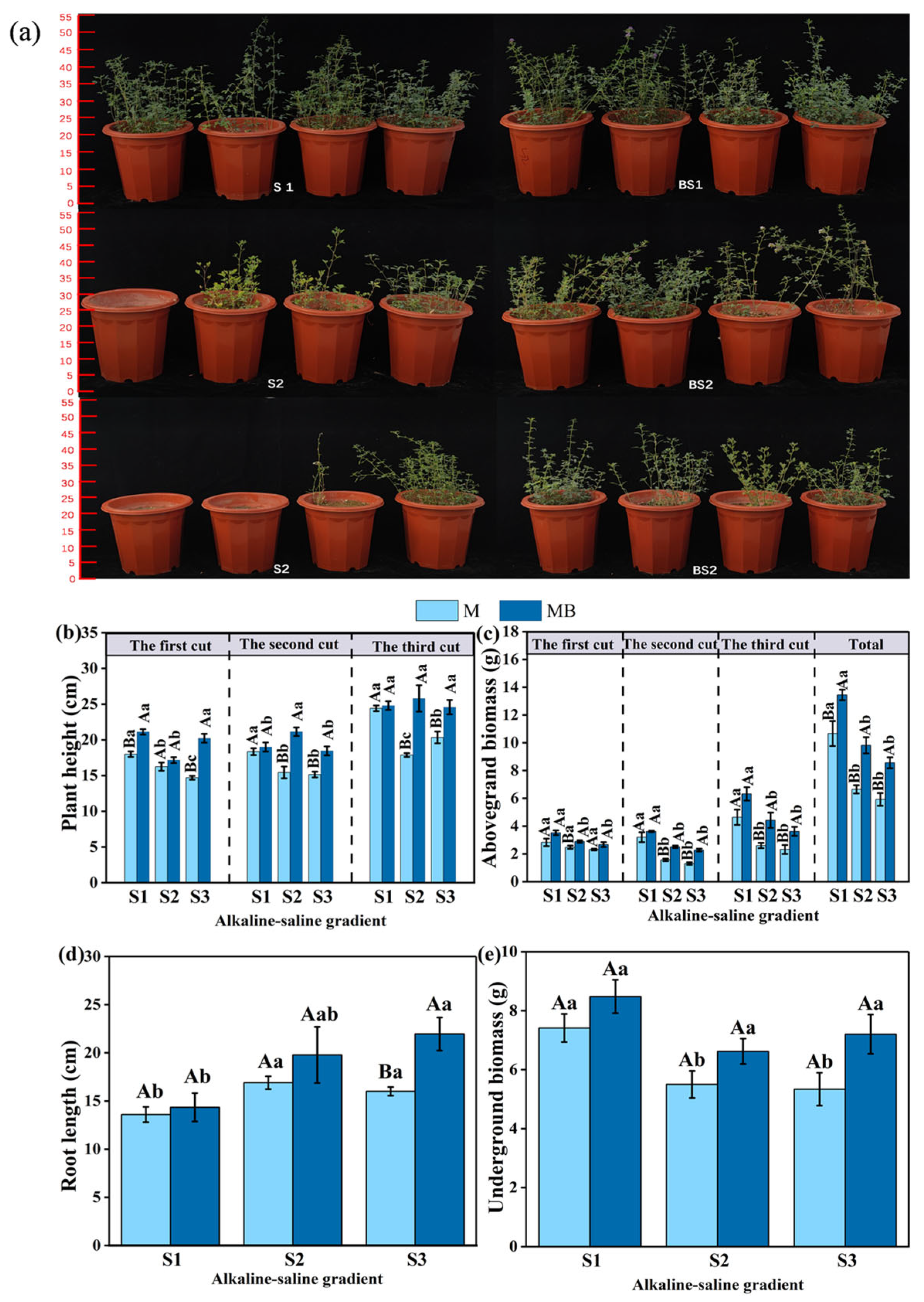
3.1. The Growth-Promoting Effect of B. tropicus YJ33 on Alfalfa Under Different Saline–Alkaline Gradients
3.2. Effects of B. tropicus YJ33 on the Nutritional Quality of Alfalfa Under Different Saline–Alkaline Gradients
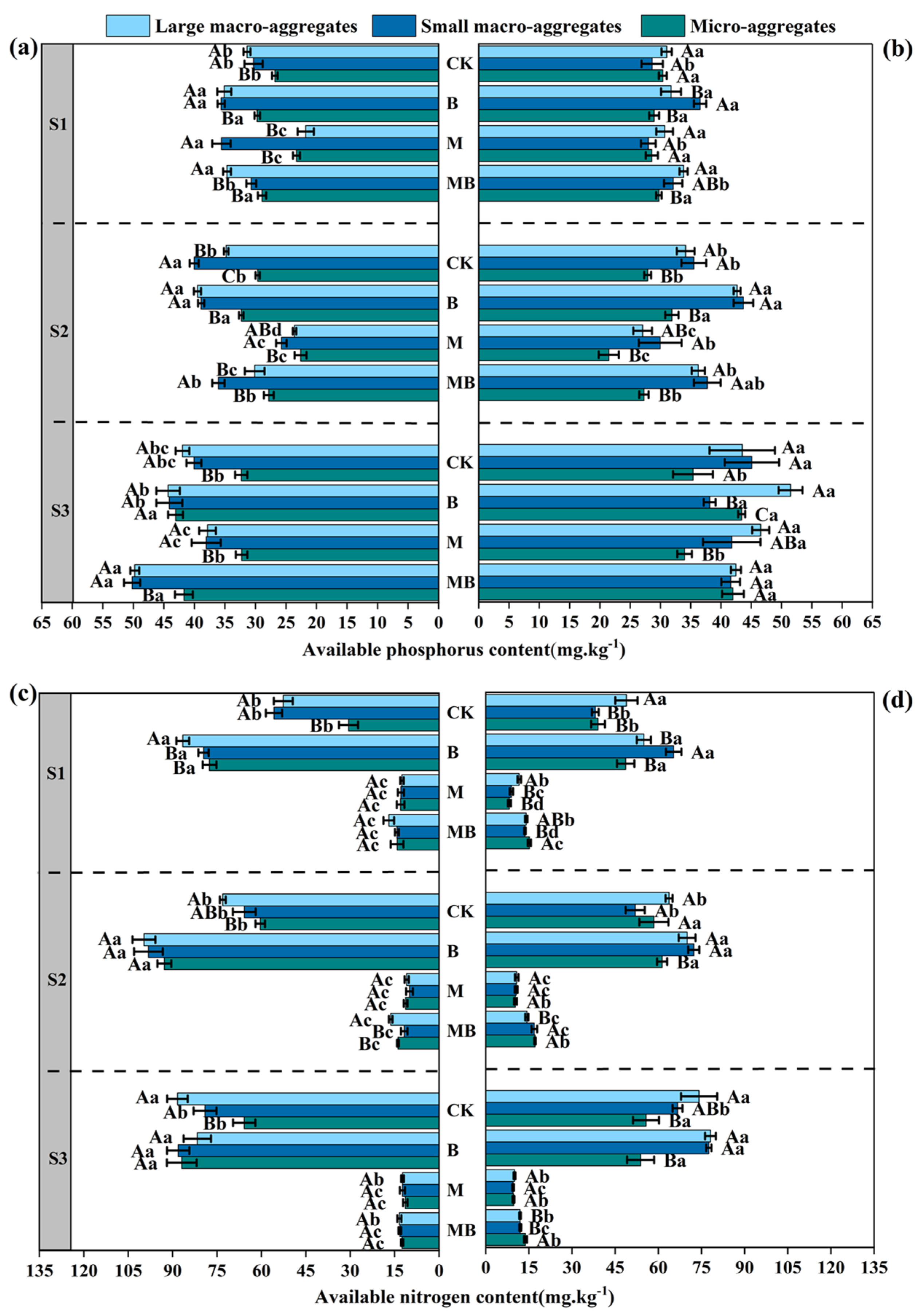
3.3. Effects of Planting Alfalfa and Inoculating B. tropicus YJ33 on Soil Nutrients
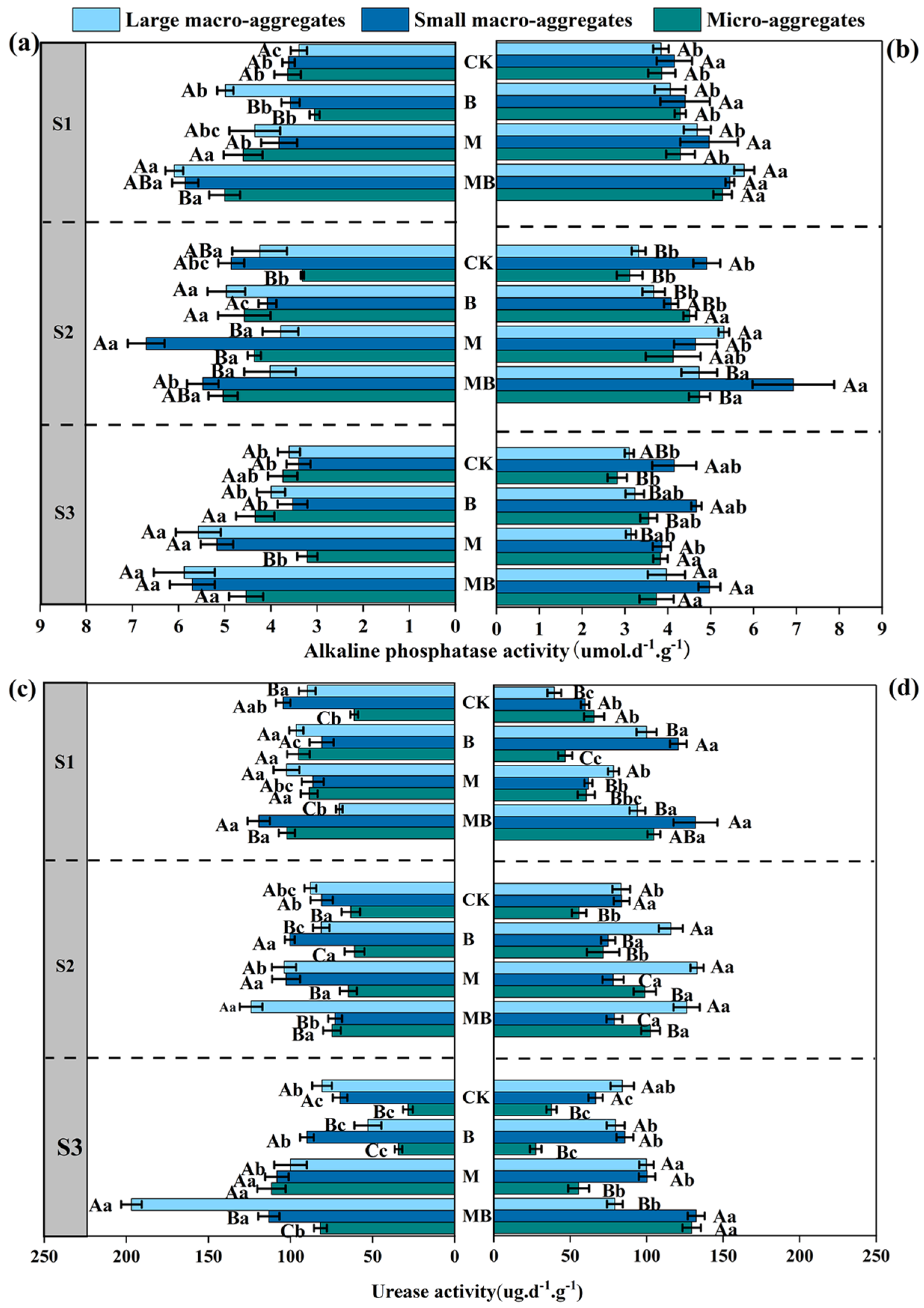
3.4. Effects of Alfalfa Planting and Inoculation with B. tropicus YJ33 on Soil Enzyme Activity in Saline–Alkali Soil
3.5. Effects of Planting Alfalfa and Inoculating B. tropicus YJ33 on Composition and Stability of Soil Aggregates in Saline–Alkali Soil
3.6. Effects of Soil Physicochemical Properties and Aggregate Stability on Alfalfa Biomass
4. Discussion
4.1. Growth-Promoting Effect of B. tropicus YJ33 on Alfalfa Grown in Saline Conditions
4.2. Improvement of Nutritional Quality of Alfalfa in Saline Soil by B. tropicus YJ33
4.3. The Effects of Alfalfa and B. tropicus YJ33 on Soil Nutrients
4.4. The Effects of Alfalfa and B. tropicus YJ33 on Enzyme Activity of Soil
4.5. Improvement of Stability of Soil Aggregates in Saline Soil by Alfalfa and B. tropicus YJ33
4.6. The Effects of Inoculation with B. tropicus YJ33 on Soil Aggregates and Alfalfa Growth
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Z.; Xu, N.; Wang, J. Satellite-Observed Evolution Dynamics of the Yellow River Delta in 1984–2018. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2020, 13, 6044–6050. [Google Scholar] [CrossRef]
- FAO. Global Map of Salt-Affected Soils (GSASmap). 2021. Available online: https://www.fao.org/global-soil-partnership/gsasmap/en (accessed on 15 January 2025).
- Lim, S.J.; Shin, M.N.; Son, J.K.; Song, J.D.; Cho, K.H.; Lee, S.H.; Ryu, J.H.; Cho, J.Y. Evaluation of soil pore-water salinity using a Decagon GS3 sensor in saline-alkali reclaimed tidal lands. Comput. Electron. Agric. 2017, 132, 49–55. [Google Scholar] [CrossRef]
- Xia, J.B.; Ren, J.Y.; Zhang, S.Y.; Wang, Y.H.; Fang, Y. Forest and grass composite patterns improve the soil quality in the coastal saline-alkali land of the Yellow River Delta, China. Geoderma 2019, 349, 25–35. [Google Scholar] [CrossRef]
- Liu, M.; Han, G.L.; Zhang, Q. Effects of agricultural abandonment on soil aggregation, soil organic carbon storage and stabilization: Results from observation in a small karst catchment, Southwest China. Agric. Ecosyst. Environ. 2020, 288, 106719. [Google Scholar] [CrossRef]
- Li, S.; Yang, Y.C.; Li, Y.C.; Gao, B.; Tang, Y.F.; Xie, J.Z.; Zhao, H.C. Remediation of saline-sodic soil using organic and inorganic amendments: Physical, chemical, and enzyme activity properties. J. Soils Sediments 2020, 20, 1454–1467. [Google Scholar] [CrossRef]
- Deng, P.B.; Guo, L.P.; Yang, H.T.; Leng, X.Y.; Wang, Y.M.; Bi, J.; Shi, C.F. Effect of an Organic Fertilizer of Residue on the Physical and Chemical Properties and Microbial Communities of Saline Alkaline Soil. Water 2023, 15, 962. [Google Scholar] [CrossRef]
- Kuzmin, A.; Tusupbekov, Z.A.; Ryapolova, N.; Nadtochiy, V. Environmental improvement of saline and alkali soils in the Kamyshlovskaya valley and revival of natural grassland efficiency. IOP Conf. Ser. Mater. Sci. Eng. 2020, 941, 012011. [Google Scholar] [CrossRef]
- Yang, C.; Liu, Y.; Zhao, W.; Wang, N. Colonization Characteristics and Diversity of Arbuscular Mycorrhizal Fungi in the Rhizosphere of Iris lactea in Songnen Saline-alkaline Grassland. Phyton 2021, 90, 719–729. [Google Scholar] [CrossRef]
- Bai, Y.; Xue, W.; Yan, Y.; Zuo, W.; Shan, Y.; Feng, K. The Challenge of Improving Coastal Mudflat Soil: Formation and Stability of Organo-mineral Complexes. Land Degrad. Dev. 2017, 29, 1074–1080. [Google Scholar] [CrossRef]
- Dai, H.C.; Chen, Y.Q.; Liu, K.C.; Li, Z.X.; Qian, X.; Zang, H.D.; Yang, X.L.; Zhao, Y.X.; Shen, Y.W.; Li, Z.J.; et al. Water-stable aggregates and carbon accumulation in barren sandy soil depend on organic amendment method: A three-year field study. J. Clean. Prod. 2019, 212, 393–400. [Google Scholar] [CrossRef]
- Gao, L.L.; Becker, E.; Liang, G.P.; Houssou, A.A.; Wu, H.J.; Wu, X.P.; Cai, D.X.; Degré, A. Effect of different tillage systems on aggregate structure and inner distribution of organic carbon. Geoderma 2017, 288, 97–104. [Google Scholar] [CrossRef]
- Duan, Y.; Chen, L.; Zhang, J.B.; Li, D.M.; Han, X.R.; Zhu, B.; Li, Y.; Zhao, B.J.; Huang, P. Long-term fertilisation reveals close associations between soil organic carbon composition and microbial traits at aggregate scales. Agric. Ecosyst. Environ. 2021, 306, 107169. [Google Scholar] [CrossRef]
- Wang, X.; Cammeraat, E.L.H.; Cerli, C.; Kalbitz, K. Soil aggregation and the stabilization of organic carbon as affected by erosion and deposition. Soil Biol. Biochem. 2014, 72, 55–65. [Google Scholar] [CrossRef]
- Dorji, T.; Field, D.J.; Odeh, I.O.A.; Bhogal, A. Soil aggregate stability and aggregate-associated organic carbon under different land use or land cover types. Soil Use Manag. 2019, 36, 308–319. [Google Scholar] [CrossRef]
- Liu, C.; Shang, H.; Han, L.; Sun, X. Effect of alkali residue and humic acid on aggregate structure of saline-alkali soil. Soil Sci. Soc. Am. J. 2024, 88, 291–303. [Google Scholar] [CrossRef]
- Tian, T.; Whalen, J.K.; Dutilleul, P. Macroaggregate persistence: Definition and applications to describe soil surface dynamics. Geoderma 2021, 397, 115096. [Google Scholar] [CrossRef]
- Totsche, K.U.; Amelung, W.; Gerzabek, M.H.; Guggenberger, G.; Klumpp, E.; Knief, C.; Lehndorff, E.; Mikutta, R.; Peth, S.; Prechtel, A.; et al. Microaggregates in soils. J. Plant Nutr. Soil Sci. 2018, 181, 104–136. [Google Scholar] [CrossRef]
- Cao, S.; Zhou, Y.Z.; Zhou, Y.Y.; Zhou, X.; Zhou, W.J. Soil organic carbon and soil aggregate stability associated with aggregate fractions in a chronosequence of citrus orchards plantations. J. Environ. Manag. 2021, 293, 112847. [Google Scholar] [CrossRef]
- Li, S.; Yao, Y.; Yang, M.; Zhang, Y.; Zhang, S.; Shen, T.; Ding, F.; Li, Z.; Liu, W.; Cui, J.; et al. Effects of different amendments on aggregate stability and microbial communities of coastal saline–alkali soil in the Yellow River Delta. Land Degrad. Dev. 2023, 34, 1694–1707. [Google Scholar] [CrossRef]
- Liu, L.; Liu, D.; Ding, X.; Chen, M.; Zhang, S. Straw incorporation and nitrogen fertilization enhance soil carbon sequestration by altering soil aggregate and microbial community composition in saline-alkali soil. Plant Soil 2023, 498, 341–356. [Google Scholar] [CrossRef]
- Han, C.L.; Zhou, W.D.; Gu, Y.J.; Wang, J.Q.; Zhou, Y.F.; Xue, Y.Y.; Shi, Z.G.; Siddique, K.H.M. Effects of tillage regime on soil aggregate-associated carbon, enzyme activity, and microbial community structure in a semiarid agroecosystem. Plant Soil 2024, 498, 543–559. [Google Scholar] [CrossRef]
- Chen, M.M.; Zhang, S.R.; Liu, L.; Liu, J.G.; Ding, X.D. Organic fertilization increased soil organic carbon stability and sequestration by improving aggregate stability and iron oxide transformation in saline-alkaline soil. Plant Soil 2022, 474, 233–249. [Google Scholar] [CrossRef]
- Gao, C.W.; Zhang, S.R.; Chen, M.M.; Cheng, L.B.; Zhang, X.G.; Ding, X.D. Appropriate Amount Application of Manure is Conducive to Improve Soil Aggregate Stability in Saline-Alkaline Soil: Analyzed from Soil Organic Carbon Mediated by Microbial Necromass Carbon. J. Soil Sci. Plant Nutr. 2024, 24, 7256–7270. [Google Scholar] [CrossRef]
- Tian, S.Y.; Zhu, B.J.; Yin, R.; Wang, M.W.; Jiang, Y.J.; Zhang, C.Z.; Li, D.M.; Chen, X.Y.; Kardol, P.; Liu, M.Q. Organic fertilization promotes crop productivity through changes in soil aggregation. Soil Biol. Biochem. 2022, 165, 108533. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, S.; Hu, R.; Li, Y. Aggregate stability and size distribution of red soils under different land uses integrally regulated by soil organic matter, and iron and aluminum oxides. Soil Tillage Res. 2017, 167, 73–79. [Google Scholar] [CrossRef]
- Burrell, L.D.; Zehetner, F.; Rampazzo, N.; Wimmer, B.; Soja, G. Long-term effects of biochar on soil physical properties. Geoderma 2016, 282, 96–102. [Google Scholar] [CrossRef]
- Duan, M.L.; Liu, G.H.; Zhou, B.B.; Chen, X.P.; Wang, Q.J.; Zhu, H.Y.; Li, Z.J. Effects of modified biochar on water and salt distribution and water-stable macro-aggregates in saline-alkaline soil. J. Soils Sediments 2021, 21, 2192–2202. [Google Scholar] [CrossRef]
- Zhang, Q.; Song, Y.; Wu, Z.; Yan, X.; Gunina, A.; Kuzyakov, Y.; Xiong, Z. Effects of six-year biochar amendment on soil aggregation, crop growth, and nitrogen and phosphorus use efficiencies in a rice-wheat rotation. J. Clean. Prod. 2020, 242, 118435. [Google Scholar] [CrossRef]
- Shao, T.Y.; Gu, X.Y.; Zhu, T.S.; Pan, X.T.; Zhu, Y.; Long, X.H.; Shao, H.B.; Liu, M.Q.; Rengel, Z. Industrial crop restored coastal saline soil quality by reducing salt and increasing diversity of bacterial community. Appl. Soil Ecol. 2019, 138, 195–206. [Google Scholar] [CrossRef]
- Yang, C.; Lv, D.; Jiang, S.; Lin, H.; Sun, J.; Li, K.; Sun, J. Soil salinity regulation of soil microbial carbon metabolic function in the Yellow River Delta, China. Sci. Total Environ. 2021, 790, 148258. [Google Scholar] [CrossRef]
- Yang, C.; Li, J.J.; Liu, N.; Zhang, Y.J. Effects of fairy ring fungi on plants and soil in the alpine and temperate grasslands of China. Plant Soil 2019, 441, 499–510. [Google Scholar] [CrossRef]
- Li, C.; Song, T.; Zhan, L.; Cong, C.; Xu, H.; Dong, L.; Cai, H. Overexpression of MsRCI2A, MsRCI2B, and MsRCI2C in Alfalfa (Medicago sativa L.) Provides Different Extents of Enhanced Alkali and Salt Tolerance Due to Functional Specialization of MsRCI2s. Front. Plant Sci. 2021, 12, 702195. [Google Scholar] [CrossRef] [PubMed]
- Land, L.; White, J.R.; Gambrell, R.P. Microbial Response to Potential Soil-Stabilizing Polymer Amendments for Coastal Wetland Restoration. Soil Sci. Soc. Am. J. 2011, 75, 2398–2406. [Google Scholar] [CrossRef]
- Vardharajula, S.; Ali, S.Z. Exopolysaccharide Production by Drought Tolerant Bacillus spp. and Effect on Soil Aggregation Under Drought Stress. J. Microbiol. Biotechnol. Food Sci. 2014, 4, 51–57. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, Q.; Qie, X.; Che, Y.; Lv, D.; Gao, Y.; Miao, F.; Li, S.; He, F.; Sun, J.; et al. Effects of Rhizosphere Microorganisms Associated with Suaeda Salsa on the Growth and Salt Stress Resistance of Alfalfa. J. Soil Sci. Plant Nutr. 2024, 24, 4033–4048. [Google Scholar] [CrossRef]
- Berna, E.; Namuk, E.; Cengiz, S. Effects of Prolonged High Salinity Stress on Alfalfa (Medicago sativa L.) Cultivars and Populations. Legume Res.—Int. J. 2024, 47, 2049–2058. [Google Scholar] [CrossRef]
- Lei, Y.; Xu, Y.; Hettenhausen, C.; Lu, C.; Shen, G.; Zhang, C.; Li, J.; Song, J.; Lin, H.; Wu, J. Comparative analysis of alfalfa (Medicago sativa L.) leaf transcriptomes reveals genotype-specific salt tolerance mechanisms. BMC Plant Biol. 2018, 18, 35. [Google Scholar] [CrossRef]
- Zhu, J.K. Abiotic Stress Signaling and Responses in Plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef]
- Negi, R.; Kaur, T.; Devi, R.; Kour, D.; Yadav, A.N. Assessment of nitrogen-fixing endophytic and mineral solubilizing rhizospheric bacteria as multifunctional microbial consortium for growth promotion of wheat and wild wheat relative Aegilops kotschyi. Heliyon 2022, 8, e12579. [Google Scholar] [CrossRef]
- Yahaghi, Z.; Shirvani, M.; Nourbakhsh, F.; Pueyo, J.J. Uptake and effects of lead and zinc on alfalfa (Medicago sativa L.) seed germination and seedling growth: Role of plant growth promoting bacteria. S. Afr. J. Bot. 2019, 124, 573–582. [Google Scholar] [CrossRef]
- Sharma, S.; Kulkarni, J.; Jha, B. Halotolerant Rhizobacteria Promote Growth and Enhance Salinity Tolerance in Peanut. Front. Microbiol. 2016, 7, 1600. [Google Scholar] [CrossRef]
- Ullah, S.; Bano, A. Isolation of plant-growth-promoting rhizobacteria from rhizospheric soil of halophytes and their impact on maize (Zea mays L.) under induced soil salinity. Can. J. Microbiol. 2015, 61, 307–313. [Google Scholar] [CrossRef]
- Xiong, Y.W.; Gong, Y.; Li, X.W.; Chen, P.; Ju, X.Y.; Zhang, C.M.; Yuan, B.; Lv, Z.P.; Xing, K.; Qin, S. Enhancement of growth and salt tolerance of tomato seedlings by a natural halotolerant actinobacterium KLBMP 5180 isolated from a coastal halophyte. Plant Soil 2019, 445, 307–322. [Google Scholar] [CrossRef]
- Parida, A.K.; Veerabathini, S.K.; Kumari, A.; Agarwal, P.K. Physiological, Anatomical and Metabolic Implications of Salt Tolerance in the Halophyte under Hydroponic Culture Condition. Front. Plant Sci. 2016, 7, 351. [Google Scholar] [CrossRef]
- Scott, S.L.; Mbifo, R.S.; Chiquette, J.; Savoie, P.; Turcotte, G. Rumen disappearance kinetics and chemical characterization of by-products from cellulosic ethanol production. Anim. Feed Sci. Technol. 2011, 165, 151–160. [Google Scholar] [CrossRef]
- Chen, W.T.; Zhou, M.Y.; Zhao, M.Z.; Chen, R.H.; Tigabu, M.; Wu, P.F.; Li, M.; Ma, X.Q. Transcriptome analysis provides insights into the root response of Chinese fir to phosphorus deficiency. BMC Plant Biol. 2021, 21, 525. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Franco, M.; Ding, Z.T.; Hao, L.; Ke, W.C.; Wang, M.E.; Xie, D.M.; Li, Z.Q.; Zhang, Y.X.; Ai, L.; et al. Effect of and on fermentation, dynamics of bacterial community and their functional shifts of whole-plant corn silage. J. Anim. Sci. Biotechnol. 2022, 13, 7. [Google Scholar] [CrossRef]
- Li, Y.B.; Li, Q.; Chen, S.F. Diazotroph BJ-18 Drives the Variation in Bacterial, Diazotrophic and Fungal Communities in the Rhizosphere and Root/Shoot Endosphere of Maize. Int. J. Mol. Sci. 2021, 22, 1460. [Google Scholar] [CrossRef] [PubMed]
- Nautiyal, C.; Dion, P. Molecular Mechanisms of Plant and Microbe Coexistence; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar] [CrossRef]
- Xiao, S.; Zhang, W.; Ye, Y.; Jie, Z.; Wang, K. Soil aggregate mediates the impacts of land uses on organic carbon, total nitrogen, and microbial activity in a Karst ecosystem. Sci. Rep. 2017, 7, 41402. [Google Scholar] [CrossRef]
- Six, J.; Conant, R.T.; Paul, E.A.; Paustian, K. Stabilization mechanisms of soil organic matter: Implications for C-saturation of soils. Plant Soil 2002, 241, 155–176. [Google Scholar] [CrossRef]
- Feng, M.; Xiang, J.; Ji, X.; Jiang, J. Larger Soil Water-Stable Aggregate May Exert a Negative Effect on Nutrient Availability: Results from Red Soil (Ultisol), in South China. Forests 2023, 14, 975. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Li, Y.B.; Tan, X.P.; He, W.X.; Xie, W.; Megharaj, M.; Wei, G.H. Effect of arsenate contamination on free, immobilized and soil alkaline phosphatases: Activity, kinetics and thermodynamics. Eur. J. Soil Sci. 2017, 68, 126–135. [Google Scholar] [CrossRef]
- Ritz, K.; Young, I.M. Interactions between soil structure and fungi. Mycologist 2004, 18, 52–59. [Google Scholar] [CrossRef]
- Huang, Y.X.; Lin, L.Y.; Zhang, L.P.; Wu, F.; Zhang, Y.; Huang, S.H. Effects of Interaction between Claroideogolmus etuicatum and Bacillus aryabhattai on the Utilization of Organic Phosphorus in Camellia oleifera Abel. J. Fungi. 2023, 9, 977. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Y.; Liu, X.S.; Zhang, Q.B.; Li, S.Y.; Sun, Y.L.; Lu, W.H.; Ma, C.H. Response of alfalfa growth to arbuscular mycorrhizal fungi and phosphate-solubilizing bacteria under different phosphorus application levels. AMB Express 2020, 10, 200. [Google Scholar] [CrossRef]
- Luo, G.W.; Ling, N.; Nannipieri, P.; Chen, H.; Raza, W.; Wang, M.; Guo, S.W.; Shen, Q.R. Long-term fertilisation regimes affect the composition of the alkaline phosphomonoesterase encoding microbial community of a vertisol and its derivative soil fractions. Biol. Fertil. Soils 2017, 53, 375–388. [Google Scholar] [CrossRef]
- Bian, Q.; Dong, Z.; Zhao, Y.; Feng, Y.; Fu, Y.; Wang, Z.; Zhu, J.; Ma, L. Micro-/nanobubble oxygenation irrigation enhances soil phosphorus availability and yield by altering soil bacterial community abundance and core microbial populations. Front. Plant Sci. 2025, 15, 1497952. [Google Scholar] [CrossRef]
- Rojo, M.J.; Carcedo, S.G.; Mateos, M.P. Distribution and characterization of phosphatase and organic phosphorus in soil fractions. Soil Biol. Biochem. 1990, 22, 169–174. [Google Scholar] [CrossRef]
- Du, Y.; Niu, W.; Zhang, Q.; Cui, B.; Gu, X.; Guo, L.; Liang, B. Effects of Nitrogen on Soil Microbial Abundance, Enzyme Activity, and Nitrogen Use Efficiency in Greenhouse Celery under Aerated Irrigation. Soil Sci. Soc. Am. J. 2018, 82, 606–613. [Google Scholar] [CrossRef]
- Richardson, A.; Simpson, R. Soil Microorganisms Mediating Phosphorus Availability Update on Microbial Phosphorus. Plant Physiol. 2011, 156, 989–996. [Google Scholar] [CrossRef]
- Six, J.; Bossuyt, H.; Degryze, S.; Denef, K. A history of research on the link between (micro)aggregates, soil biota, and soil organic matter dynamics. Soil Tillage Res. 2004, 79, 7–31. [Google Scholar] [CrossRef]
- Tisdall, J.M.; Oades, J.M. Organic matter and water-stable aggregates in soils. J. Soil Sci. 2006, 33, 141–163. [Google Scholar] [CrossRef]
- Gyssels, G.; Poesen, J.; Bochet, E.; Li, Y. Impact of plant roots on the resistance of soils to erosion by water: A review. Prog. Phys. Geogr. Earth Environ. 2005, 29, 189–217. [Google Scholar] [CrossRef]
- Morris, C.E.; Monier, J.-M. The Ecological Significance of Biofilm Formation by Plant-Associated Bacteria. Annu. Rev. Phytopathol. 2003, 41, 429–453. [Google Scholar] [CrossRef]
- Bronick, C.J.; Lal, R. Soil structure and management: A review. Geoderma 2005, 124, 3–22. [Google Scholar] [CrossRef]
- Islam, M.M.; Rengel, Z.; Storer, P.; Siddique, K.H.M.; Solaiman, Z.M. Phosphorus fertilisation differentially influences growth, morpho-physiological adaptations and nutrient uptake of industrial hemp (Cannabis sativa L.). Plant Soil 2023, 492, 301–314. [Google Scholar] [CrossRef]
- Dunn, B.; Shrestha, A.; Goad, C. Use of nondestructive sensors to quantify ornamental kale nitrogen status. J. Plant Nutr. 2016, 39, 1123–1130. [Google Scholar] [CrossRef]
- Bhattarai, S.; Lundell, S.; Biligetu, B. Effect of Sodium Chloride Salt on Germination, Growth, and Elemental Composition of Alfalfa Cultivars with Different Tolerances to Salinity. Agronomy 2022, 12, 2516. [Google Scholar] [CrossRef]
- Chen, Q.X.; Song, Y.J.; An, Y.X.; Lu, Y.L.; Zhong, G.H. Soil Microorganisms: Their Role in Enhancing Crop Nutrition and Health. Diversity 2024, 16, 734. [Google Scholar] [CrossRef]
- Goupry, S.; Gentil, E.; Akoka, S.; Robins, R.J. Co-fermentation of glucose and citrate by Lactococcus lactis diacetylactis: Quantification of the relative metabolic rates by isotopic analysis at natural abundance. Appl. Microbiol. Biotechnol. 2003, 62, 489–497. [Google Scholar] [CrossRef]
- Even, R.J.; Cotrufo, M.F. The ability of soils to aggregate, more than the state of aggregation, promotes protected soil organic matter formation. Geoderma 2024, 442, 116760. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Che, Y.; Chen, S.; Liu, M.; Diao, M.; Yang, C.; Jia, W. Bacillus tropicus YJ33 and Medicago sativa L. Synergistically Enhance Soil Aggregate Stability in Saline–Alkali Environments. Microorganisms 2025, 13, 1291. https://doi.org/10.3390/microorganisms13061291
Li J, Che Y, Chen S, Liu M, Diao M, Yang C, Jia W. Bacillus tropicus YJ33 and Medicago sativa L. Synergistically Enhance Soil Aggregate Stability in Saline–Alkali Environments. Microorganisms. 2025; 13(6):1291. https://doi.org/10.3390/microorganisms13061291
Chicago/Turabian StyleLi, Jingjing, Yajuan Che, Shiyang Chen, Mengge Liu, Mengmeng Diao, Chao Yang, and Wenke Jia. 2025. "Bacillus tropicus YJ33 and Medicago sativa L. Synergistically Enhance Soil Aggregate Stability in Saline–Alkali Environments" Microorganisms 13, no. 6: 1291. https://doi.org/10.3390/microorganisms13061291
APA StyleLi, J., Che, Y., Chen, S., Liu, M., Diao, M., Yang, C., & Jia, W. (2025). Bacillus tropicus YJ33 and Medicago sativa L. Synergistically Enhance Soil Aggregate Stability in Saline–Alkali Environments. Microorganisms, 13(6), 1291. https://doi.org/10.3390/microorganisms13061291






