The Rhizobacterium Bacillus amyloliquefaciens MHR24 Has Biocontrol Ability Against Fungal Phytopathogens and Promotes Growth in Arabidopsis thaliana
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation of Rhizobacteria, Growth Conditions, and Plant Growth-Promoting Traits
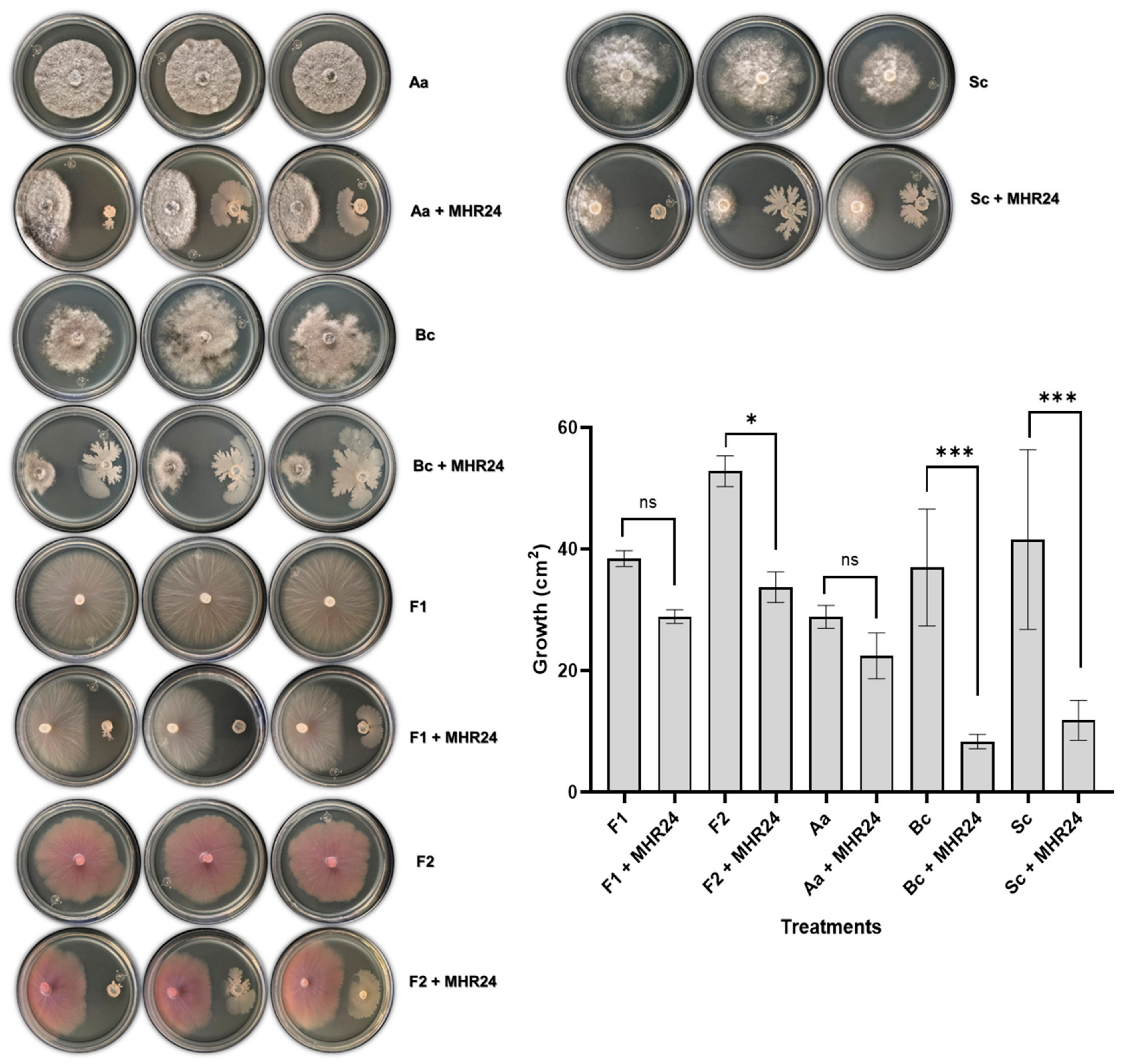
2.2. Biocontrol Assay Against Fungal Phytopathogens
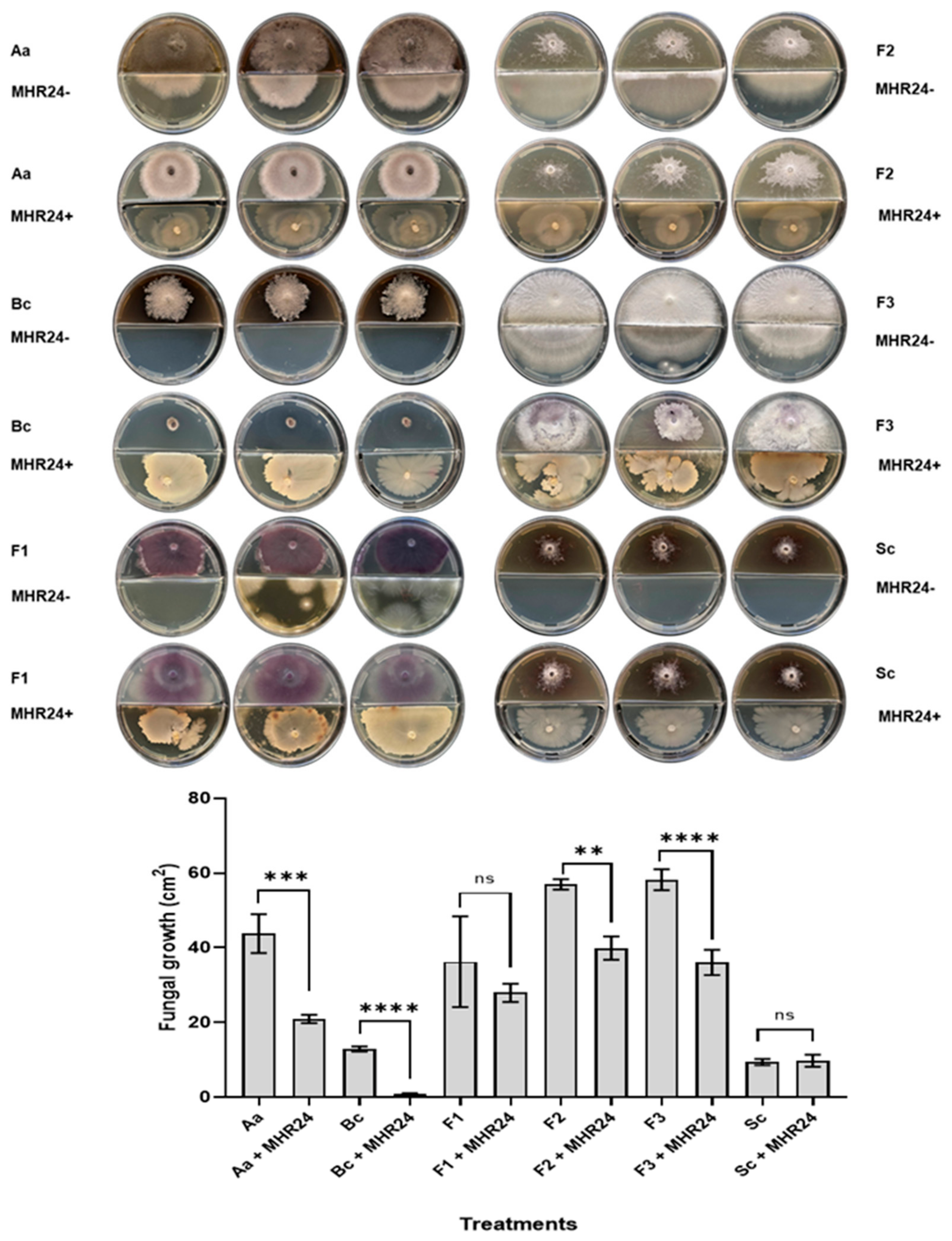
2.3. Biocontrol Assay by Bacterial Volatiles Against Fungal Phytopathogens
2.4. Saline Stress Tolerance Test in In Vitro Conditions
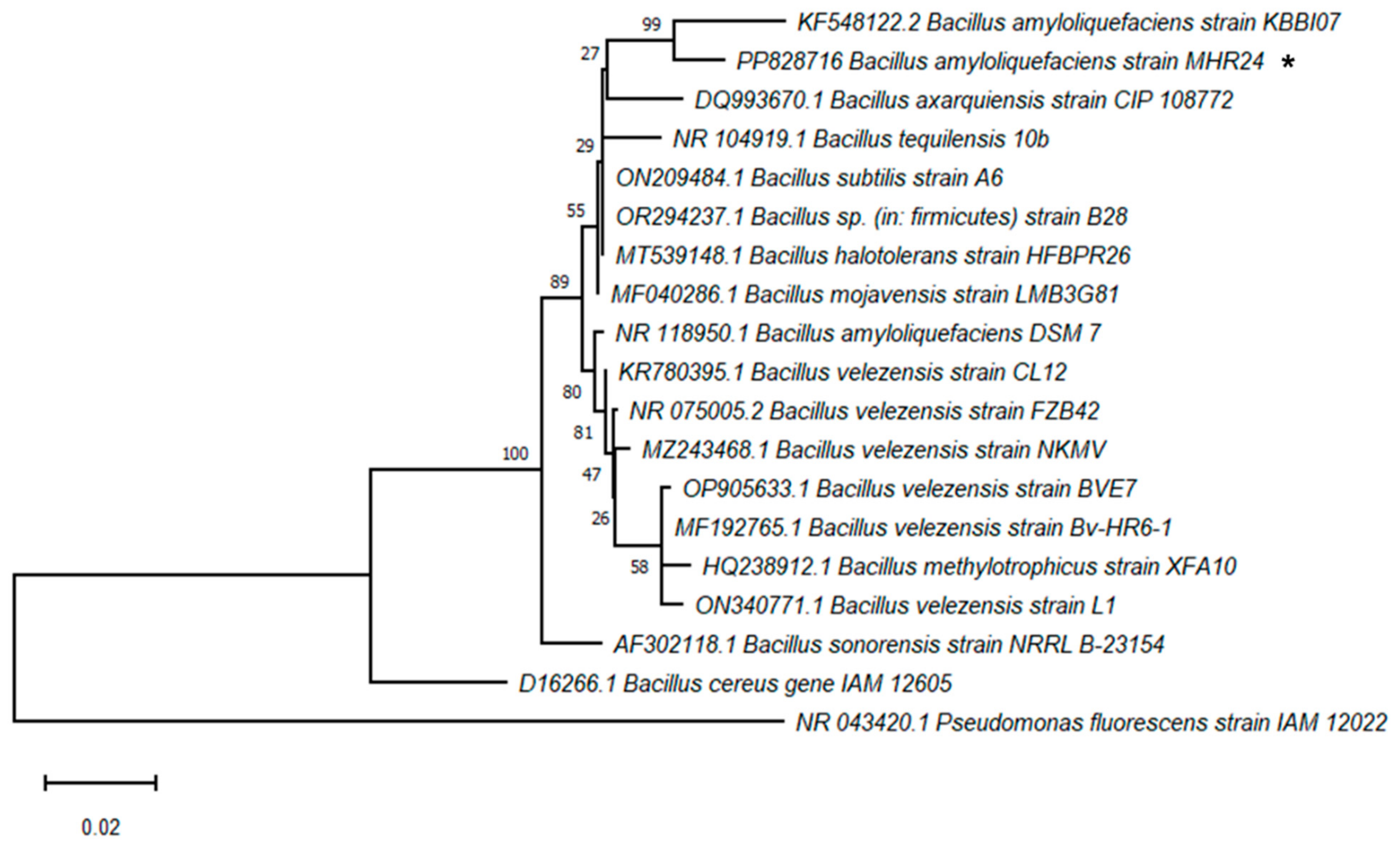
2.5. Molecular Identification of Rhizobacteria MHR24
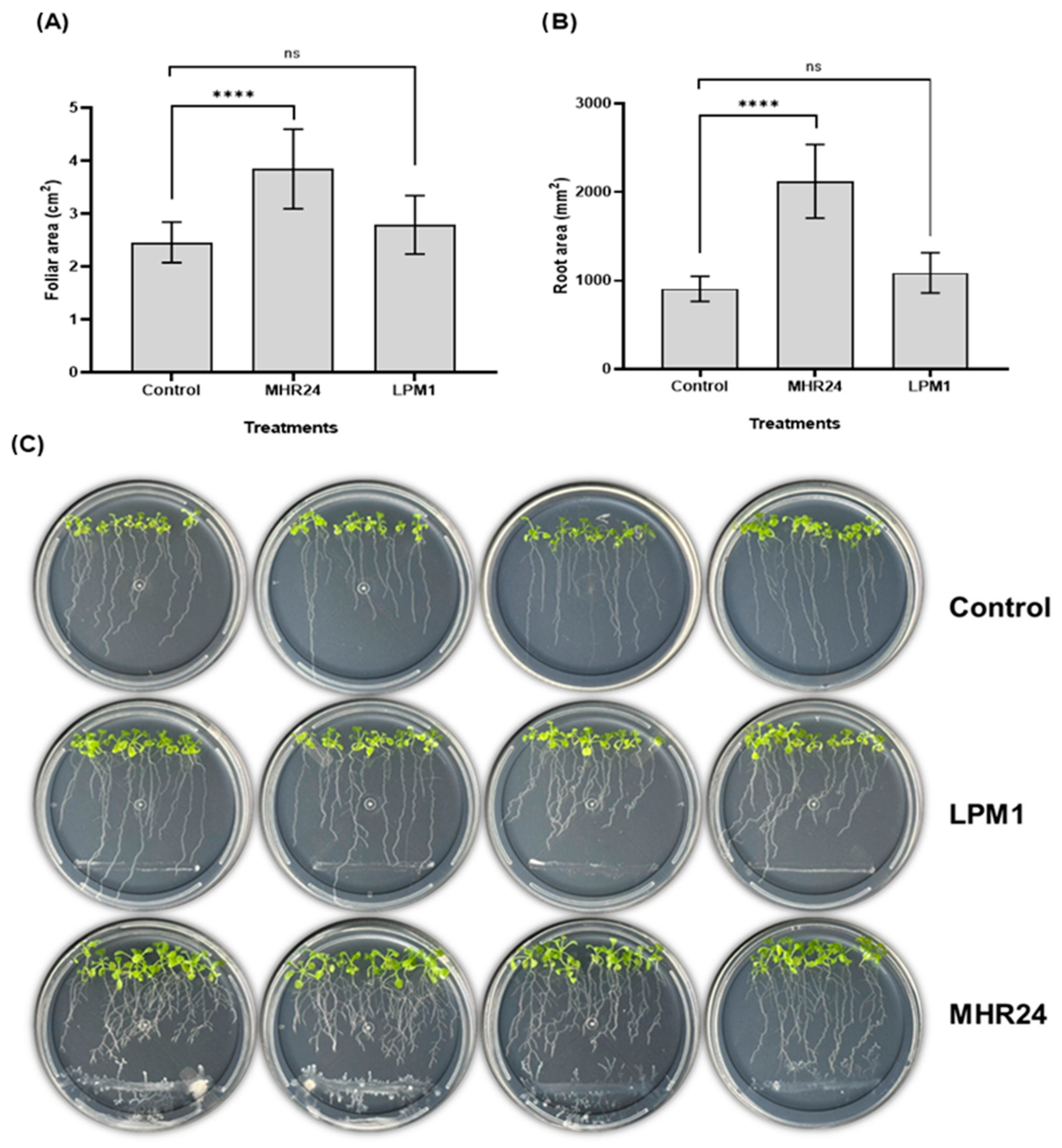
2.6. Plant Growth Promotion in Arabidopsis thaliana Col-0 Under In Vitro and Growth Chambers Conditions
2.7. Data Analysis
3. Results
3.1. PGP Traits for Isolated MHR24
3.2. Antagonistic Assays for Isolated MHR24 Against Fungal Phytopathogens
3.3. In Vitro Saline Stress Effects in MHR24 and LPM1 Inoculation
3.4. Molecular Identification of MHR24 from 16S rDNA Sequence
3.5. Growth-Promotion Effects of Strain MHR24 on Arabidopsis thaliana Col-0
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khatoon, Z.; Huang, S.; Rafique, M.; Fakhar, A.; Kamran, M.A.; Santoyo, G. Unlocking the potential of plant growth-promoting rhizobacteria on soil health and the sustainability of agricultural systems. J. Environ. Manag. 2020, 273, 111118. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, R.; You, M.P.; Barbetti, M.J.; Chen, Y. Pathogen biocontrol using plant growth-promoting bacteria (PGPR): Role of bacterial diversity. Microorganisms 2021, 9, 1988. [Google Scholar] [CrossRef] [PubMed]
- Backer, R.; Rokem, J.S.; Ilangumaran, G.; Lamont, J.; Praslickova, D.; Ricci, E.; Subramanian, S.; Smith, D.L. Plant growth-promoting rhizobacteria: Context, mechanisms of action, and roadmap to commercialization of biostimulants for sustainable agriculture. Front. Plant Sci. 2018, 9, 1473. [Google Scholar] [CrossRef] [PubMed]
- Ryu, C.M.; Farag, M.A.; Hu, C.H.; Reddy, M.S.; Wei, H.X.; Paré, P.W.; Kloepper, J.W. Bacterial volatiles promote growth in Arabidopsis. Proc. Natl. Acad. Sci. USA 2003, 100, 4927–4932. [Google Scholar] [CrossRef] [PubMed]
- Paulucci, N.S.; Gallarato, L.A.; Reguera, Y.B.; Vicario, J.C.; Cesari, A.B.; García de Lema, M.B.; Dardanelli, M.S. Arachis hypogaea PGPR isolated from Argentine soil modifies its lipids components in response to temperature and salinity. Microbiol. Res. 2015, 173, 1–9. [Google Scholar] [CrossRef]
- Guerrieri, M.C.; Fiorini, A.; Fanfoni, E.; Tabaglio, V.; Cocconcelli, P.S.; Trevisan, M.; Puglisi, E. Integrated genomic and greenhouse assessment of a novel plant growth-promoting rhizobacterium for tomato plant. Front. Plant Sci. 2021, 12, 660620. [Google Scholar] [CrossRef]
- Cárdenas-Flores, A.; Ruíz-Salas, C.E.; Baylón-Palomino, A.; Vázquez-Lee, J.; Mounzer, O.; Flores-Olivas, A.; Valenzuela-Soto, J.H. Bacillus subtilis LPM1 differentially promotes the growth of bell pepper (Capsicum annuum L.) varieties under shade house. Cogent. Food Agric. 2023, 9, 2232165. [Google Scholar] [CrossRef]
- Délano-Frier, J.P.; Flores-Olivas, A.; Valenzuela-Soto, J.H. Bio-Inoculation of tomato (Solanum lycopersicum L.) and jalapeno pepper (Capsicum annuum L.) with Enterobacter sp. DBA51 increases growth and yields under open-field conditions. Agronomy 2024, 14, 702. [Google Scholar] [CrossRef]
- Hassaan, M.A.; El-Nemr, A. Pesticides pollution: Classifications, human health impact, extraction and treatment techniques. Egypt. J. Aquat. Res. 2020, 46, 207–220. [Google Scholar] [CrossRef]
- Tudi, M.; Ruan, H.D.; Wang, L.; Lyu, J.; Sadler, R.; Connell, D.; Phung, D.T. Agriculture development, pesticide application and its impact on the environment. Int. J. Environ. Res. Public Health 2021, 18, 1112. [Google Scholar] [CrossRef]
- Aly, A.A.; El-Mahdy, O.M.; Habeb, M.M.; Elhakem, A.; Asran, A.A.; Youssef, M.M.; Mohamed, H.I.; Hanafi, R.S. Pathogenicity of Bacillus strains to cotton seedlings and their effects on some biochemical components of the infected seedlings. Plant Pathol. J. 2022, 38, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wang, W.; Zhang, X.; Gao, Z.; Cai, S.; Wang, S.; Li, Y. Bacillus velezensis BVE7 as a promising agent for biocontrol of soybean root rot caused by Fusarium oxysporum. Front. Microbiol. 2023, 14, 1275986. [Google Scholar] [CrossRef]
- Xu, S.; Liu, Y.X.; Cernava, T.; Wang, H.; Zhou, Y.; Xia, T.; Cao, S.; Berg, G.; Shen, X.X.; Wen, Z.; et al. Fusarium fruiting body microbiome member Pantoea agglomerans inhibits fungal pathogenesis by targeting lipid rafts. Nat. Microbiol. 2022, 7, 831–843. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Wu, H.; Wang, W.; Yang, Y.; Xie, S.; Xie, Y.; Gao, X. Efficient colonization and harpins mediated enhancement in growth and biocontrol of wilt disease in tomato by Bacillus subtilis. Lett. Appl. Microbiol. 2013, 57, 526–533. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, X.; He, X.; Zhao, W.; Cao, Y.; Guo, T.; Li, T.; Ni, H.; Tang, X. Phosphate-solubilizing Pseudomonas sp. strain P34-L promotes wheat growth by colonizing the wheat rhizosphere and improving the wheat root system and soil phosphorus nutritional status. J. Plant Growth Regul. 2019, 38, 1314–1324. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Li, Y.; Yu, C.; Wang, Q.Q.; Wang, M.; Sun, J.; Gao, J.X.; Chen, J. Effect of Trichoderma harzianum on maize rhizosphere microbiome and biocontrol of Fusarium stalk rot. Sci. Rep. 2017, 7, 1771. [Google Scholar] [CrossRef] [PubMed]
- Ling, L.; Han, X.; Li, X.; Zhang, X.; Wang, H.; Zhang, L.; Cao, P.; Wu, Y.; Wang, X.; Zhao, J.; et al. A Streptomyces sp. NEAU-HV9: Isolation, identification, and potential as a biocontrol agent against Ralstonia solanacearum of tomato plants. Microorganisms 2020, 8, 351. [Google Scholar] [CrossRef] [PubMed]
- Santoyo, G.; Orozco-Mosqueda, M.D.C.; Govindappa, M. Mechanisms of biocontrol and plant growth-promoting activity in soil bacterial species of Bacillus and Pseudomonas: A review. Biocontrol Sci. Technol. 2012, 22, 855–872. [Google Scholar] [CrossRef]
- Shafi, J.; Tian, H.; Ji, M. Bacillus species as versatile weapons for plant pathogens: A review. Biotechnol. Biotechnol. Equip. 2017, 31, 446–459. [Google Scholar] [CrossRef]
- Fira, D.; Dimkić, I.; Berić, T.; Lozo, J.; Stanković, S. Biological control of plant pathogens by Bacillus species. J. Biotechnol. 2018, 285, 44–55. [Google Scholar] [CrossRef]
- Solano-Alvarez, N.; Valencia-Hernández, J.A.; Rico-García, E.; Torres-Pacheco, I.; Ocampo-Velázquez, R.V.; Escamilla-Silva, E.M.; Romero-García, A.L.; Alpuche-Solís, Á.G.; Guevara-González, R.G. A novel isolate of Bacillus cereus promotes growth in tomato and inhibits Clavibacter michiganensis infection under greenhouse conditions. Plants 2021, 10, 506. [Google Scholar] [CrossRef] [PubMed]
- Fan, B.; Blom, J.; Klenk, H.P.; Borriss, R. Bacillus amyloliquefaciens, Bacillus velezensis, and Bacillus siamensis form an “operational group B. amyloliquefaciens” within the B. subtilis species complex. Front. Microbiol. 2017, 8, 22. [Google Scholar] [CrossRef] [PubMed]
- Mazzola, M.; Freilich, S. Prospects for biological soil borne disease control: Application of indigenous versus synthetic microbiomes. Phytopathology 2017, 107, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Etesami, H.; Jeong, B.R.; Glick, B.R. Biocontrol of plant diseases by Bacillus spp. Physiol. Mol. Plant Pathol. 2023, 126, 102048. [Google Scholar] [CrossRef]
- Albdaiwi, R.N.; Khyami-Horani, H.; Ayad, J.Y.; Alananbeh, K.M.; Al-Sayaydeh, R. Isolation and characterization of halotolerant plant growth promoting rhizobacteria from durum wheat (Triticum turgidum subsp. durum) cultivated in saline areas of the dead sea region. Front. Microbiol. 2019, 10, 1639. [Google Scholar] [CrossRef]
- Nautiyal, C.S. An efficient microbiological growth medium for screening phosphorus solubilizing microorganisms. FEMS Microbiol. Lett. 1999, 170, 265–270. [Google Scholar] [CrossRef]
- Subba, R. Soil Microorganisms and Plant Growth; Oxford and IBH Publishing Co.: New Delhi, India, 1977. [Google Scholar]
- Jensen, H.L. Nitrogen fixation in leguminous plants. II. Is symbiotic nitrogen fixation influenced by Azotobacter? In Proceedings of the Linnean Society of New South Wales; Sydney Linnean Society of New South Wales: Manly, Australia, 1942; pp. 205–212. [Google Scholar]
- Batista, B.B.; Bonatelli, M.L.; Quecine, M.C. Fast screening of bacteria for plant growth promoting traits. In The Plant Microbiome: Methods and Protocols; Carvalhais, L.C., Dennis, P.G., Eds.; Springer: New York, NY, USA, 2021; Chapter 7; pp. 61–75. [Google Scholar]
- Bauer, A.W.; Kirby, W.M.M.; Sherris, J.C.; Turck, M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1966, 36, 493–496. [Google Scholar] [CrossRef]
- Frank, J.A.; Reich, C.I.; Sharma, S.; Weisbaum, J.S.; Wilson, B.A.; Olsen, G.J. Critical evaluation of two primers commonly used for amplification of bacterial 16S rRNA genes. Appl. Environ. Microbiol. 2008, 74, 2461–2470. [Google Scholar] [CrossRef]
- Ortega-Ortega, Y.; Sarmiento-López, L.G.; Baylón-Palomino, A.; Vázquez-Lee, J.; Maldonado-Bonilla, L.D.; Flores-Olivas, A.; Valenzuela-Soto, J.H. Enterobacter sp. DBA51 produces ACC deaminase and promotes the growth of tomato (Solanum lycopersicum L.) and tobacco (Nicotiana tabacum L.) plants under greenhouse condition. Curr. Res. Microb. Sci. 2024, 6, 100207. [Google Scholar] [CrossRef]
- Vignesh, M.; Shankar, S.R.M.; Mubarak-Ali, D.; Hari, B.N.V. A novel rhizospheric bacterium: Bacillus velezensis NKMV-3 as a biocontrol agent against Alternaria leaf blight in tomato. Appl. Biochem. Biotechnol. 2022, 194, 1–17. [Google Scholar] [CrossRef]
- Ling, L.; Luo, H.; Yang, C.; Wang, Y.; Cheng, W.; Pang, M.; Jiang, K. Volatile organic compounds produced by Bacillus velezensis L1 as a potential biocontrol agent against postharvest diseases of wolfberry. Front. Microbiol. 2022, 13, 987844. [Google Scholar] [CrossRef] [PubMed]
- Ruckert, C.; Blom, J.; Chen, X.H.; Reva, O.; Borriss, R. Genome sequence of B. amyloliquefaciens type strain DSM7(T) reveals diferences to plant-associated B. amyloliquefaciens FZB42. J. Biotechnol. 2011, 155, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Gamboa-Becerra, R.; Desgarennes, D.; Molina-Torres, J.; Ramírez-Chávez, E.; Kiel-Martínez, A.L.; Carrión, G.; Ortiz-Castro, R. Plant growth-promoting and non-promoting rhizobacteria from avocado trees differentially emit volatiles that influence growth of Arabidopsis thaliana. Protoplasma 2022, 259, 835–854. [Google Scholar] [CrossRef]
- Cawoy, H.; Bettiol, W.; Fickers, P.; Ongena, M. Pesticides in the modern world: Bacillus-based biological control of plant diseases. FRBSF Econ. Lett. 2011, 2, 140–158. [Google Scholar]
- Wei, J.B.; Zhao, J.; Suo, M.; Wu, H.; Zhao, M.; Yang, H.Y. Biocontrol mechanisms of Bacillus velezensis against Fusarium oxysporum from Panax ginseng. Biol. Control 2023, 182, 105222. [Google Scholar] [CrossRef]
- Dunlap, C.A.; Kim, S.J.; Kwon, S.W.; Rooney, A.P. Bacillus velezensis is not a later heterotypic synonym of Bacillus amyloliquefaciens, Bacillus methylotrophicus, Bacillus amyloliquefaciens subsp. plantarum and ‘Bacillus oryzicola’ are later heterotypic synonyms of Bacillus velezensis based on phylogenomics. Int. J. Syst. Evol. Microbiol. 2016, 66, 1212–1217. [Google Scholar]
- Saxena, A.K.; Kumar, M.; Chakdar, H.; Anuroopa, N.; Bagyaraj, D.J. Bacillus species in soil as a natural resource for plant health and nutrition. J. Appl. Microbiol. 2020, 128, 1583–1594. [Google Scholar] [CrossRef]
- Shahid, I.; Han, J.; Hanooq, S.; Malik, K.A.; Borchers, C.H.; Mehnaz, S. Profiling of metabolites of Bacillus spp. and their application in sustainable plant growth promotion and biocontrol. Front. Sustain. Food Syst. 2021, 5, 37. [Google Scholar] [CrossRef]
- Sheteiwy, M.S.; Abd-Elgawad, H.; Xiong, Y.C.; Macovei, A.; Brestic, M.; Skalicky, M.; Shaghaleh, M.; Hamoud, Y.A.; El-Sawah, A.M. Inoculation with Bacillus amyloliquefaciens and mycorrhiza confers tolerance to drought stress and improve seed yield and quality of soybean plant. Physiol. Plant. 2021, 172, 2153–2169. [Google Scholar] [CrossRef]
- Xue, Y.; Zhang, Y.; Huang, K.; Wang, X.; Xing, M.; Xu, Q.; Guo, Y. A novel biocontrol agent Bacillus velezensis K01 for management of gray mold caused by Botrytis cinerea. AMB Express 2023, 13, 91. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Zhang, Y.; Yu, Z.; Zhuang, W.; Zeng, Z. Bacillus velezensis BV01 has broad-spectrum biocontrol potential and the ability to promote plant growth. Microorganisms 2023, 11, 2627. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, C.A.A.; Bettiol, W. Multifaceted intervention of Bacillus spp. against salinity stress and Fusarium wilt in tomato. J. Appl. Microbiol. 2021, 131, 2387–2401. [Google Scholar] [CrossRef] [PubMed]
- Reva, O.N.; Dixelius, C.; Meijer, J.; Priest, F.G. Taxonomic characterization and plant colonizing abilities of some bacteria related to Bacillus amyloliquefaciens and Bacillus subtilis. FEMS Microbiol. Ecol. 2004, 48, 249–259. [Google Scholar] [CrossRef]
- Chun, B.H.; Kim, K.H.; Jeong, S.E.; Jeon, C.O. Genomic and metabolic features of the Bacillus amyloliquefaciens group-B. amyloliquefaciens, B. velezensis, and B. siamensis-revealed by pan-genome analysis. Food Microbiol. 2019, 77, 146–157. [Google Scholar] [CrossRef]
- Lopez-Bucio, J.; Campos-Cuevas, J.C.; Hernandez-Calderon, E.; Velasquez-Becerra, C.; Farias-Rodriguez, R.; Macias-Rodriguez, L.I.; Valencia-Cantero, E. Bacillus megaterium rhizobacteria promote growth and alter root system architecture through an auxin and ethylene-independent signaling mechanism in Arabidopsis thaliana. Mol. Plant Microbe Interact. 2007, 20, 207–217. [Google Scholar] [CrossRef]
- Micallef, S.A.; Shiaris, M.P.; Colón-Carmona, A. Influence of Arabidopsis thaliana accessions on rhizobacterial communities and natural variation in root exudates. J. Exp. Bot. 2009, 60, 1729–1742. [Google Scholar] [CrossRef]
- Zhang, H.; Xie, X.; Kim, M.S.; Kornyeyev, D.A.; Holaday, S.; Paré, P.W. Soil bacteria augment Arabidopsis photosynthesis by decreasing glucose sensing and abscisic acid levels in planta. Plant J. 2008, 56, 264–273. [Google Scholar] [CrossRef]
- Ryu, C.M.; Farag, M.A.; Hu, C.H.; Reddy, M.S.; Kloepper, J.W.; Paré, P.W. Bacterial volatiles induce systemic resistance in Arabidopsis. Plant Physiol. 2004, 134, 1017–1026. [Google Scholar] [CrossRef]

| Test | MHR24 | LPM1 |
|---|---|---|
| P solubilization | − | − |
| Zn solubilization | + | + |
| N fixation | + | + |
| Indole acetic acid | − | − |
| Siderophores | − | − |
| Ampicillin (30 μg/mL) | S | R |
| Kanamycin (30 μg/mL) | S | R |
| Nalidixic acid (30 μg/mL) | R | R |
| Streptomycin (30 μg/mL) | R | R |
| Tetracycline (30 μg/mL) | S | R |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Rodríguez, M.; Jasso-de Rodríguez, D.; Hernández-Castillo, F.D.; Moggio, I.; Arias, E.; Valenzuela-Soto, J.H.; Flores-Olivas, A. The Rhizobacterium Bacillus amyloliquefaciens MHR24 Has Biocontrol Ability Against Fungal Phytopathogens and Promotes Growth in Arabidopsis thaliana. Microorganisms 2024, 12, 2380. https://doi.org/10.3390/microorganisms12112380
Hernández-Rodríguez M, Jasso-de Rodríguez D, Hernández-Castillo FD, Moggio I, Arias E, Valenzuela-Soto JH, Flores-Olivas A. The Rhizobacterium Bacillus amyloliquefaciens MHR24 Has Biocontrol Ability Against Fungal Phytopathogens and Promotes Growth in Arabidopsis thaliana. Microorganisms. 2024; 12(11):2380. https://doi.org/10.3390/microorganisms12112380
Chicago/Turabian StyleHernández-Rodríguez, Mónica, Diana Jasso-de Rodríguez, Francisco Daniel Hernández-Castillo, Ivana Moggio, Eduardo Arias, José Humberto Valenzuela-Soto, and Alberto Flores-Olivas. 2024. "The Rhizobacterium Bacillus amyloliquefaciens MHR24 Has Biocontrol Ability Against Fungal Phytopathogens and Promotes Growth in Arabidopsis thaliana" Microorganisms 12, no. 11: 2380. https://doi.org/10.3390/microorganisms12112380
APA StyleHernández-Rodríguez, M., Jasso-de Rodríguez, D., Hernández-Castillo, F. D., Moggio, I., Arias, E., Valenzuela-Soto, J. H., & Flores-Olivas, A. (2024). The Rhizobacterium Bacillus amyloliquefaciens MHR24 Has Biocontrol Ability Against Fungal Phytopathogens and Promotes Growth in Arabidopsis thaliana. Microorganisms, 12(11), 2380. https://doi.org/10.3390/microorganisms12112380








