Molecular Identification of Anopheles (Diptera: Culicidae) Species in Native Communities of a Northeastern Region of Peru
Abstract
1. Background
2. Methods
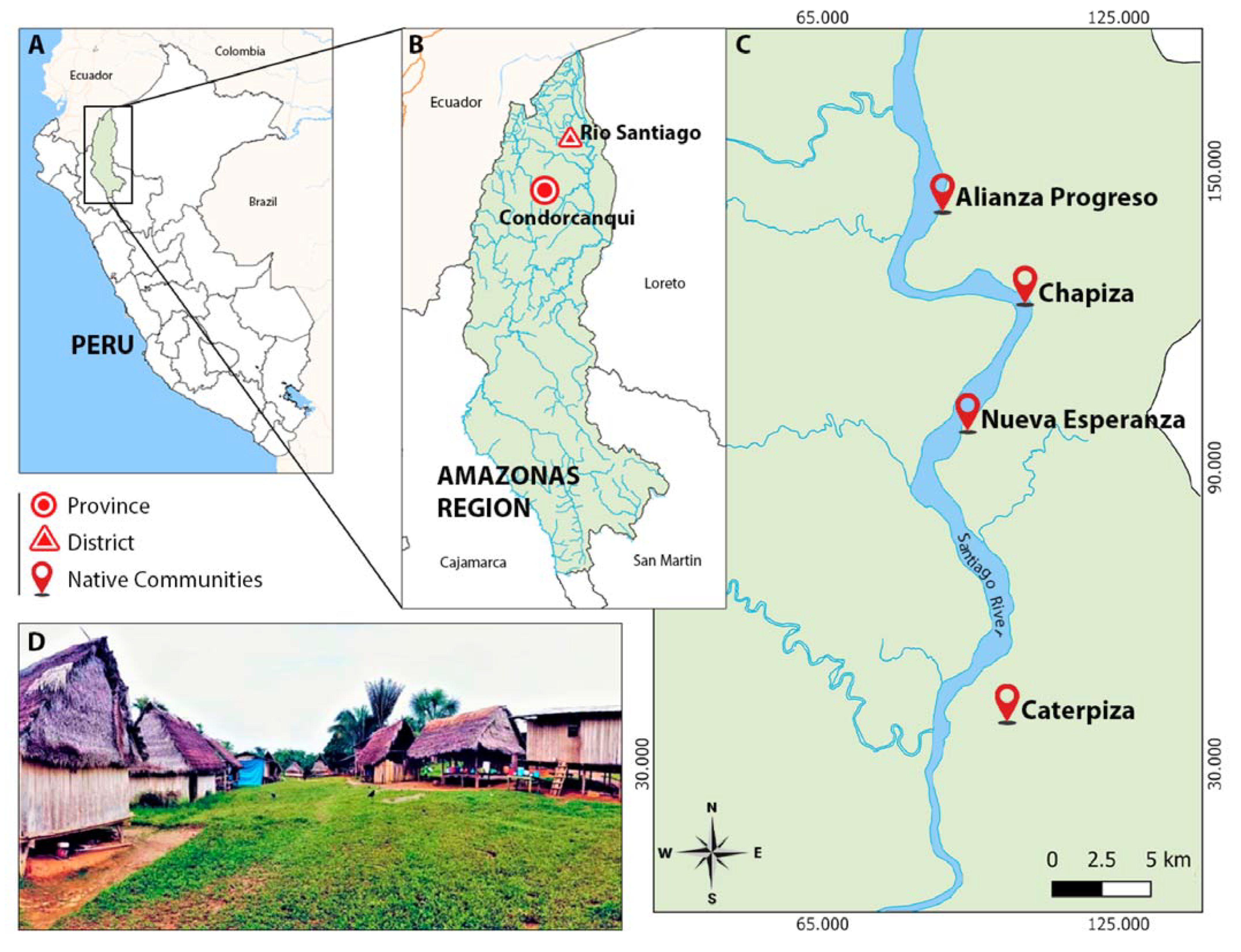
2.1. Study Site
2.2. Mosquito Collection
2.3. Molecular Methods for Anopheles Species Identification
2.4. Human Blood Meal Identification and Plasmodium Detection in Mosquitoes
2.5. Bioinformatic Analysis
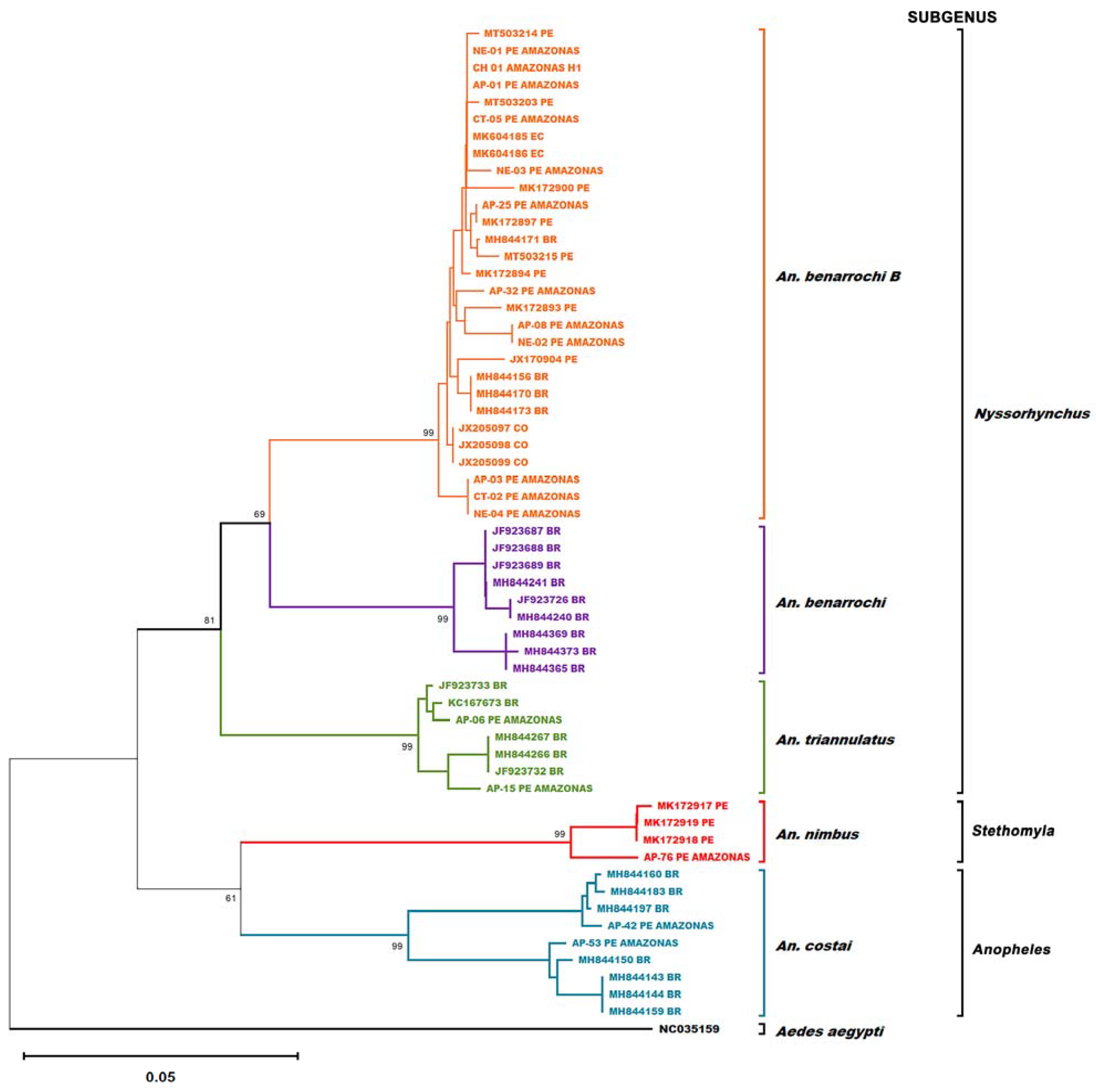
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO Malaria. Available online: https://www.who.int/health-topics/malaria (accessed on 1 August 2023).
- CDC-MINSA Boletines Epidemiológicos–CDC MINSA. Available online: https://www.dge.gob.pe/portalnuevo/publicaciones/boletines-epidemiologicos/ (accessed on 2 December 2024).
- CDC-MINSA Sala de situación nacional. CDC MINSA 2024. Available online: https://www.dge.gob.pe/portalnuevo/salas-situacionales/sala-de-situacion-nacional/ (accessed on 2 December 2024).
- Gobierno Regional de Amazonas Estudio de Diagnóstico y Zonificación Para El Tratamiento de La Demarcación Territorial de La Provincia Condorcanqui. Amazon. Dir. Nac. Técnica De Demarcación Territ. 2014, 1. Available online: https://cdn.www.gob.pe/uploads/document/file/2867803/EDZ%20UTCUBAMBA.pdf.pdf?v=1646328078 (accessed on 2 December 2024).
- Calderón, G.; Fernández, R.; Valle, J. Especies de La Fauna Anofelina, Su Distribución y Algunas Consideraciones Sobre Su Abundancia e Infectividad En El Perú. Rev. Peru. Entomol 1995, 8. Available online: https://sisbib.unmsm.edu.pe/BVrevistas/epidemiologia/v08_n1/articulo01.htm (accessed on 2 December 2024).
- Conn, J.E.; Bickersmith, S.A.; Saavedra, M.P.; Morales, J.A.; Alava, F.; Diaz Rodriguez, G.A.; del Aguila Morante, C.R.; Tong, C.G.; Alvarez-Antonio, C.; Daza Huanahui, J.M.; et al. Natural Infection of Nyssorhynchus darlingi and Nyssorhynchus benarrochi B with Plasmodium during the Dry Season in the Understudied Low-Transmission Setting of Datem Del Marañon Province, Amazonian Peru. Am. J. Trop. Med. Hyg. 2023, 109, 288–295. [Google Scholar] [CrossRef]
- Fernández, R.; Vera, H.; Calderón, G. Revisión Histórica de La Distribución de Anopheles (Nyssorhynchus) darlingi (Diptera: Culicidae) En La Amazonía Peruana. Rev. Peru. De Med. Exp. Y Salud Publica 2014, 31, 310–318. [Google Scholar] [CrossRef]
- Flores-Mendoza, C.; Fernández, R.; Escobedo-Vargas, K.S.; Vela-Perez, Q.; Schoeler, G.B. Natural Plasmodium Infections in Anopheles darlingi and Anopheles benarrochi (Diptera: Culicidae) from Eastern Peru. J. Med. Entomol. 2004, 41, 489–494. [Google Scholar] [CrossRef]
- INS. In Distribución de Los Principales Insectos Vectores de Enfermedades En El Perú; Ministerio de Salud: 2002. Available online: https://cdn.www.gob.pe/uploads/document/file/342522/Distribuci%C3%B3n_de_los_principales_insectos_vectores_de_enfermedades_en_el_Per%C3%BA20190716-19467-xdx28d.pdf?v=1563314426 (accessed on 3 December 2024).
- Calle, L.D.A.; Quiñones, M.L.; Erazo, H.F.; Jaramillo, O.N. Morphometric Discrimination of Females of Five Species of Anopheles of the Subgenus Nyssorhynchus from Southern and Northwest Colombia. Memórias Do Inst. Oswaldo Cruz 2002, 97, 1191–1195. [Google Scholar] [CrossRef] [PubMed]
- Estrada, D.A.; Quiñones, M.L.; Sierra, D.M.; Calle, D.A.; Ruiz, F.; Erazo, H.F.; Linton, Y.M. Utilidad de La Morfología de Los Huevos Como Un Método Indirecto Para Identificar Anopheles benarrochi Gabaldón, Cova García & López, Anopheles oswaldoi (Peryassu) y Anopheles rangeli Gabaldón. Biomédica 2003, 23, 388–395. [Google Scholar] [PubMed]
- Moreno, M.; Bickersmith, S.; Harlow, W.; Hildebrandt, J.; McKeon, S.N.; Silva-do-Nascimento, T.F.; Loaiza, J.R.; Ruiz, F.; Lourenço-de-Oliveira, R.; Sallum, M.A.; et al. Phylogeography of the Neotropical Anopheles triannulatus Complex (Diptera: Culicidae) Supports Deep Structure and Complex Patterns. Parasites Vectors 2013, 6, 47. [Google Scholar] [CrossRef]
- Quiñones, M.L.; Harbach, R.E.; Calle, D.A.; Ruiz, F.; Erazo, H.F.; Linton, Y.M. Variante Morfológica de Adultos Hembras de Anopheles benarrochi (Diptera: Culicidae) En Putumayo, Colombia. Biomédica 2001, 21, 351–359. [Google Scholar] [CrossRef][Green Version]
- Ruiz, F.; Quiñones, M.L.; Erazo, H.F.; Calle, D.A.; Alzate, J.F.; Linton, Y.-M. Molecular Differentiation of Anopheles (Nyssorhynchus) benarrochi and An. (N.) oswaldoi from Southern Colombia. Memórias Do Inst. Oswaldo Cruz 2005, 100, 155–160. [Google Scholar] [CrossRef]
- Matson, R.; Rios, C.T.; Chavez, C.B.; Gilman, R.H.; Florin, D.; Sifuentes, V.L.; Greffa, R.C.; Yori, P.P.; Fernandez, R.; Portocarrero, D.V.; et al. Improved Molecular Technique for the Differentiation of Neotropical Anopheline Species. Am. J. Trop. Med. Hyg. 2008, 78, 492–498. [Google Scholar] [CrossRef]
- Forattini, O.P. Culicidologia Médica. Identificação, Biología, Epidemiología; Universidad de Sao Paulo: São Paulo, Brasil, 2002; Volume 2. [Google Scholar]
- Folmer, O.; Black, M.; Wr, H.; Lutz, R.; Vrijenhoek, R. DNA Primers for Amplification of Mitochondrial Cytochrome C Oxidase Subunit I from Diverse Metazoan Invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar] [PubMed]
- Linton, Y.-M.; Pecor, J.E.; Porter, C.H.; Mitchell, L.B.; Garzón-Moreno, A.; Foley, D.H.; Pecor, D.B.; Wilkerson, R.C. Mosquitoes of Eastern Amazonian Ecuador: Biodiversity, Bionomics and Barcodes. Memórias Do Inst. Oswaldo Cruz 2013, 108 (Suppl. S1), 100–109. [Google Scholar] [CrossRef]
- Le Borgne, S.; Février, M.; Callebaut, C.; Lee, S.P.; Rivière, Y. CD8+-Cell Antiviral Factor Activity Is Not Restricted to Human Immunodeficiency Virus (HIV)-Specific T Cells and Can Block HIV Replication after Initiation of Reverse Transcription. J. Virol. 2000, 74, 4456–4464. [Google Scholar] [CrossRef]
- Singh, B.; Bobogare, A.; Cox-Singh, J.; Snounou, G.; Abdullah, M.S.; Rahman, H.A. A Genus- and Species-Specific Nested Polymerase Chain Reaction Malaria Detection Assay for Epidemiologic Studies. Am. J. Trop. Med. Hyg. 1999, 60, 687–692. [Google Scholar] [CrossRef]
- Chan-Chable, R.J.; Martínez-Arce, A.; Mis-Avila, P.C.; Ortega-Morales, A.I. DNA Barcodes and Evidence of Cryptic Diversity of Anthropophagous Mosquitoes in Quintana Roo, Mexico. Ecol. Evol. 2019, 9, 4692–4705. [Google Scholar] [CrossRef]
- Foster, P.G.; Bergo, E.S.; Bourke, B.P.; Oliveira, T.M.P.; Nagaki, S.S.; Sant’Ana, D.C.; Sallum, M.A.M. Phylogenetic Analysis and DNA-Based Species Confirmation in Anopheles (Nyssorhynchus). PLoS ONE 2013, 8, e54063. [Google Scholar] [CrossRef] [PubMed]
- McKeon, S.N.; Moreno, M.; Sallum, M.A.; Povoa, M.M.; Conn, J.E. Distinct Population Structure for Co-Occurring Anopheles goeldii and Anopheles triannulatus in Amazonian Brazil. Memórias Do Inst. Oswaldo Cruz 2013, 108, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Morales Viteri, D.; Herrera-Varela, M.; Albuja, M.; Quiroga, C.; Diaz, G.; del Aguila Morante, C.; Ramirez, D.; Vinetz, J.M.; Bickersmith, S.A.; Conn, J.E. New Records of Anopheles benarrochi B (Diptera: Culicidae) in Malaria Hotspots in the Amazon Regions of Ecuador and Peru. J. Med. Entomol. 2021, 58, 1234–1240. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Sallum, M.A.M.; Wilkerson, R.C.; Forattini, O.P. Taxonomic Study of Species Formerly Identified as Anopheles mediopunctatus and Resurrection of An. costai (Diptera: Culicidae). J. Med. Entomol. 1999, 36, 282–300. [Google Scholar] [CrossRef] [PubMed]
- Bourke, B.P.; Conn, J.E.; de Oliveira, T.M.P.; Chaves, L.S.M.; Bergo, E.S.; Laporta, G.Z.; Sallum, M.A.M. Exploring Malaria Vector Diversity on the Amazon Frontier. Malar. J. 2018, 17, 342. [Google Scholar] [CrossRef] [PubMed]
- Montenegro, C.C.; Bustamante-Chauca, T.P.; Pajuelo Reyes, C.; Bernal, M.; Gonzales, L.; Tapia-Limonchi, R.; Tejedo, J.R.; Chenet, S.M. Plasmodium falciparum Outbreak in Native Communities of Condorcanqui, Amazonas, Perú. Malar. J. 2021, 20, 88. [Google Scholar] [CrossRef]
- Fernández, L.R.; Schoeler, G.; Stancil, J. Presencia de Anopheles (Nyssorhynchus) benarrochi en Áreas de Selva Con Transmisión Malárica. Rev. Peru. De Med. Exp. Y Salud Publica 2004, 21, 217–222. [Google Scholar]
- Schoeler, G.B.; Flores-Mendoza, C.; Fernández, R.; Davila, J.R.; Zyzak, M. Geographical Distribution of Anopheles darlingi in the Amazon Basin Region of Peru. J. Am. Mosq. Control Assoc. 2003, 19, 286–296. [Google Scholar]
- Conn, J.E.; Moreno, M.; Saavedra, M.; Bickersmith, S.A.; Knoll, E.; Fernandez, R.; Vera, H.; Burrus, R.G.; Lescano, A.G.; Sanchez, J.F.; et al. Molecular Taxonomy of Anopheles (Nyssorhynchus) benarrochi (Diptera: Culicidae) and Malaria Epidemiology in Southern Amazonian Peru. Am. J. Trop. Med. Hyg. 2013, 88, 319–324. [Google Scholar] [CrossRef][Green Version]
- Moreno, M.; Saavedra, M.P.; Bickersmith, S.A.; Lainhart, W.; Tong, C.; Alava, F.; Vinetz, J.M.; Conn, J.E. Implications for Changes in Anopheles darlingi Biting Behaviour in Three Communities in the Peri-Iquitos Region of Amazonian Peru. Malar. J. 2015, 14, 290. [Google Scholar] [CrossRef] [PubMed]
- de Arruda, M.; Carvalho, M.B.; Nussenzweig, R.S.; Maracic, M.; Ferreira, A.W.; Cochrane, A.H. Potential Vectors of Malaria and Their Different Susceptibility to Plasmodium falciparum and Plasmodium vivax in Northern Brazil Identified by Immunoassay. Am. J. Trop. Med. Hyg. 1986, 5, 873–881. [Google Scholar] [CrossRef]
- Carlos, B.C.; Rona, L.D.P.; Christophides, G.K.; Souza-Neto, J.A. A Comprehensive Analysis of Malaria Transmission in Brazil. Pathog. Glob. Health 2019, 113, 1–13. [Google Scholar] [CrossRef]
- de Oliveira-Ferreira, J.; Lourenço-de-Oliveira, R.; Teva, A.; Deane, L.M.; Daniel-Ribeiro, C.T. Natural Malaria Infections in Anophelines in Rondonia State, Brazilian Amazon. Am. J. Trop. Med. Hyg. 1990, 43, 6–10. [Google Scholar] [CrossRef]
- Aramburú Guarda, J.; Ramal Asayag, C.; Witzig, R. Malaria Reemergence in the Peruvian Amazon Region. Emerg. Infect. Dis. 1999, 5, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Rosero, D.A.; Naranjo-Diaz, N.; Alvarez, N.; Cienfuegos, A.V.; Torres, C.; Luckhart, S.; Correa, M.M. Colombian Anopheles triannulatus (Diptera: Culicidae) Naturally Infected with Plasmodium spp. Int. Sch. Res. Not. 2013, 2013, 927453. [Google Scholar] [CrossRef]
- Berti, J.; Estrada, Y.; Guzmán, H.; Arias, L. Primer registro de Anopheles (Stethomyia) nimbus (Theobald, 1902) para el estado Bolívar, Venezuela. Boletín De Malariol. Y Salud Ambient. 2014, 54, 254–256. [Google Scholar]
- Natal, D.; Urbinatti, P.R.; Malafronte, R.d.S.; Rezende, H.R.; Cerutti, C., Jr.; Sallum, M.A.M. First Record of Anopheles (Anopheles) costai Fonseca & Ramos, 1939 in Espírito Santo State, Brazil. Rev. Do Inst. De Med. Trop. De São Paulo 2007, 49, 323–326. [Google Scholar] [CrossRef]
| Site | Date of Collection | UTM X | UTM Y | T° (°C) | RH (%) | No. | Cox1 Id. |
|---|---|---|---|---|---|---|---|
| NE | March 2022 | 196,280.9 | 9,581,345.6 | 27 | 75 | 5 | An. benarrochi B |
| CH | March 2022 | 199,060.7 | 9,587,806.4 | 27 | 87 | 1 | An. benarrochi B |
| AP | March and May 2022 | 195,051.2 | 9,592,391.3 | 26 | 75 | 50 | An. benarrochi B |
| March 2022 | 26 | 75 | 2 | An. triannulatus | |||
| May 2022 | 25.3 | 87 | 2 | An. costai | |||
| May 2022 | 25.3 | 87 | 1 | An. nimbus | |||
| CT | September 2022 | 198,182.6 | 9,566,709 | 25.3 | 87 | 5 | An. benarrochi B |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montalvo-Sabino, E.; Villegas-Pingo, M.; Zumaeta, J.; Gonzales, L.; Tapia-Limonchi, R.; Moreno, M.; González, C.R.; Chenet, S.M. Molecular Identification of Anopheles (Diptera: Culicidae) Species in Native Communities of a Northeastern Region of Peru. Microorganisms 2025, 13, 861. https://doi.org/10.3390/microorganisms13040861
Montalvo-Sabino E, Villegas-Pingo M, Zumaeta J, Gonzales L, Tapia-Limonchi R, Moreno M, González CR, Chenet SM. Molecular Identification of Anopheles (Diptera: Culicidae) Species in Native Communities of a Northeastern Region of Peru. Microorganisms. 2025; 13(4):861. https://doi.org/10.3390/microorganisms13040861
Chicago/Turabian StyleMontalvo-Sabino, Eddyson, Marianella Villegas-Pingo, Jhon Zumaeta, Lizandro Gonzales, Rafael Tapia-Limonchi, Marta Moreno, Christian R. González, and Stella M. Chenet. 2025. "Molecular Identification of Anopheles (Diptera: Culicidae) Species in Native Communities of a Northeastern Region of Peru" Microorganisms 13, no. 4: 861. https://doi.org/10.3390/microorganisms13040861
APA StyleMontalvo-Sabino, E., Villegas-Pingo, M., Zumaeta, J., Gonzales, L., Tapia-Limonchi, R., Moreno, M., González, C. R., & Chenet, S. M. (2025). Molecular Identification of Anopheles (Diptera: Culicidae) Species in Native Communities of a Northeastern Region of Peru. Microorganisms, 13(4), 861. https://doi.org/10.3390/microorganisms13040861






