Regulation of Ergosterol Biosynthesis in Pathogenic Fungi: Opportunities for Therapeutic Development
Abstract
1. Introduction
2. Methodology: Systematic Literature Review Framework
2.1. Data Sources and Search Strategy
2.2. Inclusion and Exclusion Criteria
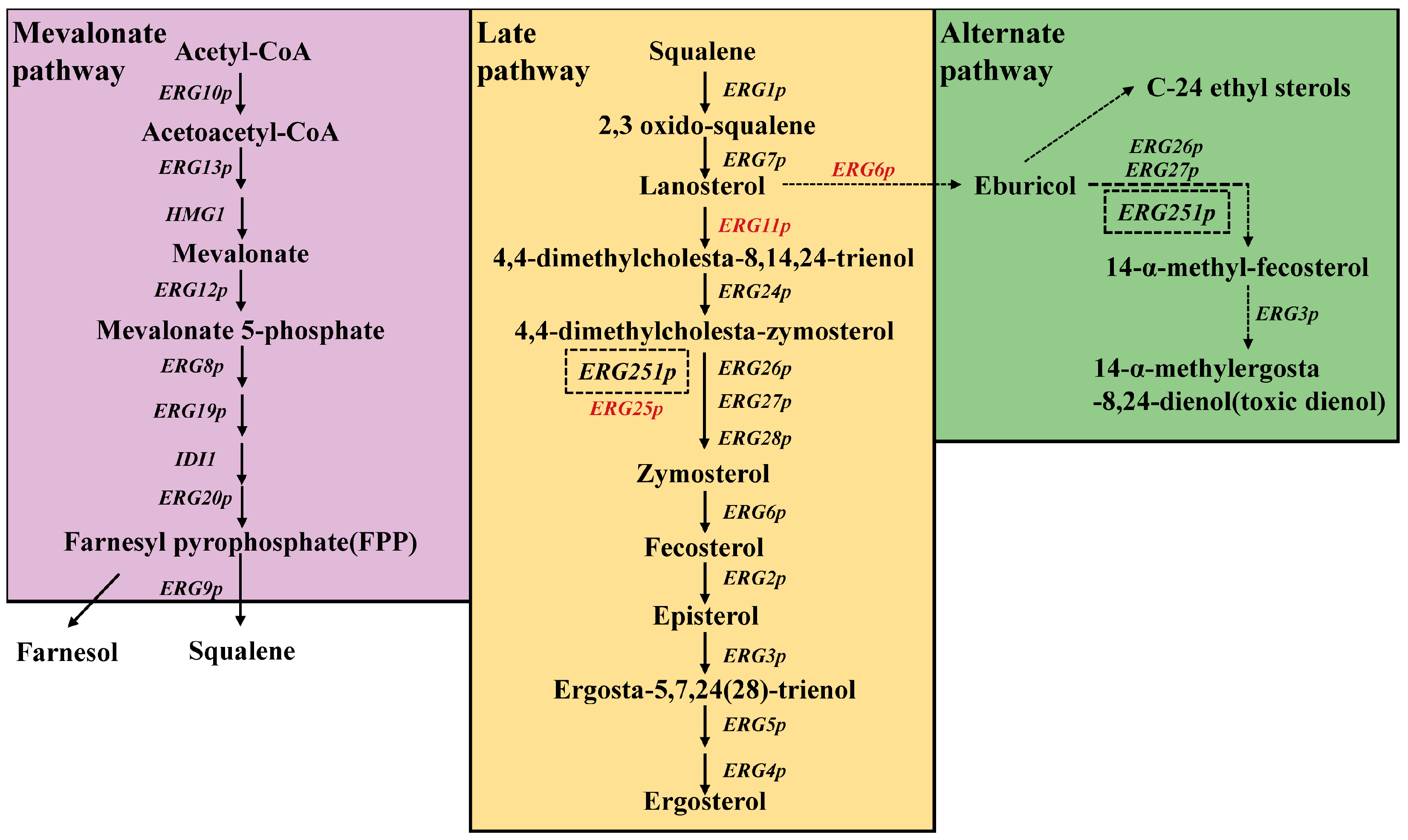
3. Mechanistic Insights and Functional Implications of Ergosterol Biosynthetic Pathway in Pathogenic Fungi
4. Regulation of Ergosterol Biosynthesis in Pathogenic Fungi
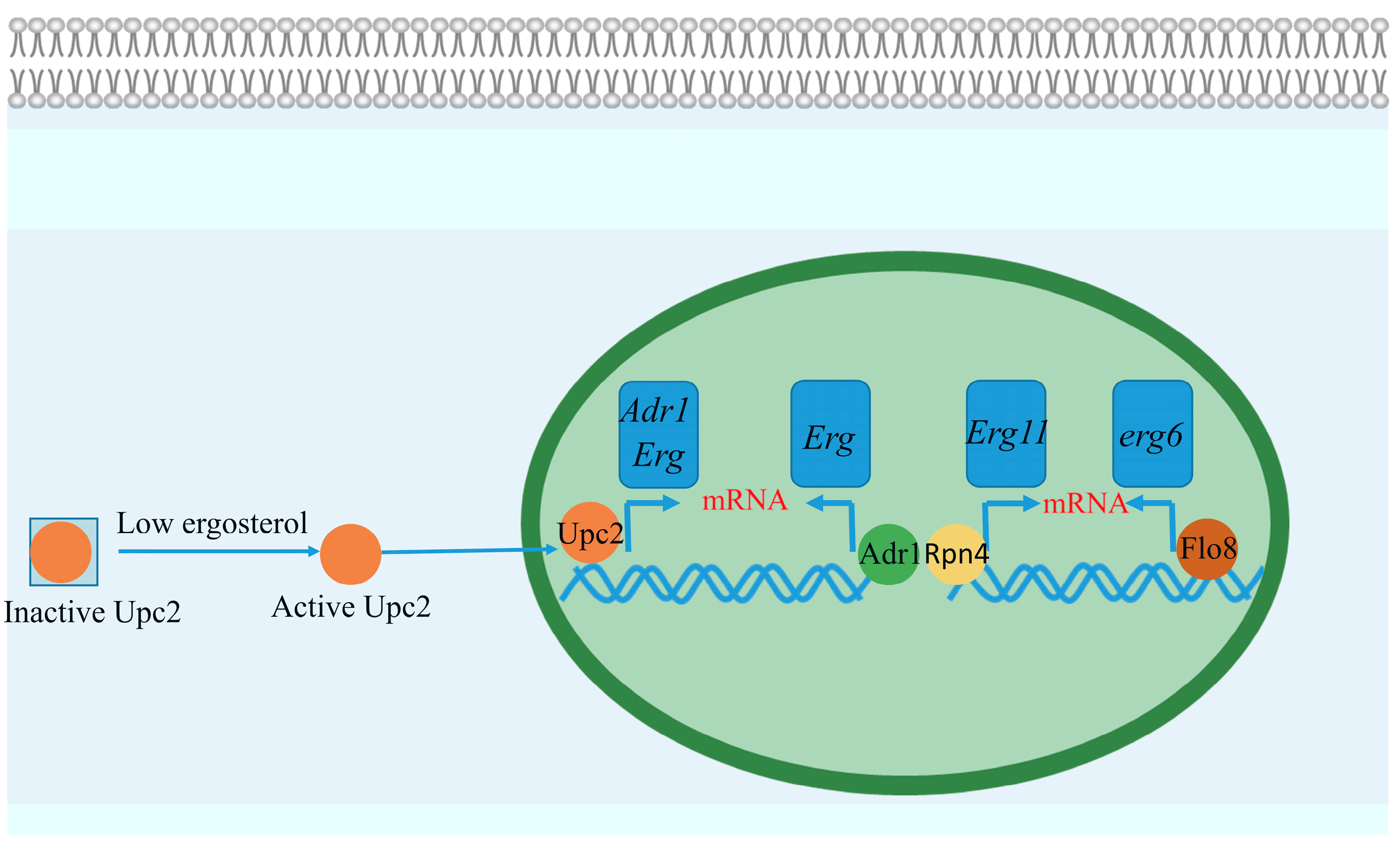
4.1. Transcriptional Regulation of Ergosterol Biosynthesis
4.1.1. Transcriptional Network in A. fumigatus
Core Regulatory Axis: SrbA-AtrR Synergy
Dynamic Repressive Networks
Beyond the 34 bp Hotspot: SltA as a Novel Master Regulator
4.1.2. Spatial Hierarchy of Transcription Factor Binding Sites in erg11A/cyp51A
Regulation
4.1.3. Azole Resistance Under Coordinated Regulation of Ergosterol Pathway Remodeling and Drug Efflux
4.1.4. Evolutionary Innovations in Ergosterol Transcriptional Networks Across Candida Species
4.2. Redox-Driven Regulation of Cyp51/Erg11p: Evolutionary Innovations in Electron Flux Management
4.2.1. Electron Donor Hierarchy and Clinical Resistance
4.2.2. Heme-Mediated Stability Control
5. The Ergosterol Biosynthesis Pathway: Innovations in Antifungal Target Discovery and Therapeutic Development
5.1. Reimagining Cyp51/Erg11p Inhibition: Beyond Traditional Azoles
5.2. Dual-Target Strategies: Synergistic Mechanisms to Combat Resistance
5.3. Unlocking Underexplored Targets: Erg6p and Early-Pathway Enzymes
5.4. Direct Ergosterol Targeting and Natural Product Exploration
5.5. Nanoparticle-Based Targeted Delivery Systems: Novel Antifungal Strategies Centered on Ergosterol
6. Identified Research Gaps and Future Directions
6.1. Mechanistic and Functional Gaps in Ergosterol Pathway Components
6.2. Regulatory Network Complexity
6.3. Therapeutic Strategy Limitations
7. Conclusions
7.1. Reinvigorating Azoles Through Synergistic Mechanisms
7.2. Targeting Fungal Virulence and Host Adaptation
7.3. Harnessing Regulatory Networks for Precision Targeting
7.4. Integrating Omics and Computational Tools for Next-Generation Inhibitors
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lass-Flörl, C.; Dietl, A.M.; Kontoyiannis, D.P.; Brock, M. Aspergillus terreus Species Complex. Clin. Microbiol. Rev. 2021, 34, e0031120. [Google Scholar]
- Chowdhary, A.; Jain, K.; Chauhan, N. Candida auris Genetics and Emergence. Annu. Rev. Microbiol. 2023, 77, 583–602. [Google Scholar] [PubMed]
- Paiva, J.A.; Pereira, J.M. Treatment of invasive candidiasis in the era of Candida resistance. Curr. Opin. Crit. Care 2023, 29, 457–462. [Google Scholar] [PubMed]
- May, R.C.; Stone, N.R.; Wiesner, D.L.; Bicanic, T.; Nielsen, K. Cryptococcus: From environmental saprophyte to global pathogen. Nat. Rev. Microbiol. 2016, 14, 106–117. [Google Scholar]
- Steenwyk, J.L.; Lind, A.L.; Ries, L.N.A.; Dos Reis, T.F.; Silva, L.P.; Almeida, F.; Bastos, R.W.; Fraga da Silva, T.F.C.; Bonato, V.L.D.; Pessoni, A.M.; et al. Pathogenic Allodiploid Hybrids of Aspergillus Fungi. Curr. Biol. 2020, 30, 2495–2507.e7. [Google Scholar]
- Huang, S.J.; Lv, G.; Song, Y.H.; Zhao, J.T.; Liu, J.Y.; Wang, L.L.; Xiang, M.J. Antifungal susceptibility, molecular epidemiology, and clinical risk factors of Candida glabrata in intensive care unit in a Chinese Tertiary Hospital. Front. Cell. Infect. Microbiol. 2024, 14, 1455145. [Google Scholar]
- Perlin, D.S.; Rautemaa-Richardson, R.; Alastruey-Izquierdo, A. The global problem of antifungal resistance: Prevalence, mechanisms, and management. The Lancet. Infect. Dis. 2017, 17, e383–e392. [Google Scholar]
- Gupta, A.K.; Venkataraman, M.; Renaud, H.J.; Summerbell, R.; Shear, N.H.; Piguet, V. The increasing problem of treatment-resistant fungal infections: A call for antifungal stewardship programs. Int. J. Dermatol. 2021, 60, e474–e479. [Google Scholar]
- Czajka, K.M.; Venkataraman, K.; Brabant-Kirwan, D.; Santi, S.A.; Verschoor, C.; Appanna, V.D.; Singh, R.; Saunders, D.P.; Tharmalingam, S. Molecular Mechanisms Associated with Antifungal Resistance in Pathogenic Candida Species. Cells 2023, 12, 2655. [Google Scholar] [CrossRef]
- Kang, Y.; Li, Q.; Yao, Y.; Xu, C.; Qiu, Z.; Jia, W.; Li, G.; Wang, P. Epidemiology and Azole Resistance of Clinical Isolates of Aspergillus fumigatus from a Large Tertiary Hospital in Ningxia, China. Infect. Drug Resist. 2024, 17, 427–439. [Google Scholar]
- Morrison, V.A. The role of caspofungin and the echinocandins in the antifungal armamentarium. Curr. Opin. Investig. Drugs 2002, 3, 1432–1436. [Google Scholar] [PubMed]
- Lockhart, S.R.; Chowdhary, A.; Gold, J.A.W. The rapid emergence of antifungal-resistant human-pathogenic fungi. Nat. Rev. Microbiol. 2023, 21, 818–832. [Google Scholar] [CrossRef] [PubMed]
- Jordá, T.; Puig, S. Regulation of Ergosterol Biosynthesis in Saccharomyces cerevisiae. Genes 2020, 11, 795. [Google Scholar] [CrossRef] [PubMed]
- Dupont, S.; Beney, L.; Ferreira, T.; Gervais, P. Nature of sterols affects plasma membrane behavior and yeast survival during dehydration. Biochim. Biophys. Acta 2011, 1808, 1520–1528. [Google Scholar] [CrossRef]
- Liu, J.; Nes, W.D. Steroidal triterpenes: Design of substrate-based inhibitors of ergosterol and sitosterol synthesis. Molecules 2009, 14, 4690–4706. [Google Scholar] [CrossRef]
- Krauß, J.; Müller, C.; Klimt, M.; Valero, L.J.; Martínez, J.F.; Müller, M.; Bartel, K.; Binder, U.; Bracher, F. Synthesis, Biological Evaluation, and Structure-Activity Relationships of 4-Aminopiperidines as Novel Antifungal Agents Targeting Ergosterol Biosynthesis. Molecules 2021, 26, 7208. [Google Scholar] [CrossRef]
- Cruz, A.; Sánchez-Hernández, E.; Teixeira, A.; Oliveira, R.; Cunha, A.; Martín-Ramos, P. Phytoconstituents and Ergosterol Biosynthesis-Targeting Antimicrobial Activity of Nutmeg (Myristica fragans Houtt.) against Phytopathogens. Molecules 2024, 29, 471. [Google Scholar] [CrossRef]
- Liu, J.F.; Xia, J.J.; Nie, K.L.; Wang, F.; Deng, L. Outline of the biosynthesis and regulation of ergosterol in yeast. World J. Microbiol. Biotechnol. 2019, 35, 98. [Google Scholar] [CrossRef]
- Jiang, Y.Q.; Lin, J.P. Recent progress in strategies for steroid production in yeasts. World J. Microbiol. Biotechnol. 2022, 38, 93. [Google Scholar] [CrossRef]
- Lu, H.; Li, W.; Whiteway, M.; Wang, H.; Zhu, S.; Ji, Z.; Feng, Y.; Yan, L.; Fang, T.; Li, L.; et al. A Small Molecule Inhibitor of Erg251 Makes Fluconazole Fungicidal by Inhibiting the Synthesis of the 14α-Methylsterols. Mbio 2023, 14, e0263922. [Google Scholar] [CrossRef]
- Blosser, S.J.; Merriman, B.; Grahl, N.; Chung, D.; Cramer, R.A. Two C4-sterol methyl oxidases (Erg25) catalyse ergosterol intermediate demethylation and impact environmental stress adaptation in Aspergillus fumigatus. Microbiology 2014, 160, 2492–2506. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Esquivel, B.D.; White, T.C. Overexpression or Deletion of Ergosterol Biosynthesis Genes Alters Doubling Time, Response to Stress Agents, and Drug Susceptibility in Saccharomyces cerevisiae. Mbio 2018, 9, e01291-18. [Google Scholar] [PubMed]
- Batliner, M.; Schumacher, F.; Wigger, D.; Vivas, W.; Prell, A.; Fohmann, I.; Köhler, T.; Schempp, R.; Riedel, A.; Vaeth, M.; et al. The Candida albicans quorum-sensing molecule farnesol alters sphingolipid metabolism in human monocyte-derived dendritic cells. Mbio 2024, 15, e0073224. [Google Scholar]
- Elsaman, H.; Golubtsov, E.; Brazil, S.; Ng, N.; Klugherz, I.; Martin, R.; Dichtl, K.; Müller, C.; Wagener, J. Toxic eburicol accumulation drives the antifungal activity of azoles against Aspergillus fumigatus. Nat. Commun. 2024, 15, 6312. [Google Scholar]
- Vandeputte, P.; Tronchin, G.; Larcher, G.; Ernoult, E.; Bergès, T.; Chabasse, D.; Bouchara, J.P. A nonsense mutation in the ERG6 gene leads to reduced susceptibility to polyenes in a clinical isolate of Candida glabrata. Antimicrob. Agents Chemother. 2008, 52, 3701–3709. [Google Scholar]
- Young, L.Y.; Hull, C.M.; Heitman, J. Disruption of ergosterol biosynthesis confers resistance to amphotericin B in Candida lusitaniae. Antimicrob. Agents Chemother. 2003, 47, 2717–2724. [Google Scholar]
- Borecká-Melkusová, S.; Moran, G.P.; Sullivan, D.J.; Kucharíková, S.; Chorvát, D., Jr.; Bujdáková, H. The expression of genes involved in the ergosterol biosynthesis pathway in Candida albicans and Candida dubliniensis biofilms exposed to fluconazole. Mycoses 2009, 52, 118–128. [Google Scholar]
- Regan, J.; DeJarnette, C.; Luna-Tapia, A.; Parker, J.E.; Reitler, P.; Barnett, S.; Tucker, K.M.; Kelly, S.L.; Palmer, G.E. Titration of C-5 Sterol Desaturase Activity Reveals Its Relationship to Candida albicans Virulence and Antifungal Susceptibility Is Dependent upon Host Immune Status. Mbio 2022, 13, e0011522. [Google Scholar]
- Scott, N.E.; Edwin Erayil, S.; Kline, S.E.; Selmecki, A. Rapid Evolution of Multidrug Resistance in a Candida lusitaniae Infection during Micafungin Monotherapy. Antimicrob. Agents Chemother. 2023, 67, e0054323. [Google Scholar]
- Branco, J.; Ola, M.; Silva, R.M.; Fonseca, E.; Gomes, N.C.; Martins-Cruz, C.; Silva, A.P.; Silva-Dias, A.; Pina-Vaz, C.; Erraught, C.; et al. Impact of ERG3 mutations and expression of ergosterol genes controlled by UPC2 and NDT80 in Candida parapsilosis azole resistance. Clin. Microbiol. Infect. 2017, 23, 575.e1–575.e8. [Google Scholar]
- Kim, S.H.; Steere, L.; Zhang, Y.K.; McGregor, C.; Hahne, C.; Zhou, Y.; Liu, C.; Cai, Y.; Zhou, H.; Chen, X.; et al. Inhibiting C-4 Methyl Sterol Oxidase with Novel Diazaborines to Target Fungal Plant Pathogens. ACS Chem. Biol. 2022, 17, 1343–1350. [Google Scholar] [PubMed]
- Okamoto, M.; Takahashi-Nakaguchi, A.; Tejima, K.; Sasamoto, K.; Yamaguchi, M.; Aoyama, T.; Nagi, M.; Tanabe, K.; Miyazaki, Y.; Nakayama, H.; et al. Erg25 Controls Host-Cholesterol Uptake Mediated by Aus1p-Associated Sterol-Rich Membrane Domains in Candida glabrata. Front. Cell Dev. Biol. 2022, 10, 820675. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Hilk, A.; Solis, N.V.; Pereira De Sa, N.; Hogan, B.M.; Bierbaum, T.A.; Del Poeta, M.; Filler, S.G.; Burrack, L.S.; Selmecki, A. Erg251 has complex and pleiotropic effects on sterol composition, azole susceptibility, filamentation, and stress response phenotypes. PLoS Pathog. 2024, 20, e1012389. [Google Scholar]
- Gao, J.; Wang, H.; Li, Z.; Wong, A.H.; Wang, Y.Z.; Guo, Y.; Lin, X.; Zeng, G.; Liu, H.; Wang, Y.; et al. Candida albicans gains azole resistance by altering sphingolipid composition. Nat. Commun. 2018, 9, 4495. [Google Scholar]
- Alcazar-Fuoli, L.; Mellado, E. Ergosterol biosynthesis in Aspergillus fumigatus: Its relevance as an antifungal target and role in antifungal drug resistance. Front. Microbiol. 2012, 3, 439. [Google Scholar]
- Furukawa, T.; van Rhijn, N.; Fraczek, M.; Gsaller, F.; Davies, E.; Carr, P.; Gago, S.; Fortune-Grant, R.; Rahman, S.; Gilsenan, J.M.; et al. The negative cofactor 2 complex is a key regulator of drug resistance in Aspergillus fumigatus. Nat. Commun. 2020, 11, 427. [Google Scholar]
- Du, W.; Zhai, P.; Wang, T.; Bromley, M.J.; Zhang, Y.; Lu, L. The C2H2 Transcription Factor SltA Contributes to Azole Resistance by Coregulating the Expression of the Drug Target Erg11A and the Drug Efflux Pump Mdr1 in Aspergillus fumigatus. Antimicrob. Agents Chemother. 2021, 65, e01839-20. [Google Scholar]
- Gsaller, F.; Hortschansky, P.; Furukawa, T.; Carr, P.D.; Rash, B.; Capilla, J.; Müller, C.; Bracher, F.; Bowyer, P.; Haas, H.; et al. Sterol Biosynthesis and Azole Tolerance Is Governed by the Opposing Actions of SrbA and the CCAAT Binding Complex. PLoS Pathog. 2016, 12, e1005775. [Google Scholar]
- Hagiwara, D.; Miura, D.; Shimizu, K.; Paul, S.; Ohba, A.; Gonoi, T.; Watanabe, A.; Kamei, K.; Shintani, T.; Moye-Rowley, W.S.; et al. A Novel Zn2-Cys6 Transcription Factor AtrR Plays a Key Role in an Azole Resistance Mechanism of Aspergillus fumigatus by Co-regulating cyp51A and cdr1B Expressions. PLoS Pathog. 2017, 13, e1006096. [Google Scholar]
- Paul, S.; Diekema, D.; Moye-Rowley, W.S. Contributions of both ATP-Binding Cassette Transporter and Cyp51A Proteins Are Essential for Azole Resistance in Aspergillus fumigatus. Antimicrob. Agents Chemother. 2017, 61, e02748-16. [Google Scholar]
- Zhang, C.; Gao, L.; Ren, Y.; Gu, H.; Zhang, Y.; Lu, L. The CCAAT-binding complex mediates azole susceptibility of Aspergillus fumigatus by suppressing SrbA expression and cleavage. MicrobiologyOpen 2021, 10, e1249. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Verweij, P.E.; Melchers, W.J.G.; Moye-Rowley, W.S. Differential Functions of Individual Transcription Factor Binding Sites in the Tandem Repeats Found in Clinically Relevant cyp51A Promoters in Aspergillus fumigatus. Mbio 2022, 13, e0070222. [Google Scholar] [CrossRef] [PubMed]
- Porter, J.R.; Burg, J.S.; Espenshade, P.J.; Iglesias, P.A. Ergosterol regulates sterol regulatory element binding protein (SREBP) cleavage in fission yeast. J. Biol. Chem. 2010, 285, 41051–41061. [Google Scholar] [CrossRef]
- Kühbacher, A.; Peiffer, M.; Hortschansky, P.; Merschak, P.; Bromley, M.J.; Haas, H.; Brakhage, A.A.; Gsaller, F. Azole Resistance-Associated Regulatory Motifs within the Promoter of cyp51A in Aspergillus fumigatus. Microbiol. Spectr. 2022, 10, e0120922. [Google Scholar] [CrossRef]
- Chung, D.; Barker, B.M.; Carey, C.C.; Merriman, B.; Werner, E.R.; Lechner, B.E.; Dhingra, S.; Cheng, C.; Xu, W.; Blosser, S.J.; et al. ChIP-seq and in vivo transcriptome analyses of the Aspergillus fumigatus SREBP SrbA reveals a new regulator of the fungal hypoxia response and virulence. PLoS Pathog. 2014, 10, e1004487. [Google Scholar] [CrossRef]
- Hortschansky, P.; Misslinger, M.; Mörl, J.; Gsaller, F.; Bromley, M.J.; Brakhage, A.A.; Groll, M.; Haas, H.; Huber, E.M. Structural basis of HapEP88L-linked antifungal triazole resistance in Aspergillus fumigatus. Life Sci. Alliance 2020, 3, e202000729. [Google Scholar] [CrossRef]
- Souza, A.C.O.; Ge, W.; Wiederhold, N.P.; Rybak, J.M.; Fortwendel, J.R.; Rogers, P.D. hapE and hmg1 Mutations Are Drivers of cyp51A-Independent Pan-Triazole Resistance in an Aspergillus fumigatus Clinical Isolate. Microbiol. Spectr. 2023, 11, e0518822. [Google Scholar] [CrossRef]
- Huber, E.M.; Hortschansky, P.; Scheven, M.T.; Misslinger, M.; Haas, H.; Brakhage, A.A.; Groll, M. Structural insights into cooperative DNA recognition by the CCAAT-binding complex and its bZIP transcription factor HapX. Structure 2022, 30, 934–946.e4. [Google Scholar] [CrossRef]
- Furukawa, T.; Scheven, M.T.; Misslinger, M.; Zhao, C.; Hoefgen, S.; Gsaller, F.; Lau, J.; Jöchl, C.; Donaldson, I.; Valiante, V.; et al. The fungal CCAAT-binding complex and HapX display highly variable but evolutionary conserved synergetic promoter-specific DNA recognition. Nucleic Acids Res. 2020, 48, 3567–3590. [Google Scholar] [CrossRef]
- Pontes, L.; Arai, T.; Gualtieri Beraquet, C.A.; Giordano, A.; Reichert-Lima, F.; da Luz, E.A.; Fernanda de Sá, C.; Ortolan Levy, L.; Tararam, C.A.; Watanabe, A.; et al. Uncovering a Novel cyp51A Mutation and Antifungal Resistance in Aspergillus fumigatus through Culture Collection Screening. J. Fungi 2024, 10, 122. [Google Scholar] [CrossRef]
- van Dijk, M.A.M.; Buil, J.B.; Tehupeiory-Kooreman, M.; Broekhuizen, M.J.; Broens, E.M.; Wagenaar, J.A.; Verweij, P.E. Azole Resistance in Veterinary Clinical Aspergillus fumigatus Isolates in the Netherlands. Mycopathologia 2024, 189, 50. [Google Scholar] [PubMed]
- Lavergne, R.A.; Albassier, M.; Hardouin, J.B.; Alvarez-Moreno, C.; Pagniez, F.; Morio, F.; Le Pape, P.; Ourliac-Garnier, I. Impact of TR(34)/L98H, TR(46)/Y121F/T289A and TR(53) Alterations in Azole-Resistant Aspergillus fumigatus on Sterol Composition and Modifications after In Vitro Exposure to Itraconazole and Voriconazole. Microorganisms 2022, 10, 104. [Google Scholar] [CrossRef] [PubMed]
- Vasicek, E.M.; Berkow, E.L.; Flowers, S.A.; Barker, K.S.; Rogers, P.D. UPC2 is universally essential for azole antifungal resistance in Candida albicans. Eukaryot. Cell 2014, 13, 933–946. [Google Scholar]
- Li, J.; Aubry, L.; Brandalise, D.; Coste, A.T.; Sanglard, D.; Lamoth, F. Upc2-mediated mechanisms of azole resistance in Candida auris. Microbiol. Spectr. 2024, 12, e0352623. [Google Scholar] [CrossRef]
- Vu, B.G.; Stamnes, M.A.; Li, Y.; Rogers, P.D.; Moye-Rowley, W.S. The Candida glabrata Upc2A transcription factor is a global regulator of antifungal drug resistance pathways. PLoS Genet. 2021, 17, e1009582. [Google Scholar] [CrossRef]
- Hoot, S.J.; Smith, A.R.; Brown, R.P.; White, T.C. An A643V amino acid substitution in Upc2p contributes to azole resistance in well-characterized clinical isolates of Candida albicans. Antimicrob. Agents Chemother. 2011, 55, 940–942. [Google Scholar]
- Shrivastava, M.; Kouyoumdjian, G.S.; Kirbizakis, E.; Ruiz, D.; Henry, M.; Vincent, A.T.; Sellam, A.; Whiteway, M. The Adr1 transcription factor directs regulation of the ergosterol pathway and azole resistance in Candida albicans. Mbio 2023, 14, e0180723. [Google Scholar]
- Yau, K.P.S.; Weerasinghe, H.; Olivier, F.A.B.; Lo, T.L.; Powell, D.R.; Koch, B.; Beilharz, T.H.; Traven, A. The proteasome regulator Rpn4 controls antifungal drug tolerance by coupling protein homeostasis with metabolic responses to drug stress. PLoS Pathog. 2023, 19, e1011338. [Google Scholar] [CrossRef]
- Pais, P.; Califórnia, R.; Galocha, M.; Viana, R.; Ola, M.; Cavalheiro, M.; Takahashi-Nakaguchi, A.; Chibana, H.; Butler, G.; Teixeira, M.C. Candida glabrata Transcription Factor Rpn4 Mediates Fluconazole Resistance through Regulation of Ergosterol Biosynthesis and Plasma Membrane Permeability. Antimicrob. Agents Chemother. 2020, 64, e00554-20. [Google Scholar] [CrossRef]
- Jin, X.; Luan, X.; Xie, F.; Chang, W.; Lou, H. Erg6 Acts as a Downstream Effector of the Transcription Factor Flo8 To Regulate Biofilm Formation in Candida albicans. Microbiol. Spectr. 2023, 11, e0039323. [Google Scholar]
- Crešnar, B.; Petrič, S. Cytochrome P450 enzymes in the fungal kingdom. Biochim. Biophys. Acta 2011, 1814, 29–35. [Google Scholar] [PubMed]
- Kühbacher, A.; Merschak, P.; Haas, H.; Liebl, M.; Müller, C.; Gsaller, F. The cytochrome P450 reductase CprA is a rate-limiting factor for Cyp51A-mediated azole resistance in Aspergillus fumigatus. Antimicrob. Agents Chemother. 2023, 67, e0091823. [Google Scholar]
- Zhang, C.; Ren, Y.; Gao, L.; Gu, H.; Lu, L. Electron donor cytochrome b5 is required for hyphal tip accumulation of sterol-rich plasma membrane domains and membrane fluidity in Aspergillus fumigatus. Appl. Environ. Microbiol. 2021, 87, e02571-20. [Google Scholar] [PubMed]
- Truan, G.; Epinat, J.C.; Rougeulle, C.; Cullin, C.; Pompon, D. Cloning and characterization of a yeast cytochrome b5-encoding gene which suppresses ketoconazole hypersensitivity in a NADPH-P-450 reductase-deficient strain. Gene 1994, 142, 123–127. [Google Scholar] [PubMed]
- Misslinger, M.; Gsaller, F.; Hortschansky, P.; Müller, C.; Bracher, F.; Bromley, M.J.; Haas, H. The cytochrome b5 CybE is regulated by iron availability and is crucial for azole resistance in A. fumigatus. Metallomics 2017, 9, 1655–1665. [Google Scholar]
- Craven, R.J.; Mallory, J.C.; Hand, R.A. Regulation of iron homeostasis mediated by the heme-binding protein Dap1 (damage resistance protein 1) via the P450 protein Erg11/Cyp51. J. Biol. Chem. 2007, 282, 36543–36551. [Google Scholar]
- Hughes, A.L.; Powell, D.W.; Bard, M.; Eckstein, J.; Barbuch, R.; Link, A.J.; Espenshade, P.J. Dap1/PGRMC1 binds and regulates cytochrome P450 enzymes. Cell Metab. 2007, 5, 143–149. [Google Scholar] [CrossRef]
- Song, J.; Zhai, P.; Zhang, Y.; Zhang, C.; Sang, H.; Han, G.; Keller, N.P.; Lu, L. The Aspergillus fumigatus Damage Resistance Protein Family Coordinately Regulates Ergosterol Biosynthesis and Azole Susceptibility. mBio 2016, 7, e01919-15. [Google Scholar]
- Kane, A.; Carter, D.A. Augmenting Azoles with Drug Synergy to Expand the Antifungal Toolbox. Pharmaceuticals 2022, 15, 482. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, Y.; Wu, A.; Wang, J.; Zhang, J. Strategies of targeting CYP51 for IFIs therapy: Emerging prospects, opportunities and challenges. Eur. J. Med. Chem. 2023, 259, 115658. [Google Scholar]
- Howard, K.C.; Dennis, E.K.; Watt, D.S.; Garneau-Tsodikova, S. A comprehensive overview of the medicinal chemistry of antifungal drugs: Perspectives and promise. Chem. Soc. Rev. 2020, 49, 2426–2480. [Google Scholar] [PubMed]
- Agnello, S.; Brand, M.; Chellat, M.F.; Gazzola, S.; Riedl, R. A Structural View on Medicinal Chemistry Strategies against Drug Resistance. Angew. Chem. Int. Ed. 2019, 58, 3300–3345. [Google Scholar]
- Sobel, J.D.; Nyirjesy, P. Oteseconazole: An advance in treatment of recurrent vulvovaginal candidiasis. Future Microbiol. 2021, 16, 1453–1461. [Google Scholar]
- Warrilow, A.G.; Parker, J.E.; Price, C.L.; Nes, W.D.; Garvey, E.P.; Hoekstra, W.J.; Schotzinger, R.J.; Kelly, D.E.; Kelly, S.L. The Investigational Drug VT-1129 Is a Highly Potent Inhibitor of Cryptococcus Species CYP51 but Only Weakly Inhibits the Human Enzyme. Antimicrob. Agents Chemother. 2016, 60, 4530–4538. [Google Scholar]
- Ghobadi, E.; Saednia, S.; Emami, S. Synthetic approaches and structural diversity of triazolylbutanols derived from voriconazole in the antifungal drug development. Eur. J. Med. Chem. 2022, 231, 114161. [Google Scholar] [CrossRef]
- Wiederhold, N.P.; Patterson, H.P.; Tran, B.H.; Yates, C.M.; Schotzinger, R.J.; Garvey, E.P. Fungal-specific Cyp51 inhibitor VT-1598 demonstrates in vitro activity against Candida and Cryptococcus species, endemic fungi, including Coccidioides species, Aspergillus species and Rhizopus arrhizus. J. Antimicrob. Chemother. 2018, 73, 404–408. [Google Scholar]
- Kim, J.; Lee, J.E.; Lee, J.S. Histone deacetylase-mediated morphological transition in Candida albicans. J. Microbiol. 2015, 53, 805–811. [Google Scholar]
- Lee, I.; Oh, J.H.; Shwab, E.K.; Dagenais, T.R.; Andes, D.; Keller, N.P. HdaA, a class 2 histone deacetylase of Aspergillus fumigatus, affects germination and secondary metabolite production. Fungal Genet. Biol. 2009, 46, 782–790. [Google Scholar]
- Lohse, M.B.; Johnson, A.D. Temporal anatomy of an epigenetic switch in cell programming: The white-opaque transition of C. albicans. Mol. Microbiol. 2010, 78, 331–343. [Google Scholar] [CrossRef]
- Hnisz, D.; Schwarzmüller, T.; Kuchler, K. Transcriptional loops meet chromatin: A dual-layer network controls white-opaque switching in Candida albicans. Mol. Microbiol. 2009, 74, 1–15. [Google Scholar] [CrossRef]
- Kmetzsch, L. Histone deacetylases: Targets for antifungal drug development. Virulence 2015, 6, 535–536. [Google Scholar] [PubMed]
- Su, S.; Li, X.; Yang, X.; Li, Y.; Chen, X.; Sun, S.; Jia, S. Histone acetylation/deacetylation in Candida albicans and their potential as antifungal targets. Future Microbiol. 2020, 15, 1075–1090. [Google Scholar] [PubMed]
- Pfaller, M.A.; Messer, S.A.; Georgopapadakou, N.; Martell, L.A.; Besterman, J.M.; Diekema, D.J. Activity of MGCD290, a Hos2 histone deacetylase inhibitor, in combination with azole antifungals against opportunistic fungal pathogens. J. Clin. Microbiol. 2009, 47, 3797–3804. [Google Scholar]
- Liu, W.; Sun, Z.; An, Y.; Liu, Y.; Fan, H.; Han, J.; Sun, B. Construction and activity evaluation of novel dual-target (SE/CYP51) anti-fungal agents containing amide naphthyl structure. Eur. J. Med. Chem. 2022, 228, 113972. [Google Scholar] [CrossRef]
- Zhu, T.; Chen, X.; Li, C.; Tu, J.; Liu, N.; Xu, D.; Sheng, C. Lanosterol 14α-demethylase (CYP51)/histone deacetylase (HDAC) dual inhibitors for treatment of Candida tropicalis and Cryptococcus neoformans infections. Eur. J. Med. Chem. 2021, 221, 113524. [Google Scholar]
- Li, Z.J.; Liu, M.; Dawuti, G.; Dou, Q.; Ma, Y.; Liu, H.G.; Aibai, S. Antifungal Activity of Gallic Acid In Vitro and In Vivo. Phytother. Res. PTR 2017, 31, 1039–1045. [Google Scholar]
- Prajapati, J.; Goswami, D.; Dabhi, M.; Acharya, D.; Rawal, R.M. Potential dual inhibition of SE and CYP51 by eugenol conferring inhibition of Candida albicans: Computationally curated study with experimental validation. Comput. Biol. Med. 2022, 151, 106237. [Google Scholar]
- Diener, A.C.; Li, H.; Zhou, W.; Whoriskey, W.J.; Nes, W.D.; Fink, G.R. Sterol methyltransferase 1 controls the level of cholesterol in plants. Plant Cell 2000, 12, 853–870. [Google Scholar]
- Kristan, K.; Rižner, T.L. Steroid-transforming enzymes in fungi. J. Steroid Biochem. Mol. Biol. 2012, 129, 79–91. [Google Scholar]
- Nes, W.D.; Zhou, W.; Ganapathy, K.; Liu, J.; Vatsyayan, R.; Chamala, S.; Hernandez, K.; Miranda, M. Sterol 24-C-methyltransferase: An enzymatic target for the disruption of ergosterol biosynthesis and homeostasis in Cryptococcus neoformans. Arch. Biochem. Biophys. 2009, 481, 210–218. [Google Scholar] [CrossRef]
- Ganapathy, K.; Kanagasabai, R.; Nguyen, T.T.; Nes, W.D. Purification, characterization and inhibition of sterol C24-methyltransferase from Candida albicans. Arch. Biochem. Biophys. 2011, 505, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Hou, X.; Wang, X.; Zhang, M.; Chen, J.; Song, M.; Zhang, J.; Zheng, H.; Chang, W.; Lou, H. Characterization of an allosteric inhibitor of fungal-specific C-24 sterol methyltransferase to treat Candida albicans infections. Cell Chem. Biol. 2023, 30, 553–568.e7. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Rybak, J.M.; Martin-Vicente, A.; Guruceaga, X.; Thorn, H.I.; Nywening, A.V.; Ge, W.; Parker, J.E.; Kelly, S.L.; Rogers, P.D.; et al. The sterol C-24 methyltransferase encoding gene, erg6, is essential for viability of Aspergillus species. Nat. Commun. 2024, 15, 4261. [Google Scholar] [CrossRef] [PubMed]
- Nes, W.D. Sterol methyl transferase: Enzymology and inhibition. Biochim. Biophys. Acta 2000, 1529, 63–88. [Google Scholar] [CrossRef]
- Leaver, D.J. Synthesis and Biological Activity of Sterol 14α-Demethylase and Sterol C24-Methyltransferase Inhibitors. Molecules 2018, 23, 1753. [Google Scholar] [CrossRef]
- Argüelles, J.C.; Sánchez-Fresneda, R.; Argüelles, A.; Solano, F. Natural Substances as Valuable Alternative for Improving Conventional Antifungal Chemotherapy: Lights and Shadows. J. Fungi 2024, 10, 334. [Google Scholar] [CrossRef]
- Li, S.; Wang, Y.; Zhou, J.; Wang, J.; Zhang, M.; Chen, H. Structural Characterization, Cytotoxicity, and the Antifungal Mechanism of a Novel Peptide Extracted from Garlic (Allium sativa L.). Molecules 2023, 28, 3098. [Google Scholar] [CrossRef]
- Gucwa, K.; Kusznierewicz, B.; Milewski, S.; Van Dijck, P.; Szweda, P. Antifungal Activity and Synergism with Azoles of Polish Propolis. Pathogens 2018, 7, 56. [Google Scholar] [CrossRef]
- Oliveira, V.M.; Carraro, E.; Auler, M.E.; Khalil, N.M. Quercetin and rutin as potential agents antifungal against Cryptococcus spp. Braz. J. Biol. 2016, 76, 1029–1034. [Google Scholar] [CrossRef]
- Argüelles, A.; Sánchez-Fresneda, R.; Guirao-Abad, J.P.; Belda, C.; Lozano, J.A.; Solano, F.; Argüelles, J.C. Novel Bi-Factorial Strategy against Candida albicans Viability Using Carnosic Acid and Propolis: Synergistic Antifungal Action. Microorganisms 2020, 8, 749. [Google Scholar] [CrossRef]
- Pippi, B.; Lana, A.J.; Moraes, R.C.; Güez, C.M.; Machado, M.; de Oliveira, L.F.; Lino von Poser, G.; Fuentefria, A.M. In vitro evaluation of the acquisition of resistance, antifungal activity and synergism of Brazilian red propolis with antifungal drugs on Candida spp. J. Appl. Microbiol. 2015, 118, 839–850. [Google Scholar] [PubMed]
- Voltan, A.R.; Quindós, G.; Alarcón, K.P.; Fusco-Almeida, A.M.; Mendes-Giannini, M.J.; Chorilli, M. Fungal diseases: Could nanostructured drug delivery systems be a novel paradigm for therapy? Int. J. Nanomed. 2016, 11, 3715–3730. [Google Scholar]
- Agbadamashi, D.J.; Price, C.L. Novel Strategies for Preventing Fungal Infections-Outline. Pathogens 2025, 14, 126. [Google Scholar] [CrossRef]
- Nami, S.; Aghebati-Maleki, A.; Aghebati-Maleki, L. Current applications and prospects of nanoparticles for antifungal drug delivery. EXCLI J. 2021, 20, 562–584. [Google Scholar]
- Kischkel, B.; Rossi, S.A.; Santos, S.R.; Nosanchuk, J.D.; Travassos, L.R.; Taborda, C.P. Therapies and Vaccines Based on Nanoparticles for the Treatment of Systemic Fungal Infections. Front. Cell. Infect. Microbiol. 2020, 10, 463. [Google Scholar]
- Bhatt, P.; Lalani, R.; Vhora, I.; Patil, S.; Amrutiya, J.; Misra, A.; Mashru, R. Liposomes encapsulating native and cyclodextrin enclosed paclitaxel: Enhanced loading efficiency and its pharmacokinetic evaluation. Int. J. Pharm. 2018, 536, 95–107. [Google Scholar]
- Escárcega-González, C.E.; Garza-Cervantes, J.A.; Vázquez-Rodríguez, A.; Montelongo-Peralta, L.Z.; Treviño-González, M.T.; Díaz Barriga Castro, E.; Saucedo-Salazar, E.M.; Chávez Morales, R.M.; Regalado Soto, D.I.; Treviño González, F.M.; et al. In vivo antimicrobial activity of silver nanoparticles produced via a green chemistry synthesis using Acacia rigidula as a reducing and capping agent. Int. J. Nanomed. 2018, 13, 2349–2363. [Google Scholar] [CrossRef]
- Sousa, F.; Ferreira, D.; Reis, S.; Costa, P. Current Insights on Antifungal Therapy: Novel Nanotechnology Approaches for Drug Delivery Systems and New Drugs from Natural Sources. Pharmaceuticals 2020, 13, 248. [Google Scholar] [CrossRef]
- Huang, Q.; Yu, H.; Ru, Q. Bioavailability and delivery of nutraceuticals using nanotechnology. J. Food Sci. 2010, 75, R50–R57. [Google Scholar]
- Hamill, R.J. Amphotericin B formulations: A comparative review of efficacy and toxicity. Drugs 2013, 73, 919–934. [Google Scholar] [CrossRef]
- Füredi, P.; Pápay, Z.E.; Kovács, K.; Kiss, B.D.; Ludányi, K.; Antal, I.; Klebovich, I. Development and characterization of the voriconazole loaded lipid-based nanoparticles. J. Pharm. Biomed. Anal. 2017, 132, 184–189. [Google Scholar] [PubMed]
- Hassanpour, P.; Hamishehkar, H.; Bahari Baroughi, B.; Baradaran, B.; Sandoghchian Shotorbani, S.; Mohammadi, M.; Shomali, N.; Aghebati-Maleki, L.; Nami, S. Antifungal Effects of Voriconazole-Loaded Nano-Liposome on Fluconazole-Resistant Clinical Isolates of Candida albicans, Biological Activity and ERG11, CDR1, and CDR2 Gene Expression. Assay Drug Dev. Technol. 2021, 19, 453–462. [Google Scholar] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, L.; Wang, S.; Zou, H.; Yi, X.; Jia, S.; Li, R.; Song, J. Regulation of Ergosterol Biosynthesis in Pathogenic Fungi: Opportunities for Therapeutic Development. Microorganisms 2025, 13, 862. https://doi.org/10.3390/microorganisms13040862
Song L, Wang S, Zou H, Yi X, Jia S, Li R, Song J. Regulation of Ergosterol Biosynthesis in Pathogenic Fungi: Opportunities for Therapeutic Development. Microorganisms. 2025; 13(4):862. https://doi.org/10.3390/microorganisms13040862
Chicago/Turabian StyleSong, Lingyun, Sha Wang, Hang Zou, Xiaokang Yi, Shihan Jia, Rongpeng Li, and Jinxing Song. 2025. "Regulation of Ergosterol Biosynthesis in Pathogenic Fungi: Opportunities for Therapeutic Development" Microorganisms 13, no. 4: 862. https://doi.org/10.3390/microorganisms13040862
APA StyleSong, L., Wang, S., Zou, H., Yi, X., Jia, S., Li, R., & Song, J. (2025). Regulation of Ergosterol Biosynthesis in Pathogenic Fungi: Opportunities for Therapeutic Development. Microorganisms, 13(4), 862. https://doi.org/10.3390/microorganisms13040862





